Disagreements in the therapeutic use of mesenchymal stem cellderived secretome
2022-07-05FerencSiposGyrgyizes
Ferenc Sipos,Györgyi Műzes
Ferenc Sipos,Györgyi Műzes,Department of Internal Medicine and Hematology,Semmelweis University,Budapest 1088,Hungary
Abstract In a recent article,the authors provide a detailed summary of the characteristics and biological functions of mesenchymal stem cells(MSCs),as well as a discussion on the potential mechanisms of action of MSC-based therapies.They describe the morphology,biogenesis,and current isolation techniques of exosomes,one of the most important fractions of the MSC-derived secretome.They also summarize the characteristics of MSC-derived exosomes and highlight their functions and therapeutic potential for tissue/organ regeneration and for kidney,liver,cardiovascular,neurological,and musculoskeletal diseases,as well as cutaneous wound healing.Despite the fact that MSCs are regarded as an important pillar of regenerative medicine,their regenerative potential has been demonstrated to be limited in a number of pathological conditions.The negative effects of MSC-based cell therapy have heightened interest in the therapeutic use of MSC-derived secretome.On the other hand,MSC-derived exosomes and microvesicles possess the potential to have a significant impact on disease development,including cancer.MSCs can interact with tumor cells and promote mutual exchange and induction of cellular markers by exchanging secretome.Furthermore,enzymes secreted into and activated within exosomes can result in tumor cells acquiring new properties.As a result,therapeutic applications of MSC-derived secretomes must be approached with extreme caution.
Key Words: Mesenchymal stem cells;Secretome;Exosomes;Regeneration;Therapy;Cancer
INTRODUCTION
Commentary on hot topics
Stem cell and tissue engineering studies appear to be critical components of regenerative medicine.Stem cells are characterized as totipotent,pluripotent,multipotent,or unipotent depending on their ability to differentiate into new cell lines.While allogeneic cells can create complications such as immunological rejection,when autologous cells are utilized,rejection can be avoided,making this a less risky mode of treatment.
Adult stem cells,such as mesenchymal stem cells(MSCs)and hematopoietic stem cells,are the most commonly used types in clinical practice,owing to their availability from individuals with various medical conditions(e.g.,aplastic anemia,Duchenne muscular dystrophy,ankylosing spondylitis,etc.)[1].
MSCs have the ability to self-renew while also possessing a limited potential to distinguish from one another.Bone marrow,adipose tissue,liver,skin,lungs,cord blood,and fallopian tubes are their primary sources[2].
MSC-based treatments are widely used around the world,with their effects mediatedviainduced differentiation,immunological modulation,cell fusion,paracrine actions,mRNA or micro-RNA(miRNA)carriage,and mitochondrial metastasis.MSCs for therapeutic purposes face challenges such as maintaining a homogeneous culture and,further,characterization of the cells[3].In addition to cell replacement,MSCs possess a diverse array of functional characteristics(i.e.,angiogenesis,fibrosis inhibitory as well as anti-apoptotic capacity,directed migration,immunomodulation,growth and differentiation supporting activity on other stem cells)[4-7].The release of bioactive components,referred to as the secretome,into the conditioned media of cell culture is one of their most intriguing qualities[8].The secretome is composed of two fractions: Soluble and vesicular.Immunomodulatory molecules,chemokines,cytokines,and growth factors are abundant in the soluble fraction.The vesicular fraction consists of extracellular vesicles that can be categorized as apoptotic bodies,microvesicles,and exosomes based on their diameter and synthesis route.Exosomes and microvesicles containing lipids,proteins,or nucleic acids comprise the secretome derived from MSCs[8].As indicated above,the secretome has the potential to directly stimulate target cells through endocytosis and to exert a wide range of actions[9].However,it is critical to keep in mind that,depending on where the MSCs come from,the secretome's therapeutic potential may differ[10].
MSCs are an important pillar in regenerative medicine due to their wide range of functional capabilities.As a result,to ensure that no functional or genetic alterations occur during clinical use,their biosafety characteristics should be examined.MSCs have a number of disadvantages,including their detrimental effect on the pulmonary microvasculature,host cell rejection,and ectopic tissue formation[11-13].Additionally,it has been demonstrated that MSCs have a very limited capacity for regeneration,particularly in pathological conditions.While MSCs are found in a variety of tissues,their numbers are relatively small.Furthermore,transplanted cells’ viability and uptake into host tissues are frequently compromised[14].Also,a variety of factors,such as the donor’s age,the number of passages and culture conditions used duringin vitrogrowth,administration procedure,and the deleterious host microenvironment encountered by the relocated MSCs,may have a negative effect on the cells’ proclivity for survival and engraftment in host tissues[15].Recent studies have also indicated possible protumorigenic activities of MSCs[16,17],along with pro-fibrogenic and pro-coagulant potentials[18,19],a higher risk of infections(e.g.,zoonotic illnesses)during thein vitrogrowth process[20],and the unfavorable heterogeneity of their differentiation potential(Figure 1)[21].Due to these drawbacks,their clinical application has been limited.As a result,it is necessary to develop alternative,complication-free MSC-based therapeutic strategies.
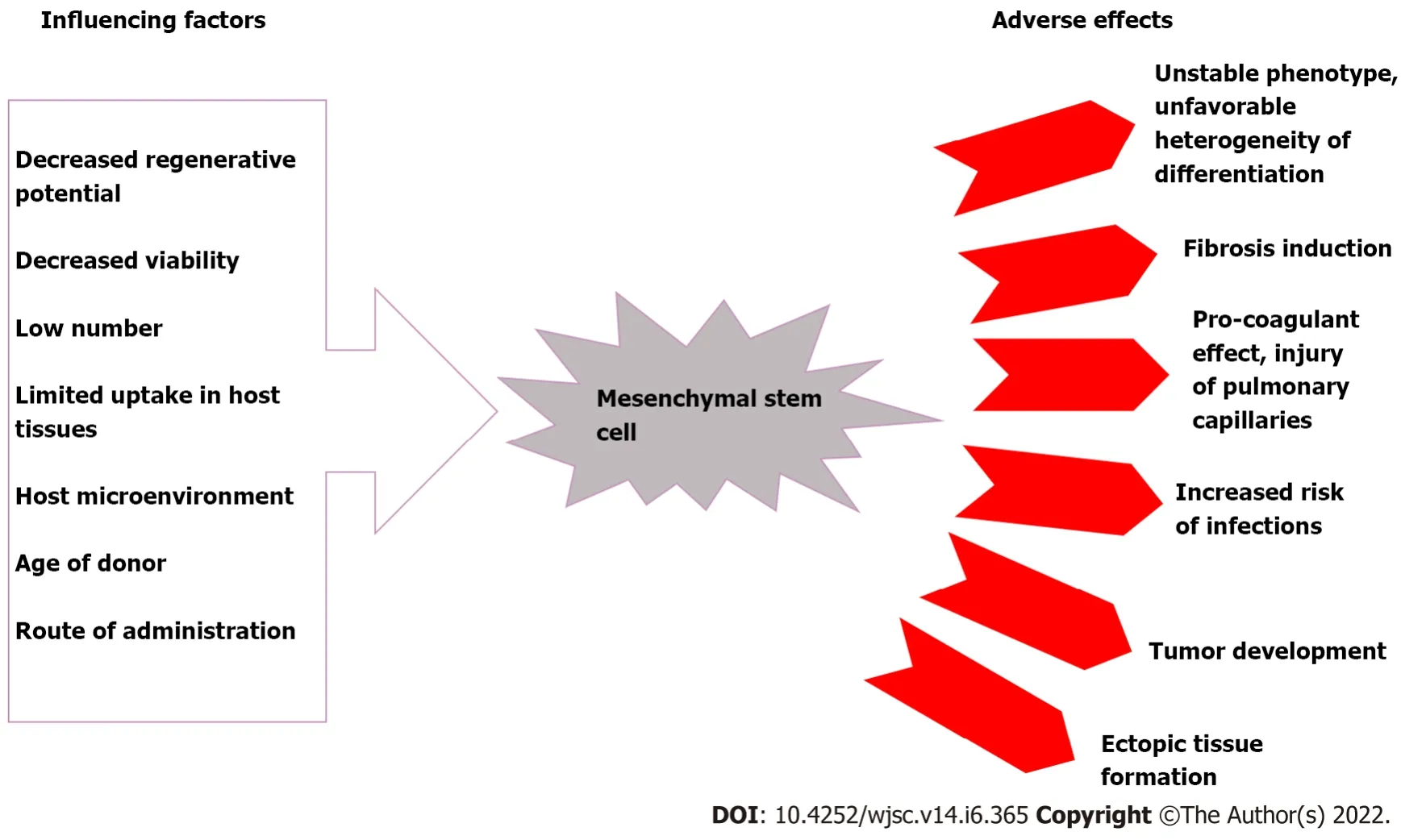
Figure 1 Factors influencing the therapeutic potential of mesenchymal stem cells and their consequences.
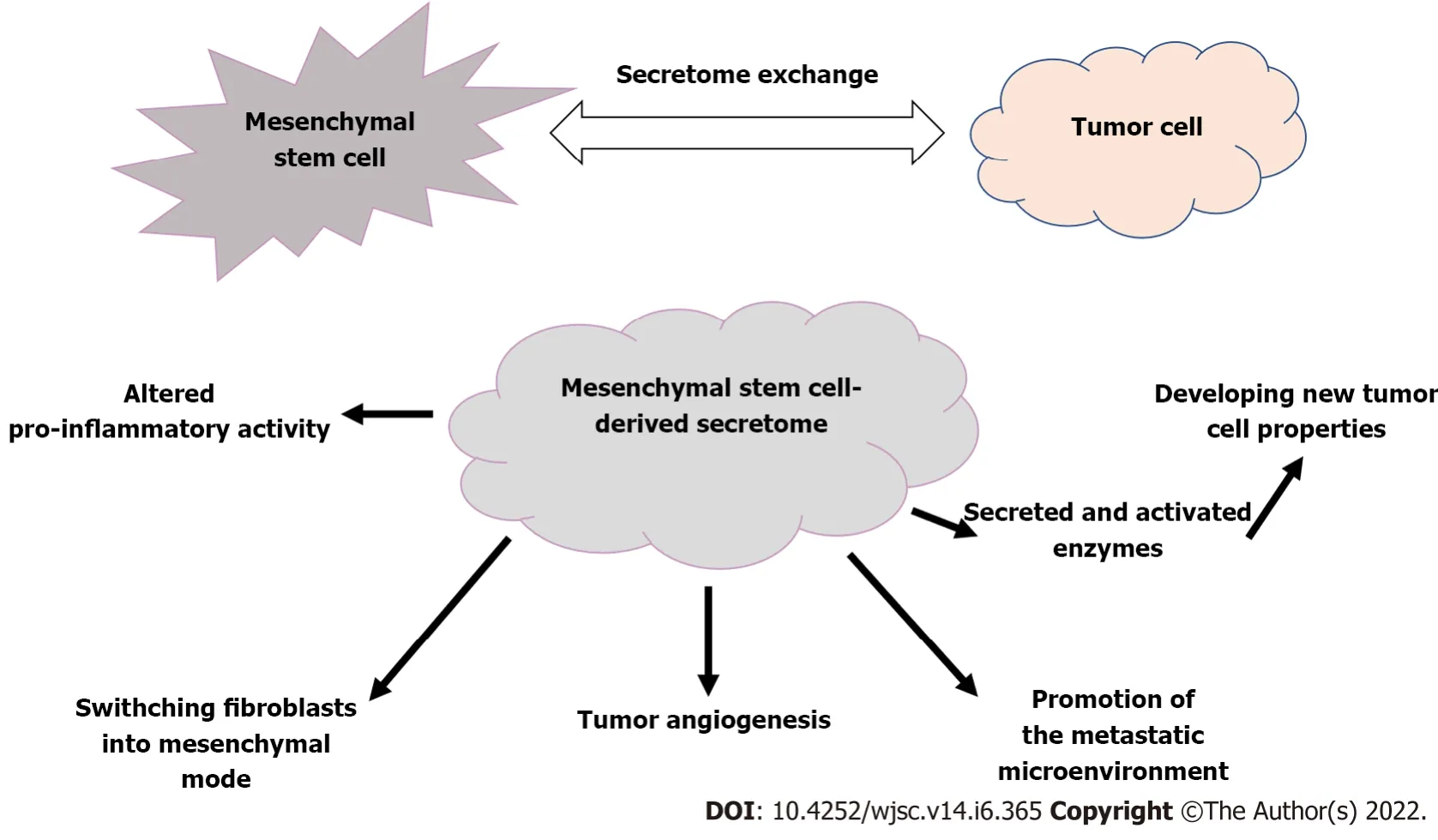
Figure 2 The secretome exchange between mesenchymal stem cells and tumor cells has unfavorable effects.
In a recent review by Maet al[22],the authors provide a detailed summary of the characteristics and biological functions of MSCs and discuss the potential mechanisms of action of MSC-based therapies.They describe the morphology,biogenesis,and current isolating techniques of exosomes,one of the most important fractions of the MSC-derived secretome.
UNDESIRABLE EFFECTS OF THE MSC SECRETOME
The consequences of the treatments with MSC-derived cells have heightened interest in the MSCs’secretome for therapeutic purposes.The application of MSCs’ secretome has a number of significant benefits,including the complete absence of the necessity for an invasive solution to obtain cells,the capability of conducting pharmacological dosage and safety tests,the convenience of application,and the possibility of manipulating the composition[23].Soluble and vesicular factors derived from MSCs exhibit a variety of unique properties that may make them a precious tool for therapeutic reasons[8].Maet al[22]compiled a list of the numerous regenerative medicine benefits of MSC-derived exosomes[22].Simple collection,long-term stability,safety,optimal drug transport capacity,and tissue or microenvironment-specific targeting are the most critical of these.Additionally,they summarized recent research on the actions of MSC-derived exosomes in different diseases affecting the skin,bone,muscle,kidney,cardiovascular system,liver,and nervous system.
However,practical difficulties appear in cases of those entities,as their physical and biochemical properties frequently cause complications to obtain them as perfect and correctly characterized preparations.As a result,the International Society for Extracellular Vesicles developed guidelines for the field in 2014(i.e.,Minimal Information for Studies of Extracellular Vesicles),which were recently revised in 2018[24].
We must not forget that exosomes can also play a significant role in the development of diseases such as cancer.When tissue is damaged,MSCs are recruited to aid in the repair and regeneration of wounds.Also,aggressive tumor development results in inflammation-related tissue injury as a result of intense cell recruitment and cross-modulation.By exchanging secretome,MSCs have the potential to interact with tumor cells[25-28],promoting reciprocal interchange and induction of biological markers[29,30].
Not only the direct effect of the MSC-secreted soluble fraction,but enzymes excreted into and activated inside exosomes(primarily matrix metalloproteinases and their regulators)could make malignant cells have novel properties[25].The secretome's vesicular fraction is involved in the formation of the pre-metastatic niche and tumor neovascularization.In addition,abnormalities in the extracellular matrix may influence cancer progression by promoting fibroblastic switching and acquisition of mesenchymal mode[26].
The incorporation of MSC-derived exosomes has been linked to the development of ecto-5′-nucleotidase activity in a subset of tumor cells[25].Tumor cells equipped with this unique ability are capable of suppressing and modulating inflammation-inducing activity by way of the stimulation of adenosine receptor signaling located in the external membrane of the majority of immunocompetent cells,(e.g.,tumor-infiltrating T-cell function)[31,32].
In the opposite direction,tumor cells can also affect and modify MSCs through the use of their secretome[22,26].Extracellular vesicles produced by cancer stem cells are capable of establishing a metastasis supportive compartment and inducing an epithelial to mesenchymal transition,allowing tumors to spread more easily(Figure 2)[26].
Along with undesirable biological properties,current methods for isolating the vesicular secretome(e.g.,membrane filtration,ultracentrifugation,precipitation,immunoaffinity capture technology,and size exclusion chromatography)are inefficient,yielding small quantities of low-purity,occasionally distorted extracellular vesicles.As a result,their further application presents difficulties[22,33-35].
CONCLUSION
In accordance with ClinicalTrials.gov,the number of studies utilizing the MSC-derived secretome is fairly small(i.e.,ten),notwithstanding the fact that just three have been completed so far.While the restorative potential of MSC-originated secretome appears auspicious,care is advised.Not only is the content and function of the secretome formed from MSCs largely dependent on the environment from which they were derived(i.e.,healthy,inflammatory or tumorous environment),but the therapeutic targeting of the secretome is also difficult at the moment[36].Whichever method of application is employed,it is not yet feasible to be assured that the biologically active chemical will work on a particular cell type,nor is it totally likely to identify how the intended physiological action of the secretome is altered by the surrounding milieu.
Currently,we also lack knowledge on how drug combinations used in disease conditions affect MSCs and their secretome.By altering MSCs to carry anticancer miRNAs,oncolytic viruses,and anticancer drugs into tumor areas,scientists are able to overcome a number of barriers[37].However,additional research is required to determine the influence of probable epigenetic or genetic alterations in MSCs on the content and biological functions of the secretome.This is critical to prevent the possibility of tumorigenicity[38].
Along with the technical challenges associated with locating and separating MSCs,laboratory approaches that are novel and efficient are required to extract the MSC-derived secretome in sufficient quality and quantity for application in daily routines.In addition,it would be advantageous to minimize the time and expense involved in these novel procedures,thereby effectively promoting their spread.In conclusion,there is no doubt that,in relation to cell-based techniques,cell-free bioactive components such as the secretome could serve as a significant option in translational medicine.
ACKNOWLEDGEMENTS
We would like to express our gratitude to Anika Scott for her assistance.
FOOTNOTES
Author contributions:Sipos F and Műzes G contributed to study conception and design,data collection,analysis and interpretation of results,and manuscript preparation.
Supported byStartup Program of Semmelweis University Faculty of Medicine,No.SE10332470.
Conflict-of-interest statement:All the authors report no relevant conflicts of interest for this article.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial(CC BYNC 4.0)license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is noncommercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:Hungary
ORCID number:Ferenc Sipos 0000-0002-2767-7746;Györgyi Műzes 0000-0002-9099-0372.
S-Editor:Fan JR
L-Editor:Webster JR
P-Editor:Fan JR
杂志排行
World Journal of Stem Cells的其它文章
- Adipose tissue in bone regeneration-stem cell source and beyond
- Application and prospects of high-throughput screening for in vitro neurogenesis
- Role of stem cells-based in facial nerve reanimation:A meta-analysis of histological and neurophysiological outcomes
- Long noncoding RNAs in mesenchymal stromal/stem cells osteogenic differentiation:Implications in osteoarthritis pathogenesis
