木犀草素对缺氧缺血性脑损伤新生大鼠的神经保护作用*
2022-07-06王明鹤杨峰常成高晓群
王明鹤, 杨峰, 常成, 高晓群△
木犀草素对缺氧缺血性脑损伤新生大鼠的神经保护作用*
王明鹤1, 杨峰1, 常成2, 高晓群2△
(1郑州卫生健康职业学院基础教学部,河南 郑州 450122;2郑州大学基础医学院人体解剖学系,河南 郑州 450001)
探究木犀草素(Lut)对缺氧缺血性脑损伤(HIBD)新生大鼠是否具有神经保护作用及其作用机制。将7日龄新生大鼠分为假手术组、模型组(HIBD组)和Lut治疗组(HIBD+Lut组)。用Rice-Vannucci法建立新生大鼠HIBD模型。HIBD+Lut组在造模后即刻通过腹腔注射给予50 mg/kg Lut,连续3 d,模型组和假手术组同时腹腔注射等体积生理盐水。3 d后,用氯化三苯基四氮唑(TTC)染色评估脑梗死范围;用干/湿重脑含水量法评估缺血脑半球的脑水肿情况;用苏木精-伊红(HE)染色以及原位末端转移酶标记(TUNEL)和神经元核抗原(NeuN)荧光双标共定位法观察缺血脑半球海马和皮质中神经元损伤情况;用商用试剂盒检测缺血脑半球中超氧化物歧化酶(SOD)、谷胱甘肽过氧化物酶(GSH-Px)和过氧化氢酶(CAT)活性及丙二醛(MDA)水平;用Western blot法检测缺血脑半球的海马和皮质中核因子E2相关因子2(NRF-2)和血红素加氧酶1(HO-1)的蛋白表达水平。取35日龄大鼠,用水迷宫实验评估大鼠的认知功能情况。与假手术组比较,HIBD组大鼠脑梗死、脑水肿、海马和皮质中神经元损伤均显著增加(<0.05或<0.01);而Lut治疗显著改善了HIBD大鼠的上述情况(<0.05或<0.01)。同时,Lut逆转了HIBD导致的缺血脑半球中SOD、CAT和GSH-Px活性降低和MDA水平升高(<0.05或<0.01)。Western blot结果显示,与假手术组比较,HIBD组缺血半球脑海马和皮质中NRF-2和HO-1的蛋白表达水平均增加(<0.05或<0.01);Lut治疗进一步增加了HIBD大鼠缺血半球海马和皮质中NRF-2和HO-1的蛋白表达水平(<0.01)。Lut可能通过抗氧化作用减轻HIBD新生大鼠脑梗死、脑水肿及皮质和海马CA1区神经元损伤,改善后期的认知功能。
缺氧缺血性脑损伤;木犀草素;神经元凋亡;认知功能;NRF-2/HO-1信号通路
新生儿缺血缺氧性脑损伤(hypoxic-ischemic brain damage, HIBD)是一种以脑缺氧、脑血流量减少和短暂通气障碍为特征的新生儿临床综合征[1]。统计数据表明,每1 000名新生儿中约有3~6名患有HIBD[2]。目前新生儿HIBD的临床疗法包含亚低温、吸氧、甘露醇和脑细胞营养药等[3]。然而,经过这些方法治疗后,仍有高达40%以上的HIBD新生儿出现如脑瘫、癫痫、认知障碍、发育迟缓以及社交障碍等多种神经系统后遗症甚至死亡[4]。因此,迫切需要寻找其他安全且有效的治疗新生儿HIBD措施。
新生儿HIBD的病理生理机制包括兴奋性毒性、氧化应激、神经炎症和细胞凋亡等,涉及多种病理生理过程的相互作用[1,5]。越来越多的证据表明,氧化应激与新生儿HIBD的发病机理密切相关[6-7]。HIBD后,新生儿大脑由于对氧的需求大,活性氧簇(reactive oxygen species, ROS)的过量产生和细胞抗氧化剂系统的崩溃会触发脂质过氧化、蛋白质氧化和核酸损伤,继而导致脑功能障碍和神经元损伤[7]。
木犀草素(luteolin, Lut)是一种存在于多种植物中具有如抗氧化、抗炎和神经保护等广泛药理活性的黄酮化合物[8]。近来研究显示,Lut具有在中风和脑缺血性损伤中具有神经保护作用[9]。另外,7 d龄新生大鼠的大脑发育情况在组织学上与32~34周胎儿和新生儿相似,且Rice-Vannucci模型也常被用于模拟新生儿HIBD的研究[10-12]。因此,本研究主要探索Lut在Rice-Vannucci模型新生大鼠中是否具有神经保护及其机制,以期为新生儿HIBD提供潜在的候选治疗药物。
材料和方法
1 试剂
Lut(纯度98%;#L812409)、焦油紫(#C861450)和氯化三苯基四氮唑(2,3,5-triphenyltetrazolium chloride, TTC; #T819366)购自上海麦克林生化科技有限公司;一步法FITC标记TUNEL细胞凋亡原位检测试剂盒(#KGA7072)购自江苏凯基生物技术股份有限公司;谷胱甘肽过氧化物酶(glutathione peroxidase, GSH-Px)测定试剂盒(#A005)、超氧化物歧化酶(superoxide dismutase, SOD)测定试剂盒(#A001)、过氧化氢酶(catalase, CAT)测定试剂盒(#A007)和丙二醛(malondialdehyde, MDA)测定试剂盒(#A003)均购自南京建成生物工程研究所有限公司;神经元核抗原(neuronal nuclear antigen, NeuN)兔源抗体(#BS6808)、核因子E2相关因子2(nuclear factor E2-related factor-2, NRF-2)兔源抗体(#GCP86)、血红素加氧酶1(heme oxygenase-1, HO-1)兔源抗体(#BS6626)、羊抗兔TRITC荧光Ⅱ抗(#BD5005)和羊抗兔IgG (H+L)-HRP(#BS13278)购自南京巴傲得生物科技有限公司;GAPDH兔源抗体(#WL01114)购自万类生物科技有限公司;RIPA裂解液(#P0013C)和BeyoECL Plus试剂盒(#P10018S)购自上海碧云天生物技术有限公司。
2 实验动物
12只6~7周龄SPF级SD大鼠,雌雄各6只,来源于河南省动物实验中心,生产许可证号为SCXK(豫)2017-0001。将大鼠常规饲养在SPF环境中,待饲养约3周(体重约240 g左右),用阴道涂片检测到雌鼠在发情期时,将其与雄鼠分别合笼交配后,将孕鼠继续常规饲养,待产下仔鼠后,将仔鼠与母鼠共同饲养。取7日龄新生大鼠进行后续实验。
3 方法
3.1构建HIBD大鼠模型和分组将每窝7 d龄的新生大鼠均随机分成3组:假手术组(sham组)、模型组(HIBD组)和Lut治疗组(HIBD+Lut组)。HIBD模型制作参考Rice-Vannucci模型[10-12],首先将7日龄大鼠通过吸入乙醚麻醉,在颈部中线位置切开,暴露左颈总动脉并剥离迷走神经和静脉,然后用6-0手术线结扎两头并在结扎之间切断动脉,外科缝合手术伤口,每次手术控制在5 min内,手术过程的动物体温用保温毯维持在37 ℃,待麻醉苏醒2 h后,将其置于一个低氧环境中(8%氧气和92%氮气的混合气)2.5 h,温度维持在37 ℃。假手术组除不结扎外和低氧处理外,其余手术同造模。HIBD+Lut组在造模后即刻通过腹腔注射给予50 mg/kg Lut(Lut的剂量参阅文献[8, 13-14]和预实验),连续3 d。模型组和假手术组同时腹腔注射等量生理盐水。
3.2脑梗死范围评估每组任选5只10日龄大鼠,麻醉后,取脑并在-20 ℃冷冻10 min,切为2 mm厚的连续大脑冠状切片。在37 ℃下,将切片浸泡在2%TTC溶液(PBS配制)中25 min。用ImageJ软件量化每个切片的梗死(苍白色部位)面积。梗死体积表示为所有切片的梗死面积×层厚(2 mm)之和。
3.3脑含水量检测每组任选5只10日龄大鼠,麻醉后,取脑缺血半球(左侧脑半球),立刻测量样本湿重,然后将样本置于65 ℃的烤箱中24 h,再次测量其干重。脑含水量(%)=(湿重-干重)/湿重×100%。
3.4HE染色每组任选5只10日龄大鼠,麻醉后,取左侧脑半球,用4%多聚甲醛在4 ℃固定24 h后进行石蜡包埋,并分离海马和皮质组织,制作石蜡切片(5 μm厚)。切片经脱蜡和梯度乙醇水合后,对切片进行HE染色,在光学显微镜下观察细胞形态。
3.5NeuN和TUNEL双标荧光染色取石蜡镶嵌石蜡的海马和皮质脑切片,经脱蜡和梯度乙醇水合后,用不含DNase的蛋白酶K(终浓度为20 mg/L)孵育10 min,PBS浸洗3次×3 min,滴加NeuN(1∶500)抗体和FITC标记的TUNEL检测液(50 μL),37 ℃下在避光的潮湿环境中孵育60 min。PBS浸洗3次×3 min,滴加羊抗兔TRITC荧光Ⅱ抗(1∶500),37 ℃下在避光的潮湿环境中孵育45 min。用DAPI孵育2 min。在显微镜下拍摄图像。每个切片任选5个不重叠的随机视野,并计数每个视野下的皮层和海马中凋亡神经元(TUNEL+NeuN+细胞)数量和NeuN+细胞数量,每个视野下凋亡神经元的比例(%)=TUNEL+NeuN+细胞数/TUNEL+细胞数×100%。每组纳入5只大鼠,每只大鼠至少计数6个切片。
3.6氧化应激指标的检测将10 d龄大鼠左侧半球的海马和皮质组织用0.9%生理盐水匀浆以制备10%的脑组织匀浆液,然后以2 500×、4 ℃离心10 min获得上清液后,利用商用试剂盒通过酶标仪来对SOD、GSH-Px、CAT和MDA的水平以及蛋白质浓度进行测定。GSH-Px的水平由其在反应体系中消耗每1 mg中含有1 μmol/L GSH的蛋白质的速率来表示,单位为U/mg。用亚硝酸盐法测定SOD的活性(生成的紫红色化合物,检测550 nm处吸光度,SOD活性用U/mg表示)。用钼酸铵法测定CAT活性(生成蜡黄色络合物,检测405 nm处吸光度,CAT活性用U/mg表示)。用巯基苯甲酸法检测MDA的含量(生成红色化合物,检测532 nm处吸光度,MDA含量用nmol/mg表示)。
3.7Western blot检测p-PI3K、PI3K、p-Akt、Akt和VEGF的蛋白水平通过RIPA裂解缓冲液裂解各组左侧半球海马和皮质组织,超声破碎后,在4 ℃下以11 000×离心12 min,取上清。并通过BCA试剂盒对上清进行蛋白浓度检测。取等量蛋白通过SDS-PAGE凝胶电泳分离蛋白质样品,并转移到PVDF膜上并封闭。4 ℃下,将膜分别与1∶1 000稀释的NRF-2、HO-1和GADPH抗体孵育过夜,用TBS浸洗3次×5 min,之后将膜与1∶1 000稀释羊抗兔IgG (H+L)-HRP Ⅱ抗室温孵育2 h,用TBS浸洗3次×5 min。用BeyoECL Plus试剂盒显示条带蛋白,并用eBlot化学发光成像系统采集图像和分析目的蛋白相对GADPH的表达水平。
3.8水迷宫实验将大鼠饲养至35日龄后,进行水迷宫实验。将35日龄大鼠置于站台(置于水下1.5 cm)外的一个象限入水(水温25 ℃),连续5 d,每天用摄影仪监测的大鼠的游泳路径并计算逃避潜伏期。第6天,撤除站台,监测大鼠穿越原站台位置的次数和原站台所在象限滞留的时间。
4 统计学处理
数据表示为均数±标准差(mean±SD),用SPSS 20.0软件采用单因素方差分析法对数据进行分析,均数的两两比较采用事后Bonferroni校正。以<0.05为差异有统计学意义。
结果
1 Lut减轻HIBD大鼠的脑梗死和脑水肿
如图1所示,与sham组相比,HIBD组脑梗死体积(图1A)和左半球脑含水量(图1B)均显著增加(<0.05或<0.01);与HIBD组相比,HIBD+Lut组脑梗死体积(图1A)和脑含水量(图1B)均显著减少(<0.05或<0.01)。

Figure 1. Luteolin (Lut) alleviated cerebral infarction and cerebral edema after HIBD. A: representative TTC staining images and quantitative analysis of cerebral infarct size in each group (red represented normal brain tissue, and pale represented infarcted brain tissue); B: quantitative analysis of brain water content in each group. Mean±SD. n=5. #P<0.05,###P<0.01 vs sham group;*P<0.05,**P<0.01 vs HIBD group.
2 Lut抑制HIBD大鼠的神经元损伤
HE染色(图2A)显示,sham组脑组织中细胞核大、细胞浆少,且密度多正常;HIBD组缺血半球的皮质和海马区细胞呈现肿胀、球囊样变、细胞稀疏、间隙增宽、细胞排列紊乱,且部分细胞发生核固缩;HIBD+Lut组缺血半球的皮质和海马区中细胞形态和密度趋向正常。NeuN和TUNEL双标荧光共定位染色显示,与sham组相比,HIBD组缺血半球脑组织皮质(图2B)和海马CA1区(图2C)凋亡神经元均显著增多(<0.01);与HIBD组相比,HIBD+Lut组缺血半球脑组织皮质(图2B)和海马CA1区(图2C)凋亡神经元均显著减少(<0.01)。

Figure 2. Luteolin (Lut) reduced neuronal injury after HIBD. A: representative HE staining images of the cortex and hippocampal CA1 region in each group (scale bar=20 μm); B: representative NeuN (red) and TUNEL (green) co-location staining images and quantified results of apoptotic neurons in the cortex (scale bar=20 μm); C: representative NeuN (red) and TUNEL (green) co-location staining images and quantified results of apoptotic neurons in the hippocampal CA1 region (scale bar=20 μm). Mean±SD. n=5. ##P<0.01 vs sham group;**P<0.01 vs HIBD group.
3 Lut降低HIBD大鼠脑内氧化应激水平
如图3所示,与sham组相比,HIBD组缺血半球脑组织中MDA含量(图3A)显著升高(<0.01),SOD(图3B)、CAT(图3C)和GSH-Px(图3D)活性均显著降低(<0.01);与HIBD组相比,HIBD+Lut组缺血半球脑组织中MDA含量(图3A)显著降低(<0.05),SOD(图3B)、CAT(图3C)和GSH-Px(图3D)活性均显著升高(<0.05或<0.01)。
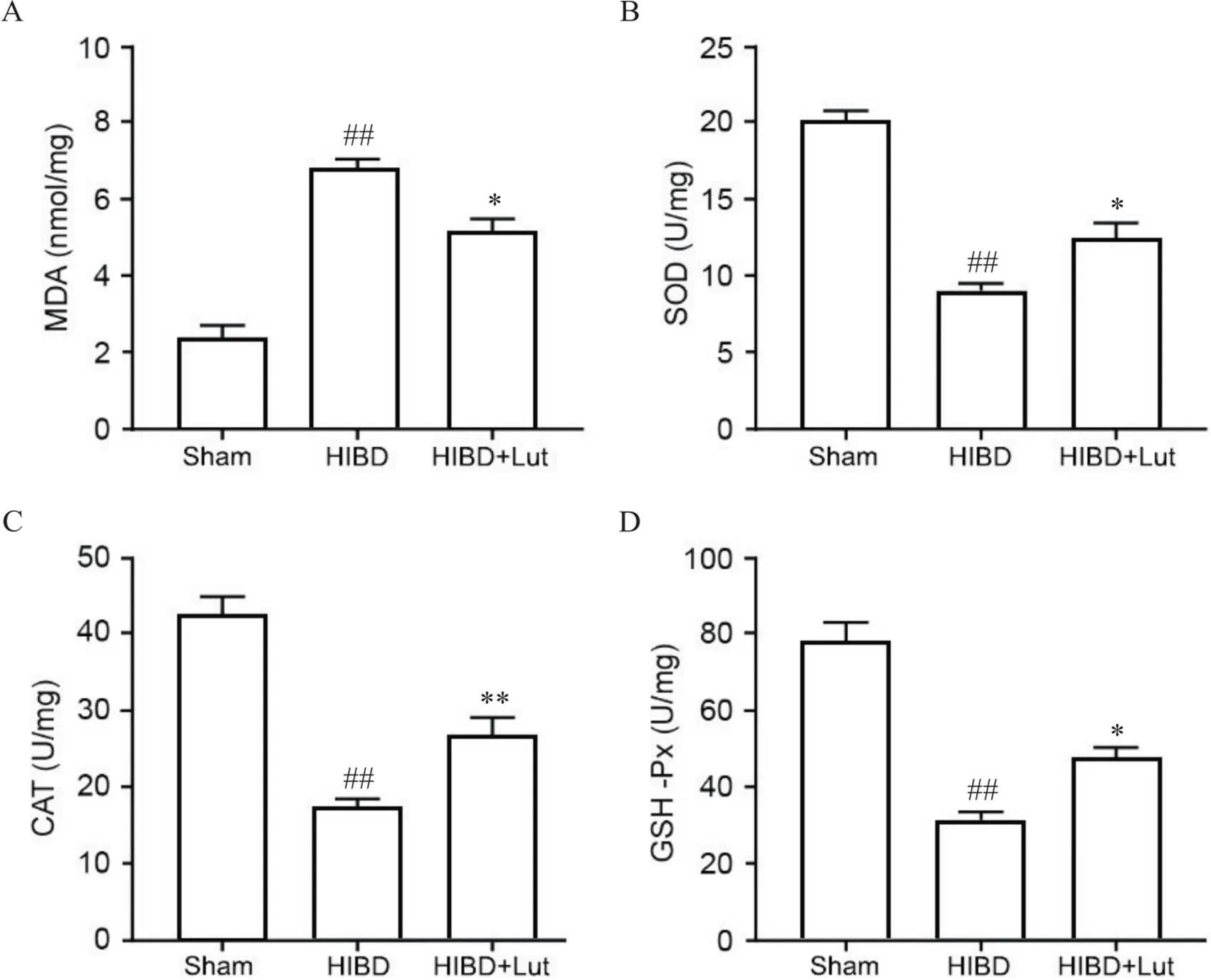
Figure 3. Luteolin (Lut) reduced oxidative stress in brain tissues after HIBD. A: quantitative analysis of MDA content; B: quantitative analysis of SOD activity; C: quantitative analysis of CAT activity; D: quantitative analysis of GSH-Px activity. Mean±SD. n=5. ##P<0.01 vs sham group;*P<0.05,**P<0.01 vs HIBD group.
4 Lut进一步增强HIBD大鼠脑组织中NRF-2/HO-1信号活性
Western blot结果显示,与sham组相比,HIBD组缺血半球的海马和皮质中NRF-2和HO-1的蛋白表达水平均显著增加(<0.05或<0.01);与HIBD组相比,HIBD+Lut组缺血半球海马和皮质中NRF-2和HO-1的蛋白表达水平均显著增加(<0.01),见图4。

Figure 4. Luteolin (Lut) promoted the protein expression of NRF-2 and HO-1 in brain tissues after HIBD. A: the protein expression levels of NRF-2 and HO-1 in the cortex were detected by Western blot; B: the protein expression levels of NRF-2 and HO-1 in the hippocampal CA1 region were detected by Western blot. Mean±SD. n=5. #P<0.05,##P<0.01 vs sham group;**P<0.01 vs HIBD group.
5 Lut改善HIBD大鼠认知功能
水迷宫实验分析结果(图5)显示,在35日龄时,与sham组相比,HIBD组大鼠的逃避潜伏期显著延长(<0.01),与HIBD组相比,HIBD+Lut组大鼠的逃避潜伏期显著缩短(<0.01);各组大鼠的游泳速度并未见显著差异(>0.05);移除站台后,HIBD+Lut组较HIBD组大鼠探索隐藏站台时间显著延长(<0.01),且穿越隐藏站台次数显著增多(<0.01)。

Figure 5. Luteolin (Lut) improved cognitive function in HIBD rats. A: swimming track diagram of each group; B: escape latency of rats in each group; C: swimming speed of rats in each group; D: the time of exploring hidden platform in each group; E: the number of crossing hidden platform in each group. Mean±SD. n=5. ##P<0.01 vs sham group;**P<0.01 vs HIBD group.
讨论
由宫内窒息、围产期窒息或产后窒息造成的新生儿HIBD是导致全球新生儿因神经系统致残的常见病因之一[1-4]。目前并没有根治新生儿HIBD的办法。基于7日龄新生大鼠建立的Rice-Vannucci模型[10-12]是用于模拟新生儿HIBD的常用模型,本研究采用该模型研究Lut对新生大鼠HIBD的影响。脑梗死和脑水肿是评估药物对缺氧缺血性脑病有效性的关键指标,脑梗死和脑水肿程度不仅能反映此病的急性期脑损伤状况,而且也能影响此病的长期神经恢复情况[15]。另外,未发育成熟的大脑海马CA1区易受到缺血缺氧诱导的损伤,而海马CA1区也是影响认知和学习功能的关键区域[16]。本研究显示,Lut不仅在脑损伤的急性期改善HIBD新生大鼠的脑梗死和脑水肿,减轻HIBD引起的皮质和海马CA1区神经元损伤,并且在后期改善认知功能,提示Lut可在HIBD新生大鼠中能发挥神经保护作用,是潜在的HIBD候选治疗药物。
氧化应激被认为是新生儿脑损伤后最早出现的病理变化。新生儿脑代谢旺盛,脑耗氧需求量高,又含有高浓度的不饱和脂肪酸并且内源性的抗氧化酶含量低,加之活性铁的存在,极易受到氧化损伤[1, 5-7, 17]。Bratek等[18]研究表明,乙酰天冬氨酰谷氨酸治疗可通过降低脑内氧化应激反应进而改善新生大鼠HIBD导致的脑损伤和认知功能障碍。本研究显示,Lut治疗显著增加了脑组织中内源性抗氧化酶SOD、CAT和GSH-Px的活性,且降低了脂质过氧化,体现了Lut在HIBD模型新生大鼠脑内的抗氧化应激的作用。NRF-2/HO-1信号通路是机体内主要的抗氧化系统信号通路之一,其在受到氧化损伤时被激活[19]。已有研究[20]报道,在新生大鼠的脑缺血组织中NRF-2和HO-1表达上调,进一步增加NRF-2/HO-1信号活性则能增强下游抗氧化酶(如HO、SOD、CAT和GSH-Px等)活性,降低脑损伤。本研究也显示,Lut能增强HIBD模型新生大鼠缺血脑半球皮质和海马中NRF-2/HO-1活性。
综上所述,本研究证明了Lut在HIBD模型新生大鼠脑损伤的急性期可减轻脑梗死、脑水肿及皮质和海马CA1区神经元损伤,降低脑内氧化应激反应,增强NRF-2/HO-1活性,并且在后期可改善认知功能。
[1] Yıldız EP, Ekici B, Tatlı B. Righini, neonatal hypoxic ischemic encephalopathy: an update on disease pathogenesis and treatment[J]. Expert Rev Neurother, 2017, 17(5):449-459.
[2] Lee BL, Glass HC. Cognitive outcomes in late childhood and adolescence of neonatal hypoxic-ischemic encephalo-pathy[J]. Clin Exp Pediatr, 2021, 64(12):608-618.
[3] Nair J, Kumar VHS. Current and emerging therapies in the management of hypoxic ischemic encephalopathy in neonates[J]. Children (Basel), 2018, 5(7):99.
[4] Papazian O. Neonatal hypoxic-ischemic encephalopathy[J]. Medicina (B Aires), 2018, 78(Suppl 2):36-41.
[5] Greco P, Nencini G, Piva I, et al. Pathophysiology of hypoxic-ischemic encephalopathy: a review of the past and a view on the future[J]. Acta Neurol Belg, 2020, 120(2):277-288.
[6] Solevåg AL, Schmölzer GM, Cheung PY. Novel interventions to reduce oxidative-stress related brain injury in neonatal asphyxia[J]. Free Radic Biol Med, 2019, 142:113-122.
[7] Thornton C, Baburamani AA, Kichev A, et al. Oxidative stress and endoplasmic reticulum (ER) stress in the deve-lopment of neonatal hypoxic-ischaemic brain injury[J]. Biochem Soc Trans, 2017, 45(5):1067-1076.
[8]吴兵,生梦飞,张想旺,等. 木犀草素对脊髓损伤大鼠Nrf2/HO-1通路的影响[J]. 中国药师, 2021, 24(10):1833-1837.
Wu B, Sheng MF, Zhang XW, et al. Effects of luteolin on Nrf2/HO-1 pathway in rats with spinal cord injury[J]. Chin Pharm, 2021, 24(10):1833-1837.
[9] Dong R, Huang R, Shi X, et al. Exploration of the mechanism of luteolin against ischemic stroke based on network pharmacology, molecular docking and experimental verification[J]. Bioengineered, 2021, 12(2):12274-12293.
[10]蔡晨晨,叶丽霞,朱将虎,等. 鞣花酸通过降低自噬作用减轻缺氧缺血性脑损伤[J]. 中国病理生理杂志, 2019, 35(2):311-319.
Cai CC, Ye LX, Zhu JH, et al. Ellagic acid attenuates hypoxic-ischemic brain injury by alleviating autophagy[J]. Chin J Pathophysiol, 2019, 35(2):311-319.
[11]欧阳颖,苏浩彬,薛红漫,等. 丙酮酸乙酯对缺氧缺血性脑损伤新生大鼠脑组织的保护作用[J]. 中国病理生理杂志, 2013, 29(7):1181-1185.
Ou-yang Y, Su HB, Xue HM, et al. Protective effect of ethyl pyruvate on brain tissues in neonatal rats with hypoxic-ischemic brain damage[J]. Chin J Pathophysiol, 2013, 29(7):1181-1185.
[12] Lan XB, Wang Q, Yang JM, et al. Neuroprotective effect of vanillin on hypoxic-ischemic brain damage in neonatal rats[J]. Biomed Pharmacother, 2019, 118:109196.
[13] Su J, Xu HT, Yu JJ, et al. Luteolin ameliorates lipopolysaccharide-induced microcirculatory disturbance through inhibiting leukocyte adhesion in rat mesenteric venules[J]. BMC Complement Med Ther, 2021, 21(1):33.
[14] Huang Y, Zhang X. Luteolin alleviates polycystic ovary syndrome in rats by resolving insulin resistance and oxidative stress[J]. Am J Physiol Endocrinol Metab, 2021, 320(6):E1085-E1092.
[15] Nakano T, Nishigami C, Irie K, et al. Goreisan prevents brain edema after cerebral ischemic stroke by inhibiting aquaporin 4 upregulation in mice[J]. J Stroke Cerebrovasc Dis, 2018, 27(3):758-763.
[16] Li Z, Fang F, Wang Y, et al. Resveratrol protects CA1 neurons against focal cerebral ischemic reperfusion-induced damage via the ERK-CREB signaling pathway in rats[J]. Pharmacol Biochem Behav, 2016, 146/147:21-27.
[17] Zhao M, Zhu P, Fujino M, et al. Oxidative stress in hypoxic-ischemic encephalopathy: molecular mechanisms and therapeutic strategies[J]. Int J Mol Sci, 2016, 17(12):2078.
[18] Bratek E, Ziembowicz A, Salinska E, et al.-acetylaspartylglutamate (NAAG) pretreatment reduces hypoxic-ischemic brain damage and oxidative stress in neonatal rats[J]. Antioxidants (Basel), 2020, 9(9):877.
[19] Yang BB, Zou M, Zhao L, et al. Astaxanthin attenuates acute cerebral infarction via Nrf-2/HO-1 pathway in rats[J]. Curr Res Transl Med, 2021, 69(2):103271.
[20] Qiu J, Chao D, Sheng S, et al. δ-opioid receptor-Nrf-2-mediated inhibition of inflammatory cytokines in neonatal hypoxic-ischemic encephalopathy[J]. Mol Neurobiol, 2019, 56(7):5229-5240.
Neuroprotective effect of luteolin on neonatal rats with hypoxic-ischemic brain damage
WANG Ming-he1, YANG Feng1, CHANG Cheng2, GAO Xiao-qun2△
(1,,450122,;2,,,450001,)
To investigate the effects of luteolin (Lut) on hypoxic-ischemic brain damage (HIBD) in neonatal rats and its mechanism.Seven-day-old neonatal rats were divided into sham group, model group (HIBD group) and Lut treatment group (HIBD+Lut group). The HIBD model of neonatal rats was established by Rice-Vannucci method. The rats in HIBD+Lut group were given 50 mg/kg Lut by intraperitoneal injection immediately after modeling for 3 consecutive days, while the rats in model group and sham group were intraperitoneally injected with the same volume of normal saline. Three days later, the size of cerebral infarction was evaluated by 2,3,5-triphenyltetrazolium chloride (TTC) staining. The cerebral edema of ischemic hemisphere was assessed by dry/wet weight brain water content method. The injury of neurons in the hippocampus and cortex of ischemic cerebral hemisphere was observed by HE staining and TUNEL-NeuN fluorescence co-location. The activity of superoxide dismutase (SOD), glutathione peroxidase (GSH-Px) and catalase (CAT), and the content of malondialdehyde (MDA) in ischemic cerebral hemisphere were measured with commercial kits. The expression levels of nuclear factor E2-related factor-2(NRF-2) and heme oxygenase-1 (HO-1) proteins in the hippocampus and cortex of ischemic cerebral hemisphere were detected by Western blot. The cognitive function of 35-day-old rats was evaluated by water maze test.Compared with sham group, cerebral infarction, cerebral edema, and neuronal injury in the hippocampus and cortex were all significantly increased in HIBD group (<0.05 or<0.01). However, the above situation of HIBD rats was significantly improved after Lut treatment (<0.05 or<0.01). Meanwhile, Lut reversed the decrease in SOD, CAT and GSH-Px activity and the increase in MDA content in ischemic cerebral hemisphere induced by HIBD (<0.05 or<0.01). Western blot results showed that compared with sham group, the expression levels of NRF-2 and HO-1 proteins in the hippocampus and cortex of ischemic hemisphere in HIBD group were increased (<0.05 or<0.01), while Lut treatment further increased NRF-2 and HO-1 protein expression levels in the hippocampus and cortex of ischemic hemispheres of HIBD rats (<0.01).Treatment with Lut alleviates cerebral infarction, cerebral edema, neuronal damage in the cortex and hippocampal CA1 region and later cognitive dysfunction in neonatal HIBD rats through antioxidant effect.
Hypoxic-ischemic brain damage; Luteolin; Neuronal apoptosis; Cognitive function; NRF-2/HO-1 signaling pathway
R722.1; R363.2
A
10.3969/j.issn.1000-4718.2022.06.005
1000-4718(2022)06-0993-08
2022-02-09
2022-03-18
国家卫生健康委出生缺陷预防重点实验室2019年开放课题(No. ZD201901)
Tel: 0371-66658363; E-mail: gxq@zzu.edu.cn
(责任编辑:林白霜,李淑媛)
