Long noncoding RNAs in mesenchymal stromal/stem cells osteogenic differentiation:Implications in osteoarthritis pathogenesis
2022-07-05DanielQuinteroHugoRodriguezAnishPottyDimitriosKouroupisAshimGupta
Daniel Quintero,Hugo C Rodriguez,Anish G Potty,Dimitrios Kouroupis,Ashim Gupta
Daniel Quintero,Department of Orthopaedics,Division of Sports Medicine,University of Miami,Miller School of Medicine,Miami,FL 33136,United States
Hugo C Rodriguez,Holy Cross Orthopedic Institute: Fort Lauderdale Practice,Oakland Park,FL 33334,United States
Anish G Potty,Ashim Gupta,South Texas Orthopedic Research Institute,Laredo,TX 78045 United States
Dimitrios Kouroupis,Diabetes Research Institute,Cell Transplant Center,University of Miami,Miller School of Medicine,Miami,FL 33136,United States
Ashim Gupta,BioIntegrate,Lawrenceville,GA 30043,United States
Ashim Gupta,Future Biologics,Lawrenceville,GA 30043,United States
Abstract This letter focuses on a recently published article that provided an exceptional description of the effect of epigenetic modifications on gene expression patterns related to skeletal system remodeling.Specifically,it discusses a novel modality of epigenetic regulation,the long noncoding RNAs(lncRNAs),and provides evidence of their involvement in mesenchymal stromal/stem cells osteo-/adipogenic differentiation balance.Despite focus on lncRNAs,there is an emerging cross talk between lncRNAs and miRNAs interaction as a novel mechanism in the regulation of the function of the musculoskeletal system,by controlling bone homeostasis and bone regeneration,as well as the osteogenic differentiation of stem cells.Thus,we touched on some examples to demonstrate this interaction.In addition,we believe there is still much to discover from the effects of lncRNAs on progenitor and non-progenitor cell differentiation.We incorporated data from other published articles to review lncRNAs in normal progenitor cell osteogenic differentiation,determined lncRNAs involved in osteoarthritis pathogenesis in progenitor cells,and provided a review of lncRNAs in non-progenitor cells that are differentially regulated in osteoarthritis.In conclusion,we really enjoyed reading this article and with this information we hope to further our understanding of lncRNAs and mesenchymal stromal/stem cells regulation.
Key Words: Long noncoding RNAs;Epigenetics;Mesenchymal stromal/stem cells;Degenerative bone diseases;Osteoarthritis;Osteoporosis
TO THE EDITOR
We read with great interest the review article by Xiaet al[1],titled “Epigenetic regulation by long noncoding RNAs in osteo-/adipo-genic differentiation of mesenchymal stromal cells and degenerative bone diseases”.We believe the article provides an exceptional description of the effect of epigenetic modifications on gene expression patterns related to skeletal system remodeling.Specifically,it discusses a novel modality of epigenetic regulation,the long noncoding RNAs(lncRNAs),and provides evidence of their involvement in mesenchymal stromal/stem cells(MSCs)osteo-/adipo-genic differentiation balance.We agree with the authors’ insight that lncRNAs are relevant to clinical practice as altered MSCs differentiation status can be implicated in the initiation/progression of various musculoskeletal pathologies such as osteoarthritis and osteoporosis.We do,however,have several clarifications we wish to provide.
In the introduction,MSCs are defined as “a heterogenous population of cells which include fibroblast,myofibroblast and progenitor cells”[1].Even though this definition was previously introduced by International Society for Cell &Gene Therapy Mesenchymal Stromal Cell Committee[2],it can be misleading within the present article as authors evaluate the effect of lncRNAs on cells that possess differentiation capacity and not fully differentiated cells(such as fibroblasts).Instead,authors could introduce MSCs as mesenchymal stromal/stem cells are fibroblast-like cells capable of multilineage differentiation at leastin vitrothat possess strong paracrine and immunomodulatory propertiesin vivo.Additionally,even though MSCs are originated from a single cell population during embryogenesis,authors should acknowledge that MSCs show intrinsic propensities to osteo-/adipo-genic differentiation strongly related to their tissue of origin and functional MSC subset heterogeneity[3].This may significantly affect the role of specific lncRNAs on the overall epigenetic regulation of MSCs differentiation.
In the present article authors have nicely presented the interactions between lncRNAs and epigenetic modifiers during osteo-/adipo-genic MSCs’ differentiation.However,in recent years the crosstalk between lncRNAs and miRNAs interaction has emerged as a novel mechanism in the regulation of the function of the musculoskeletal system,by controlling bone homeostasis and bone regeneration,as well as the osteogenic differentiation of stem cells[4].We totally acknowledge that the topic of the present article is not miRNAs,however authors could elaborate more on this significant interaction.For example,ANRIL lncRNA was correlated with increased MSCs osteogenic differentiation in the present article.According to recent studies,the molecular mechanism of ANRIL lncRNA effects is based on its direct binding to circulating miR-7a involved in activating the NFKB signaling pathway[5].Other lncRNAs that exert their osteoinductive activities on progenitor cellsviabinding to miRNAs are MALAT1 and PGC1β-OT1[6,7].Similarly,HOTAIR lncRNAviamiR-17-5p interaction inhibits osteogenic differentiation in individuals with a traumatic osteonecrosis of the femoral head.This is in relation to a variable activation of SMAD7 which directly influences osteoblastic differentiation[8].On this basis of lncRNAs and miRNAs interactions,it seems that H19 lncRNA is a major regulator of MSCs osteogenic differentiation.Specifically,H19 lncRNA actviathree modes of action:(1)Up-regulate miR-675 expression and inhibit the phosphorylation of TGF-β1 and Smad3;(2)inhibit the expression of miR-141 and miR-22 and promote Wnt/β-catenin signal transduction pathway;and(3)inhibit the expression of miR-107,miR-27b,miR-106b,miR-125a,and miR-17 resulting in Notch signaling pathway regulation[9-11].
Pathological mechanisms of osteoarthritis(OA)development involve the interplay of different OA symptoms,including inflammatory and degenerative changes that lead to destruction of articular cartilage,deranged chondrocyte regeneration,osteophyte formation,subchondral sclerosis and hyperplasia of synovial tissue.Yet,we must make a distinction between lncRNAs expression in progenitor cells and lncRNAs expression changes in terminally differentiated cells such as chondrocytes as their implication on cell differentiation and protein expression are remarkably different.Herein,in addition to the present article data we incorporated data from other literature to:(1)Review MSCs/progenitor cells lncRNAs involved in osteogenic differentiation;(2)determine MSCs/progenitor cells lncRNAs involved in OA pathogenesis;and(3)provide a review of lncRNAs in non-progenitor cells that are differentially regulated in OA.
On this basis,we identified four lncRNAs that are upregulated in MSCs/progenitor cells: DANCR,MALAT1,THRIL and LINC0051;and five lncRNAs are downregulated in MSCs/progenitor cells,specifically chondrogenic cell line ATDC5: XIST,NR024118,HULC,LncRNA-ATB,OIP5-AS1.A summary of these findings is featured in Figure 1 and Table 1[12-20].
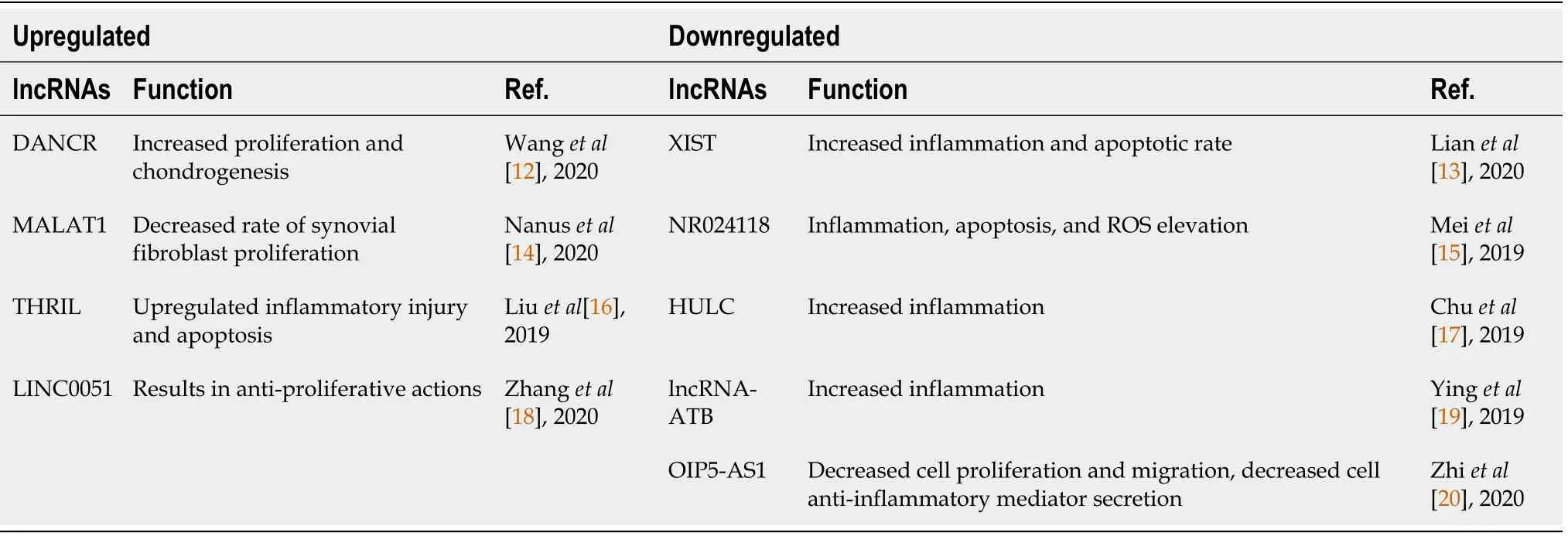
Table 1 Supplementary information to Figure 1 detailing source and mechanism of activity associated with modified long noncoding RNAs
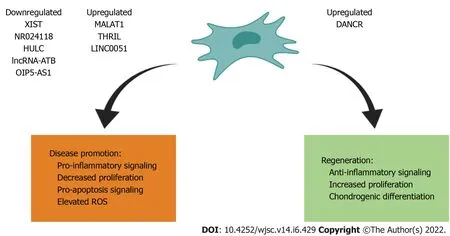
Figure 1 Effects of various long noncoding RNAs on mesenchymal stromal/stem cells/progenitor cells for disease promotion and regeneration.
lncRNAs strongly regulate chondrocytes expression patterns in both physiological and pathological conditions.Twelve different lncRNAs were upregulated in terminally differentiated chondrocytes.We summarize these findings in Figure 2 and Table 2[21-32].
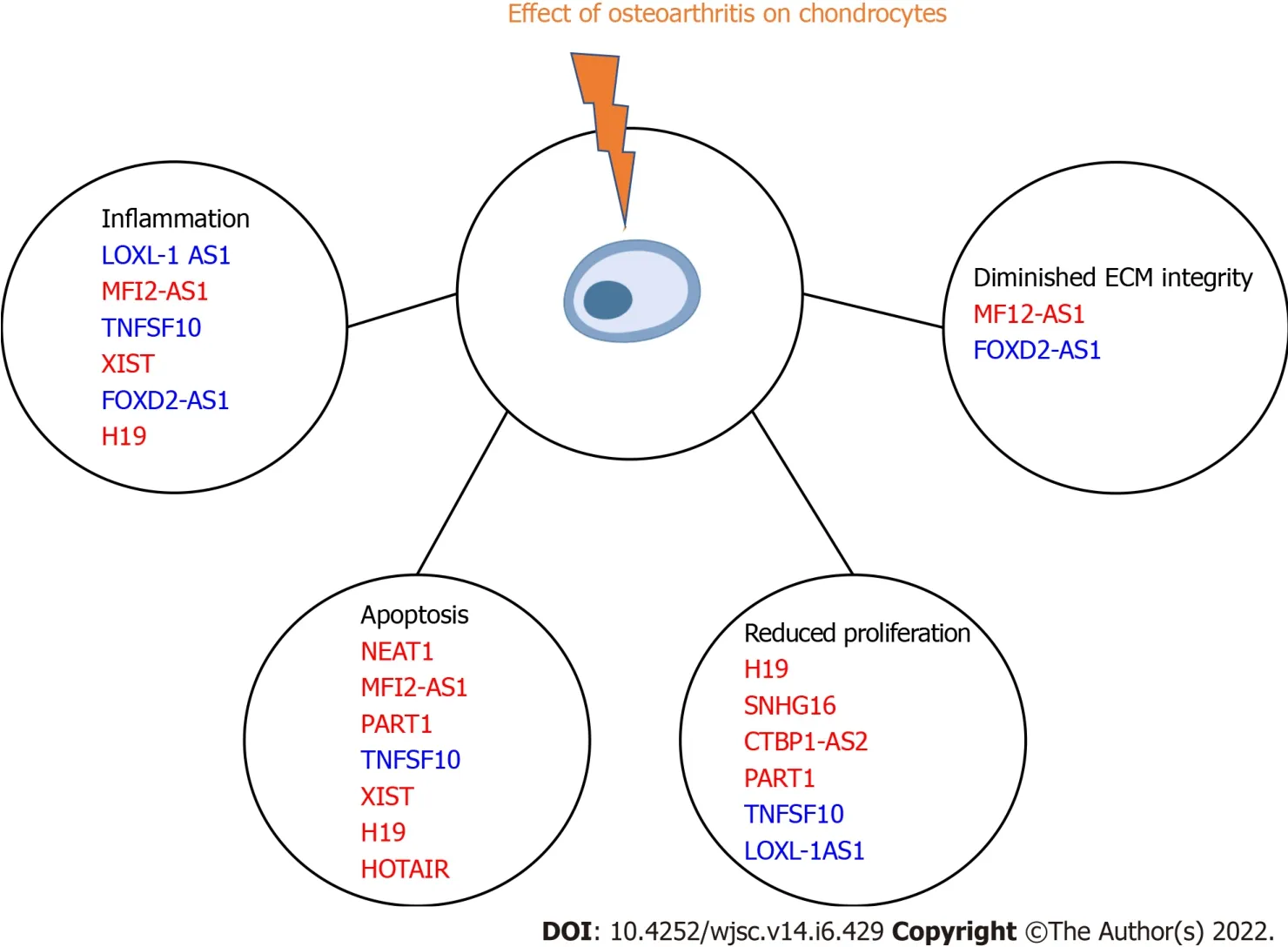
Figure 2 Effects of various long noncoding RNAs on chondrocytes in osteoarthritis.Red text indicates promotion of pathogenesis,while blue text indicated regeneration by opposing pathogenic signaling.ECM: Extracellular matrix.
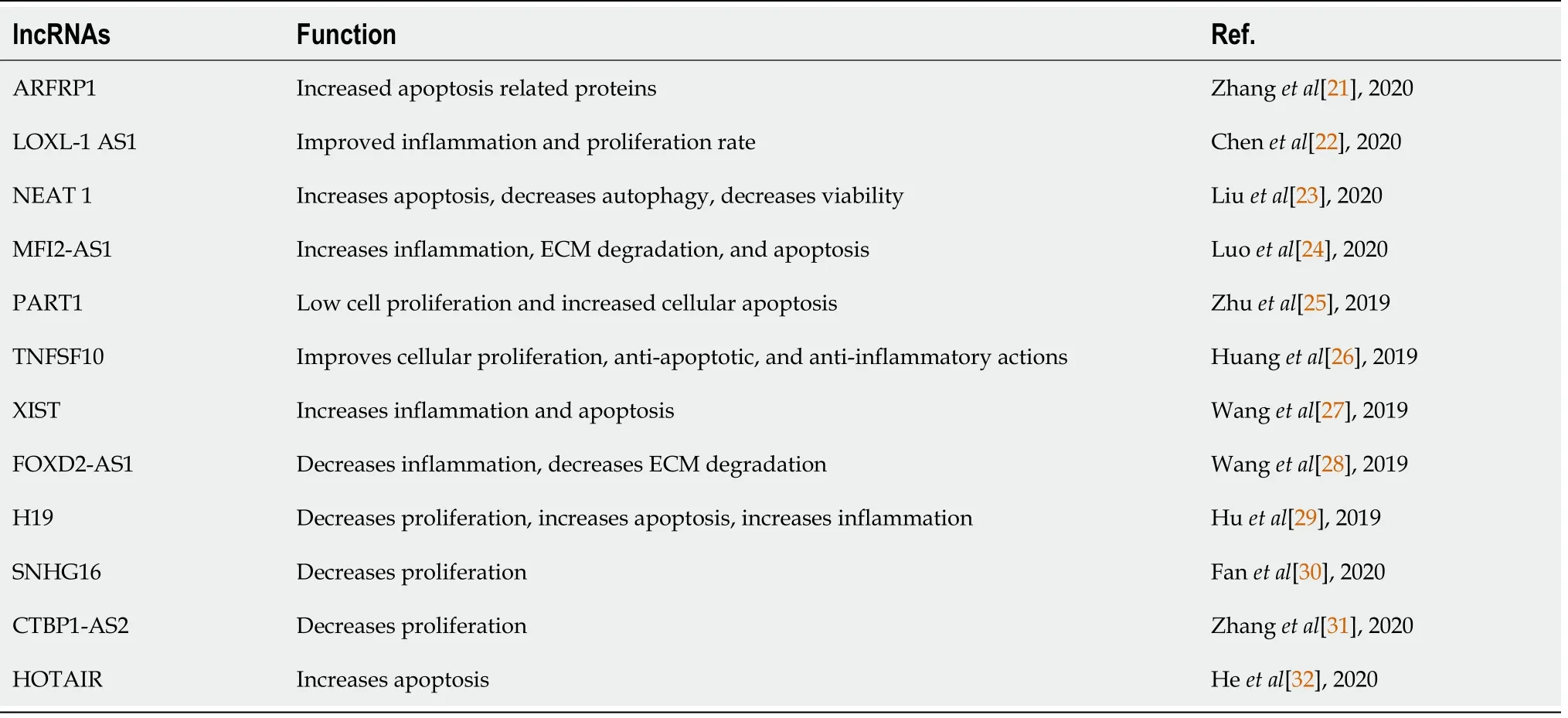
Table 2 Supplementary information to Figure 2 detailing source and mechanism of activity associated with modified long noncoding RNAs
In conclusion,we believe there is still much to discover from the effects of lncRNAs on progenitor and non-progenitor cell differentiation.We incorporated data from a recent review article by Ghafouri-Fardet al[33]among other articles to:(1)Review lncRNAs in normal progenitor cell osteogenic differentiation;(2)determine lncRNAs involved in OA pathogenesis in progenitor cells;and(3)provide a review of lncRNAs in non-progenitor cells that are differentially regulated in OA.We provided a superficial review of lncRNAs expression and osteoarthritis to clarify what was mentioned and separated the regulation in progenitor and non-progenitor cells,which was not previously published.Again,we really enjoyed the reading by Xiaet al[1]and with this information we hope to further our understanding of lncRNAs and mesenchymal stromal/stem cells regulation.
FOOTNOTES
Author contributions:Gupta A and Kouroupis D conceptualized the study;Quintero D,Rodriguez HC,Potty AG,Kouroupis D,and Gupta A outlined and designed the manuscript;Quintero D,Rodriguez HC,Kouroupis D and Gupta A drafted the manuscript;Potty AG,Kouroupis D and Gupta A critically reviewed and edited the manuscript;all authors approved the final version of the article for publication.
Conflict-of-interest statement:All the authors report no relevant conflicts of interest for this article.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial(CC BYNC 4.0)license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is noncommercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:United States
ORCID number:Daniel Quintero 0000-0002-4018-5850;Hugo C Rodriguez 0000-0003-3566-1776;Anish G Potty 0000-0001-9894-1500;Dimitrios Kouroupis 0000-0002-3892-9013;Ashim Gupta 0000-0003-1224-2755.
Corresponding Author's Membership in Professional Societies:American Academy of Regenerative Medicine;American College of Sports Medicine;International Society for Extracellular Vesicles;American Society of Regional Anesthesia and Pain Medicine;North American Neuromodulation Society;and Orthopedic Research Society.
S-Editor:Gong ZM
L-Editor:A
P-Editor:Gong ZM
杂志排行
World Journal of Stem Cells的其它文章
- Disagreements in the therapeutic use of mesenchymal stem cellderived secretome
- Adipose tissue in bone regeneration-stem cell source and beyond
- Application and prospects of high-throughput screening for in vitro neurogenesis
- Role of stem cells-based in facial nerve reanimation:A meta-analysis of histological and neurophysiological outcomes
