Adipose tissue in bone regeneration-stem cell source and beyond
2022-07-05LuminitaLabusca
Luminita Labusca
Luminita Labusca,Magnetic Materials and Sensors,National Institute of Research and Development for Technical Physics,Iasi 700050,Romania
Luminita Labusca,Orthopedics and Traumatology,County Emergency Hospital Saint Spiridon Iasi,Iasi 700050,Romania
Abstract Adipose tissue(AT)is recognized as a complex organ involved in major homeostatic body functions,such as food intake,energy balance,immunomodulation,development and growth,and functioning of the reproductive organs.The role of AT in tissue and organ homeostasis,repair and regeneration is increasingly recognized.Different AT compartments(white AT,brown AT and bone marrow AT)and their interrelation with bone metabolism will be presented.AT-derived stem cell populations-adipose-derived mesenchymal stem cells and pluripotentlike stem cells.Multilineage differentiating stress-enduring and dedifferentiated fat cells can be obtained in relatively high quantities compared to other sources.Their role in different strategies of bone and fracture healing tissue engineering and cell therapy will be described.The current use of AT-or AT-derived stem cell populations for fracture healing and bone regenerative strategies will be presented,as well as major challenges in furthering bone regenerative strategies to clinical settings.
Key Words: Adipose tissue;Bone metabolism;Fracture healing;Adipose-derived stem cells;Multilineage differentiating stress-enduring;Dedifferentiated fat cells;Bone engineering
INTRODUCTION
Adipose tissue(AT)has multiple roles in body energy balance,regulation of food intake,immunomodulation and growth and functioning of the reproductive organs[1].In recent years,the understanding of AT functioning has evolved from considering it a lipid storage,cushioning and thermal insulating mass to its recognition as the largest endocrine organ within the mammalian body[2].AT-derived signaling molecules,adipokines and cytokines have a crucial role in local and systemic regulation by controlling energy expenditure,glucose homeostasis,insulin metabolism and immune cell function to support cell proliferation in normal and pathological states.AT has received increased attention in recent years mainly due to its abnormal expansion in obesity and metabolic syndrome.Normal AT has intricate roles in maintaining healthy body functioning.Not surprisingly,obesity is very often accompanied by many endocrine and metabolic disturbances,such as insulin resistance,type 2 diabetes mellitus(T2D),disorders in immune regulation and response to pathogens,and tumor occurrence,progression and metastasis[3].The pathological lack of AT(congenital,human immunodeficiency virus-or age-related lipodystrophy)is linked to multiple metabolic and immune abnormalities,such as insulin resistance,liver steatosis and dyslipidaemia[4].Given its multifaceted roles in controlling body homeostatic mechanisms,AT involvement in tissue and organ healing and regeneration is complex and only partially recognized.Understanding AT involvement in regeneration and repair is a relatively new concept.Consistent research in recent decades has focused on AT as a mesenchymal stem cell source.Pluripotent stem cells extracted from white AT(WAT)under special conditions together with transdifferentiated adipocytes have the potential to accelerate progress in the field of bone engineering.AT,with its complex paracrine and endocrine signaling and angiogenetic potential,might also be used to support the bone regenerative niche.The types of AT-WAT,brown(and beige)AT(BAT)and bone marrow AT(BMAT)-will be very briefly introduced with emphasis on BMAT given its direct involvement in bone metabolism.Current strategies that employ AT or AT-derived cell populations for fracture healing or bone regeneration will be presented.
WAT IS A COMPLEX ENDOCRINE ORGAN
In humans,WAT is formed starting from the second semester of gestation and continues throughout life,even in adults[5].In mice,WAT adipocytes are derived from mesenchymal progenitors within the somites or lateral plate mesoderm,which could be the case for humans[6],except for minor fat deposits of the skull derived from the ectodermal neural crest[7].WAT therefore shares a developmental origin with all the components of connective tissue(muscle,bone,tendon and fascia).WAT is composed of mature cells(adipocytes)that contain unilocular deposits of lipids(triacylglycerol)occupying up to 90%of the cytoplasm.Only one-third of the tissue is represented by mature adipocytes,and other cellular components are preadipocytes,stromal cells,mesenchymal progenitors and immune components[monocytes and macrophages(Mcfs)].WAT represents the major energy storage system of the body and is the main lipid deposit[8].It has a mechanical role in thermal insulation,organ cushioning and protection from trauma.In humans,mature adipocytes store lipids synthetized in the liver and,to a minor extent,within AT itself by lipogenesis,an insulin-dependent enzymatic process.Fatty acid(FA)and triglycerides(TG)availability in other organs in the case of energy demand are dependent on the activity of AT lipolytic enzymes[9].Two main compartments of WAT are described based on their anatomic location: Subcutaneous and visceral.Their characteristic distribution is sex hormonedependent with visceral compartment testosterone and subcutaneous oestrogen-controlled[10].Mature adipocytes release bioactive molecules(commonly denominated adipokines)that exert paracrine and endocrine functions in maintaining body energetic metabolism,insulin sensitivity,food intake,immune modulation,haematopoiesis,bone metabolism,angiogenesis,coagulation and fibrinolysis.Adipokines are a set of cytokine(leptin,adiponectin visfatin)chemokine(nitric oxide,hydrogen peroxide)growth factors,vascular endothelial growth factor(VEGF),hepatocyte growth factor(HGF),and colonystimulating factor 1 complement factors,such as adipsin,B,C,and C3[11].It is beyond the scope of this paper to describe their role in body balance;however,it should be noted that numerous bioactive molecules released by normal mature adipocytes are among crucial factors implicated in all the stages of wound and bone healing.
WAT is a very dynamic tissue that largely fluctuates in quantity but also its functional qualities across growth,maturity and ageing,physiological stages and diseases.Obesity as a disease of excess and lipodystrophy as a sum of conditions where WAT does not form or becomes atrophic are both associated with important perturbations in adipocyte quality and functioning.In obesity,adipocytes increase in number(hyperplasia)and size(hypertrophy),while in lipodystrophy,de novoadipogenesis and lipid droplet formation are impaired.Obesity and lipodystrophy are accompanied by severe metabolic disorders,such as hypertriglyceridemia,insulin resistance,diabetes,and fatty liver,as well as by severe perturbation in adipokine release.Impaired metabolic status and abnormal WAT paracrine and endocrine signalling in diseases of lack and excess have consequently impaired wound and bone healing.
ROLE OF WAT IN TISSUE REGENERATION
WAT deposits and healthy functioning mature adipocytes are involved in homeostatic maintenance,turnover and repair of several organs and tissues,such as hair follicles,skin and mammary glands[12].Bone morphogenetic protein(BMP)expression by mature adipocytes may be regulators of the quiescent stage of hair follicle stem cells and supportive of hair lineage specification and differentiation during hair growth[13].Adipogenic progenitors(AP)within dermal WAT stimulate hair stem cell follicle activation.Intradermal AP injection was shown to increase the growth of hair cell follicles,while leptin expressed by mature adipocytes induced the activation of hair cell follicles and hair shaft growth[14].Mature adipocytes are a major component of the dermis that supports the skin epithelial layer and keratinocytes.Dermal WAT was previously thought to exert a cushioning and insulating function;however,its role in supporting skin integrity and promoting wound healing is increasingly recognized and explored.Mature adipocyte-secreted adiponectin and leptin were shown to increase keratinocyte proliferationin vitroand to consistently increase wound re-epithelialization in mouse models[15],while adiponectin-deficient mice suffer from severely delayed wound epithelialization[16].Adiponectin regulates local apoptosis,and its absence in diabetic subjects might explain hyperkeratosis and thickened wound margins characteristic of chronic ulcers[17].Another adipokine,leptin,was shown to increase re-epithelialization,angiogenesis and wound contraction after injury[18].Functional mature adipocytes are required for the third stage of wound healing and extracellular matrix(ECM)deposition by fibroblasts.Mouse strains that lack mature adipocytes have impaired fibroblast repopulation during wound healing,and incomplete ECM deposition leads to recurrent wounding in this model[19].Mature adipocytes are required for the development of a functional mammary gland ductal tree.In lipodystrophic and inducible adipocyte loss mouse strains,mammary gland formation is impaired[20].
WAT presence within muscles is commonly associated with tissue degeneration.Muscle fatty atrophy is a frequent clinical correlation with insulin resistance and increased body max index(BMI).However,if mature adipocytes in muscle tissue are a witness of impaired muscle function,common adipogenic and fibroblast progenitor(FAP)multipotent muscle resident stromal cells are required for muscle wound healing and tissue growth.FAPs enhance the rate of differentiation of primary myogenic progenitorsin vitroand expand during muscle injury.FAPs were shown to supply transient pro-differentiation signals for proliferating myogenic progenitor cells after muscle injury in animal models[21].Interestingly,when FAPs are transplanted within subcutaneous or dermal tissue,they differentiate into WAT,demonstrating the role of the environment in their activation and differentiation.FAPs are present in human skeletal muscle tissue and could be the source of fatty infiltration during muscle degeneration[22].These progenitors have dual responsiveness to environmental cues,being able to generate either adipocytes or to support muscle hypertrophy.FAPs respond to metabolic stress during metabolic disorders by conversion to adipocyte lineage,as well as mechanical stress,during physical activity by fibrogenic conversion and contribution to satellite cell activation.This response might explain their opposite role in fatty infiltration and muscle healing and hypertrophy[23].
WAT IN BONE METABOLISM AND FRACTURE HEALING
WAT and bone metabolism are coordinated directly by the central nervous system through sympathetic and parasympathetic innervation and indirectly by circulating hormones.Sympathetic innervation controls WAT metabolism,while the parasympathetic role in this tissue is less agreed upon.Indirectly,sympathetic innervation of adrenal glands controls glucocorticoid release.Elevated levels of glucocorticoids are clinically associated with bone loss and hypertrophic WAT expansion during obesity[24].Ghrelin,a neuroendocrine hormone produced in the gastrointestinal tract,has a dual role in regulating white adipocyte metabolism by increasing lipoprotein lipase and FA synthase and increasing peroxisome proliferator-activated receptor-β(PPAR-β),stimulating the synthesis of TG and their mobilization.Ghrelin was found to directly promote osteoblast proliferation and differentiation,resulting in increased bone mineral density(BMD)in animal models[25].Several WAT-released adipocytes have dual roles in bone and adipose maintenance and turnover.Leptin is known to inhibit food intake,increase energy expenditure and reduce WAT by increasing lipolysis.Leptin has a direct effect in promoting bone marrow stem cell(BMSC)differentiation to osteoblasts and preventing their adypogenic conversion[26].Leptin increases the expression of osteocalcin(OC),alkaline phosphatase and collagen I,which are required for osteoblast maturation[27].Leptin is involved in controlling bone resorption by increasing osteoclast-inhibiting osteoprotegerin(OPG)[28].Direct administration was found to increase BMD and femur length in leptin-deficient mice[29].Adiponectin,the adipokine that is most commonly found in plasma,improves insulin sensitivity,increases the rate of FA oxidation and reduces inflammation and fatty muscle infiltration.Low levels of circulating adiponectin are found in obese and lipodystrophic mouse models[30],have been implicated in inducing insulin resistance in obese subjects and proposed as a serum biomarker for detecting metabolic syndrome[31].Adiponectin was found to promote osteogenesis in BMSCs by indirectly increasing BMP2 expression[32],to increase alkaline phosphatase(ALP),collagen I and OC expression and to promote osteoblast proliferation and differentiation in a dose-dependent manner[33].Similar to leptin,adiponectin inhibits osteoclast activity through distinct mechanisms.Adiponectin decreases the expression of cathepsin K and acid-resistant phosphatase,which are osteoclast regulators that increase osteoclast apoptosis[34].Notably,the endocrine and paracrine roles of adipokines in bone metabolism are contradictory.The results fromin vitroandin vivostudies are sometimes contradictory,and no direct correlation between increased levels of adipokines and supported bone metabolism could be clearly stated.Several factors could be involved,such as dosage,the timing of adipokine release and their effect in mediating inflammation[35].It appears more likely that WAT and bone metabolism crosstalk is contextual.The presence of adipokine receptors on BMSCs and osteoblasts demonstrates that anabolic bone metabolism has a direct interrelation with WAT adipocytes.The WAT-bone catabolic interrelation is exerted through distinct pathways that regulate osteoclast formation or apoptosis.
WAT contains an important fraction of immune cells.Local AT-resident Mcfs(MATs)in normal/lean individuals display an anti-inflammatory phenotype described as the M2(alternatively activated)phenotype,which supports WAT expansion during adaptation to a high-fat diet.Prolonged WAT inflammation,however,leads to fibrosis and ECM stiffness,hindering adipocyte expansion and lipid storage[36].WAT expansion during obesity is associated with increased levels of macrophage chemoattractant-1,which determines the accumulation of high levels of M1(inflammatory)MATs that can induce insulin resistance[37].Other local immune cells,regulatory T cells(Tregs),which are more abundant in lean but not obese WAT,have been shown to promote the M2 MAT phenotype,and their increase in obese WAT can improve insulin sensitivity[38].
Mcfs are present during the inflammatory phase of fracture healing in humans and animals.Compared to Mcf derived from blood monocytes,tissue-resident cells seem to have a more important role in fracture healing.Bone marrow and periosteal M1 phenotype Mcf-released cytokines[interleukin(IL)-1,IL-6 and tumor necrosis factor(TNF)]are present at the fracture site during the first days after injury in animal models and humans.M1 phenotype persistence during callus formation delays or compromises healing.Bone-specific Mcfs(osteomacs)have been involved in bone healing and remodelling;however,other Mcf tissues might also contribute[39].Tissue-resident M1 and M2 MCfs are involved inde novoangiogenesis within the granulation tissue during fracture healing,as well as stem cell/progenitor cell recruitment to the fracture site and their differentiation.Oncostatin M,a cytokine of the IL-5 family produced by M1 Mcfs,was shown to induce osteoblast differentiation and matrix mineralization from human mesenchymal stem cells while inhibiting adipogenesisin vitro[40].Furthermore,the number but most of all the Mcf phenotype required for fracture healing might be dependent on the type of callus formation(enchondralvsendosteal)and the type of fracture fixation.More detailedin vivostudies are required to decipher the origin of Mcf involved in fracture healing;however,these cells apparently need to switch phenotype upon environmental stimuli during the time course of bone healing.MAT involvement in bone remodeling and fracture healing needs further investigation.Delayed fracture healing during obesity and metabolic syndrome might be[41],at least in part,explained by the presence of M1MAT,which prolongs the inflammatory stage and prevents callus maturation.
BAT
The typical BAT is located between the shoulder blades in smaller mammals.In newborn humans,the interscapular “BAT organ” contains adipocytes that are multilocular dispersed as lipid droplets within the cytoplasm and contain numerous mitochondria that express uncoupling protein-1(UCP-1).Their main function is to metabolize FAs for thermogenesis,protecting the body from cold exposure through non-shivering thermogenesis[42].Two forms of BAT are currently recognized to exist in humans:Constitutional BAT(cBAT)formed during embryonic development and beige or brite(brown-in-white)BAT.The former is recruited postnatally from WAT and has been denominated recruited BAT(rBAT).cBAT of developmental origin seems to be mesoderm closer to skeletal muscle rather than to AT,while rBAT can be formed after birth by transdifferentiating mature white to brown adipocytes or by differentiation from BAP[43].Previously considered to be represented in humans only in newborns,recent positron emission tomography/computed tomography(PET/CT)studies based on imagistic detection of UCP-1-positive adipocytes have identified functional BAT in adults.BAT seems to be more frequent in women than in men and is inversely correlated with BMI,especially for elderly subjects[44].
The main function of BAT is thermal regulation.Recently,several studies in mice revealed the role of BAT as a negative regulator of obesity since UCP-1 depletion in mice induced increased cold sensitivity and obesity[45].Apart from connections with energy metabolism and thermal regulation,recent studies correlated BAT with bone anabolism[46].BAT detected around the neck in the supraclavicular region and paravertebrae using functional PET-CT correlated positively with BMD in women[47]but not in men[48].This possible sex dependence of the positive effect of BAT on bone was not confirmed in a cross-sectional study correlating BAT volume with femoral cortical bone area and cross-sectional area in children and teenagers independent of sex[49].The transcriptional regulator and tumor suppressor retinoblastoma-associated protein(pRb)have been identified as a possible connection between bone and BAT and bone turnover.pRB functions as the switch mechanism that directs mesenchymal progenitors to the osteoblastic lineage,while deletion of pRb in the respective precursors increased the amount of BAT in mouse models[50].BMP overexpression in soft tissues after trauma seeks to recruit brown adipocytes and induce hypoxia-mediated chondrogenic differentiation of local progenitors.Subsequent ossification of chondrogenic nodules determines the formation of posttraumatic heterotopic ossification[51].
BAT AND BONE METABOLISM
Consistent research is directed to finding methods of transforming white adipocytes into brown or“beige” phenotypes for the treatment of obesity and associated metabolic disorders.Overexpression of forkhead transcription factor C2(FOXC2)in mouse WAT cells induced a BAT-like phenotype[52].Genetically modified mice that overexpress FOXC2 were found to not only be protected against dietinduced obesity and insulin resistance but also have increased trabecular bone mass and bone turnover[53].BAT-bone metabolism could be correlated through secreted paracrine factors.AT and BMAT overexpressing FOXC2 displayed increased gene expression of endocrine factors adiponectin,insulin growth factor receptor 3(IGFR2)and IGF1,as well as paracrine factors BMP4,wingless-type MMTV integration site family member 10B and angiopoietin 2.Human endolymphatic sac epithelial factors were shown to exert a pro-osteoblastic effectin vitroand could represent the modality of BAT-bone communication and bone anabolic support[46].
BMAT-A UNIQUE TYPE OF AT
Bone marrow contains a fraction of AT that fluctuates during development,growth,ageing and pathological conditions.While this fact is common knowledge,the origin,role and functions of BMAT remain largely unknown.More closely resembling WAT,which shares several microstructural commonalities[54],BMAT has a particular molecular make-up that distinguishes it from WAT and BAT[55].The unicity of BMAT seems to be related to not only its particular anatomic location and spatial constraints but also its involvement in body functioning as a whole[56].BMAT-released adipokines,inflammatory cytokines and other possible bioactive molecules are thought to exert systemic regulatory effects.Recent years have witnessed a surge in experimental investigations that challenge the passive role of BMAT as a simple space filler within the bone marrow microenvironment.The onset and progression of postmenopausal osteoporosis in the context of oestrogen depletion[57],glucose homeostasis,energy metabolism[58],or adipocyte-osteoblast balance have been recently linked with BMAT reactivity[59].These investigations point towards BMAT involvement in structural changes occurring within the skeleton with age during physiological and pathological situations and as a key player in the maintenance of body energetic expenditure.
STRUCTURE AND COMPOSITION OF BMAT
In healthy adults,BMAT represents approximately 10% of the total body AT.While the presence of adipocytes within the complexity of the bone marrow tissue environment was detected a century ago,only recently was their role as a local and systemic regulator explored[60].The availability of methods forin vivoquantification and the use of reporter transgenic mice combined with experimental induction of BMAT expansion have enabled recent insights into its function.The correlation of BMAT expansion with metabolic diseases,such as obesity,metabolic syndrome,diabetes and anorexia nervosa,is sought to point towards a role in systemic metabolic balance.Unlike any other form of AT,BMAT has physical vicinity at a cellular level with bone tissue.BMAT expansion is associated with decreased bone mass and osteoporosis experimentally,as well as in epidemiological studies[61].Multiple factors are involved in this correlation,such as bone marrow mesenchymal stem cell adipogenicvsosteoblastic conversion,adipokine release or inflammation.BMAT is also involved in normal and pathological haematopoiesis through adipocyte cellular interaction with haematopoietic progenitors and local adipokine release[62].BMAT adipocytes are responsive to producing and sustaining a local inflammatory environment that impactsde novobone formation and favors malignant conversion of haematopoietic lineages or tumor metastasis to bone[63].
Considered to originate from bone marrow mesenchymal progenitors,BMAT adipocytes are unilocular similar to WAT and can be found within the bone marrow cavity of bones.In young individuals in humans and mice,bone marrow has a red appearance and contains predominately haematopoietic and osteoblast progenitors,as well as erythroid cells.Macroscopically,bone marrow becomes yellow with a fatty structure upon BMAT development.Using magnetic resonance imaging,in human subjects,progression of red to yellow marrow was observed in the long bones(the femur)first in the diaphysis(ages 1-10 years)and then in the distal metaphysis(ages 10-20 years),with an adult pattern seen by age 24 years[64].BMAT first develops in the distal appendicular skeleton(femur,tibia)compared to the proximal and caudal vertebrae(tail bones)compared to the proximal(thoracic)vertebrae.In rats,differences were attributed to cold exposure,as well as strong erythropoietin stimuli,since retaining warm temperature,as well as induced haemolysis,prevented bone marrow “yellowing”in pre-weanling but not in mature animals.This led to the conclusion that BMAT once formed is a stable tissue[65].Two developmental and regional distinct BMAT subtypes have been identified.The distal localized,first to develop,was denominated the constitutive(cBMAT),while the proximal placed(within proximal limbs,thoracic vertebrae,hips,ribs)later occurring and more scattered was denominated the regulated(rBMAT).cBMAT was found to contain predominately unsaturated lipids,while rBMAT contains saturated fats.It has been proposed that rBMAT can mature into the more stable cBMAT phenotype under certain conditions[57].
ROLE OF BMAT IN BONE METABOLISM AND FRACTURE HEALING
BMAT adipocytes are a major participant in the BM niche alongside BMSCs and hematopoietic stem cells.Their physical presence,as well as endocrine and paracrine function,impacts osteoblast and osteoclast differentiation and functioning[66].Several mechanisms for BMAT adipocyte involvement in the maintenance of bone anabolic-catabolic balance have been proposed.
Since osteoblasts and adipocytes share a common precursor,the most important factor in regulating bone formation is intrinsic BMSC “fate decision”.Lineage determination is controlled on one side by signalling pathways that promote expansion of one lineagevsanother and on the other side by suppression of pathways promoting the competitive lineage.Bone formation occurs by inducing osteogenic key regulators runt-related transcription factor 2(RUNX2)and osterix in MSCs while inhibiting adipogenic PPAR-γ and CCAAT/enhancer-binding protein αviaa Wingless-type MMTV integration site family(Wnt)mechanism[67].Conversely,adipogenesis requires concomitant induction of key adipogenic pathways and inhibition of osteogenic Wnt and Notch[68].In BMSCs,intracellular accumulation of proteins that induce adipogenesis,such as transducing-like enhancer of split 3,increases the expression of PPAR-γ and suppresses Wnt-induced β catenin accumulation and RUNX by a histone deacetylase mechanism[69].Increased BMSC adipogenic conversion and reduced osteoblast formation are considered the main culprits for compromised bone anabolism and BMAT accumulation[70].It is currently accepted that an increase in BMAT during ageing,osteoporosis,and T2D is associated with decreased bone quality and quantity(osteoporosis,osteopenia).However,this inverse correlation is not confirmed by all clinical situations.Epidemiological studies confirm increased BMAT in osteoporotic patients compared to age-matched controls in children,young adults and elderly individuals[71,72].Increased BMAT was found to correlate with increased BMD in obese and T2D patients[73].Furthermore,decreased BMD and increased BMAT content in anorexia nervosa are associated with decreased BMI in anorexia nervosa patients[74].These findings suggest that BMD might not be the ultimate predictor of bone quality and that BMSC adipogenic and osteoblast conversion might not be mutually exclusive[75].Lineage tracking of adult adipocyte BMAT is warranted to elucidate their origin,as well as potential competition with osteoblast differentiation and maturation.
The BMAT-bone relationship does not resume cell fate decisions.MAT-released adipocytes(especially leptin and adiponectin),inflammatory cytokines(which include TNF-α and the IL family)and mRNA-containing extracellular vesicles(EVs)form a complex signalling network involved in regulating osteogenesis[76].It is worth mentioning that studies on AT-released factors and their influence on bone metabolism largely involve WAT adipocytes.Few studies specifically address the molecular mechanisms of BMAT adipocyte-secreted signalling molecules and their role in bone metabolism.
No direct evidence exists regarding the association between BMAT and fracture risk and fracture healing.Indirect observation is provided by studies on fracture healing in obese experimental models of human subjects that could have increased BMAT.Experimental studies on obese mice reported an increased incidence of delayed union associated with increased callus adiposity in obese T2D mice[77,78].A meta-analysis of eight epidemiologic studies including 39938 participants concluded that metabolic syndrome has no explicit effect on bone fractures[79].In another study,obesity was not associated with an increased incidence of nonunion after ankle fractures[80],while yet another study reports a greater risk of complications in obese patients[81].Multiple confounders,such as the association of alcohol consumption,T2D,and quality of fixation,can explain these contradictory results.Another possible indirect indication comes from the studies reporting increased fracture healing in patients with long bone fractures fixed with reamed intramedullary rodsvsnon-reamed patients.This finding can be explained by the stability of fixation,preservation of fracture haematoma that favors formation of periosteal callus or activation of MSC recruitment[82],rather than by mere removal of BMAT.
AT IN BONE REGENERATIVE MEDICINE
Regenerative medicine(RM)aims to completely restore functionality and anatomy in degenerating or ageing tissues or to replace tissues and organs lost to trauma,infection,tumor removal or congenitally absent[83].RM makes use of cells,especially stem cells,bioactive molecules and supportive/functional ECM equivalents,to induce regeneration or engineer implantable bioequivalent structures.Recent decades have witnessed a surge in regenerative interventions for improving bone health,aiming to increase bone quality and prevent or treat failures in fracture healing.Several cell types of use for RM purposes can be obtained from AT,and adipose-derived mesenchymal stem cells(ADSCs)and adiposederived pluripotent stem cells will be briefly described in the following subchapters.
ADSCS
AT is considered a convenient source of mesenchymal stem cells because of its ease of procurement and abundance of colony-forming units.Compared to adult bone marrow,the frequency of ADSCs obtained per tissue unit can be up to 500-fold higher[84].AT can be obtained by minimally invasive procedures(subcutaneous lipectomy)or as a byproduct of cosmetic liposuction procedures.ADSCs were first obtained from subcutaneous WAT lipoaspirate by enzymatic digestion and selection of plastic adherent cell populations[85].Enzymatic digestion of lipoaspirate or WAT fragments obtained by lipectomy or mechanical cell extraction from the same sources derives the stromal vascular fraction(SVF).SVF is a cell mixture that contains preadipocytes,fibroblasts,vascular cells,blood cells and Mcfs that can be readily used for regenerative purposes.ADSCs are obtained from the SVF by further cultivation and selection of mesenchymal progenitors based on their adherence to tissue culture.The anatomic location of harvest(such as abdominal,brachial,inguinal)position(superficial subcutaneousvsdeep hypodermic),age and sex of the donor influence the number of mononuclear cells extracted and the number of ADSCs obtained from subcutaneous WAT[86].ADSCs meet the criteria established by the International Society for Cell Therapy for defining mesenchymal progenitors(plastic adherence,trilineage mesenchymal differentiation and surface marker phenotype)[87].It has been reported that the SVF contains four different mesenchymal cells or progenitors or that the putative ADSCs are CD31-,CD34+/-,CD45-,CD90+,CD105-,CD117-and CD146-,the others being pericytes(CD146+/CD31-/CD34-),mature endothelial cells(CD31+/CD34-),progenitor endothelial cells(CD31+CD34+),and preadipocytes as CD31-/CD34+ cells[88].ADSCs were reported to differentiate under controlled conditionsin vitroto mesenchymal lineages(adipocytes,chondrocytes,osteoblasts and cardiomyocytes[89]and skeletal muscle[90]).Ectodermal(neurons,glia and Schwan cells)and endodermal(hepatocytes and pancreatic beta islet cells)ADSC conversion has been obtained[91].A subset of ADSCs was shown to express markers of pluripotency(Sox2,Nanog,and OCT4)and to differentiate into mesodermal and extramesodermal lineages,especially when cultured in three-dimensional suspension culture[92].An important feature of putative ADSCs is their growth factor and immunomodulatory cytokine release.ADSCs were found to express multiple growth factors,of which basic fibroblast growth factor(bFGF),VEGF,insulin-like growth factor 1,HGFs,and transforming growth factor(TGF)-β1 but as well β-nerve growth factor,stromal cell-derived factor-1α and growth factor receptors.Mass spectrometry analysis of the ADSC secretome revealed that ADSCs express 342 proteins under normoxic conditions.These proteins were found to be related to angiogenesis and blood vessel expansion,ECM formation,cell adhesion/migration,cell survival/death,and immune regulation with little variation after hypoxic preconditioning[93].Importantly,the ADSC secretome varies upon stimulation.bFGF or epidermal growth factor(EGF)preconditioning significantly increases ADSC release of HGF,a cytokine involved in haematopoiesis,vasculogenesis,and mammary epithelial duct formation[94].Neural growth factor preconditioning increased the axonal growth capability of a conditioned medium from ADSCs[95].It has been proposed that preconditioning ADSCs using low oxygen content,generation of reactive oxygen species(ROS)and activation of platelet-derived growth factor(PDGF)receptor signalling can increase the regenerative proprieties of cultivated ADSCs by mimicking thein vivoregenerating niche[96].
Inflammatory cytokine release varies upon ADSC stimulation.Exposure to lipopolysaccharides induced the release of haematopoietic(granulocyte/monocyte,granulocyte,and macrophage colonystimulating factors,IL-7)and proinflammatory mediators(IL-6,IL-8,and IL-11,TNF-α)[97].Under normal culture conditions,conditioned medium from ADSCs reduced the production of TNF-α,NO and prostaglandin E2,and the activation of nuclear factor-kappaB in blood-derived monocytes decreased their degranulation,phagocytic activity and migratory ability.Notably,using next-generation sequencing,cultivated ADSCs were found to have a more homogenous immunomodulatory gene expression profile than SVF in the natural state and upon TNF-α stimulation[98].Trophic and immunomodulatory factors released by cultivated ADSCs are strongly influenced by a large variety of factors,such as WAT origin,donor age and health status,cell culture and preconditioning[99-101](for a summary,see Table 1).While this influence opens large possibilities in manipulating cell therapeutic qualities,it calls for thorough characterization when an ADSC-based product is envisaged.
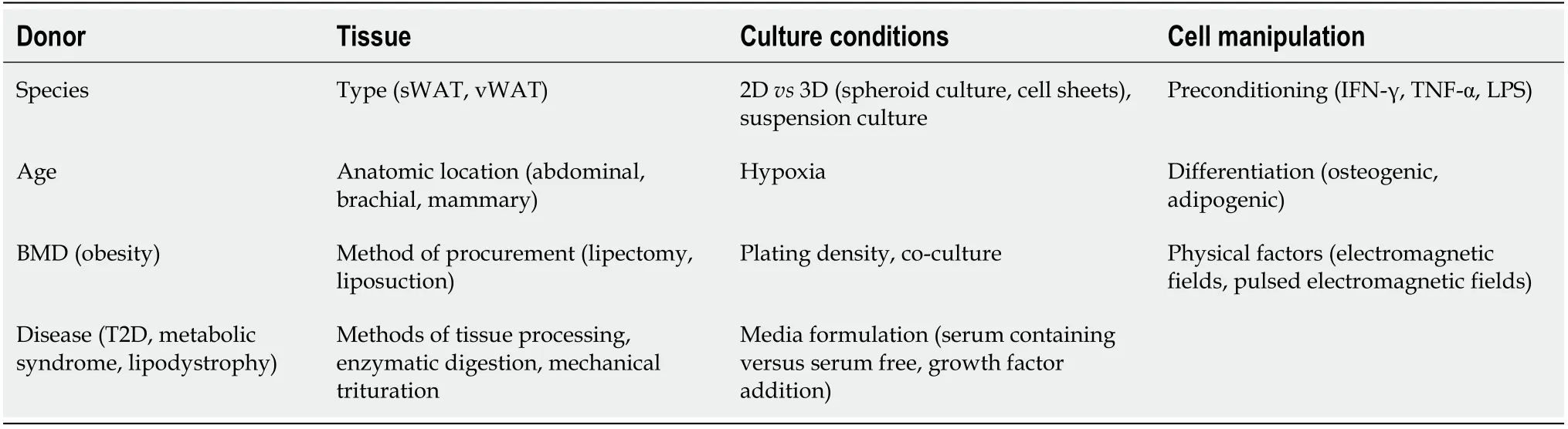
Table 1 Factors that influence adipose-derived mesenchymal stem cells secretome content and release[99-101]
Given their phenotypic characteristics,ADSCs are intensively sought for their differentiation and tissue trophic and immunomodulatory potential.ADSCs can be used as building blocks forde novobioengineered organs and are currently tested for the generation of musculoskeletal tissues[102,103].Cell therapy using ADSCs has proven useful in preclinical settings for immunomodulation in autoimmune diseases(such as inflammatory bowel disease,multiple sclerosis,and rheumatoid arthritis)[104].With the recent coronavirus disease 2019 pandemic,ADSCs have been tested in emergency clinical trials for the prevention of severe “cytokine storm” and the installation of acute respiratory distress syndrome,septic shock,and/or multiple organ failure[105].
ADSCs have been intensively testedin vitroand in preclinical studies for their direct contribution by differentiation to the osteoblastic lineage,for their supportive effect in promoting osteogenesis and for accelerating fracture healing.Deriving from these distinct roles in RM,bone tissue engineering using ADSCs as cell sources and cell therapy for the treatment of problematic bone healing,bone pathology and systemic osteoporosis are possible therapeutic scenarios.
DIRECT EFFECT OF ADSC IN REGENERATION-OSTEOBLASTIC DIFFERENTIATION AND TISSUE-ENGINEERED BONE STRUCTURES
Numerous reports exist regarding thein vitroosteogenic potential of ADSCs under defined differentiation media,and osteogenic conversion is assessed based on specific gene expression(OC,corebinding factor subunit alpha-1,also known as RUNX2,AP,osteonectin,osteopontin,BMP-2,ALP activity and mineralized ECM deposition)[106].Mechanical stimulation by dynamic compression or magnetic nanoparticle-induced remote actuation has also been reported to increasein vitroosteogenesis[107,108].To assess ADSC osteogenic potentialin vivo,several methods have been validated starting from ectopic bone formation in small animal models(rat,mice)after intramuscular delivery of osteogenic-induced ADSCs[109].More complex models consist of healing experimentally induced calvarial bone defects in rodents or long bone fractures[110,111].Generally,in vivotesting of ADSC osteogenic potential requires the use of a supportive structure for cell implantation.This strategy realizes a tissue-engineered implantable structure with variable degrees of complexity and potential for clinical translation.The classical “tissue engineering triad” is based on the use of scaffolds,cells and bioactive molecules to generate implantable bioequivalent tissues or organs.For bone engineering,the biomaterial needs to fulfil the general requirements for a scaffold structure(biocompatibility,biodegradability,porosity and interconnectivity of the pores,not to generate inflammatory responsein vivo).In addition,this material needs to be osteoconductive(to allow bone mineral and collagen deposition)and osteoinductive(to favor osteogenic differentiation).Three main types of biomaterials have been used for scaffolds in bone tissue engineering: Ceramics(such as tricalcium phosphate,hydroxyapatite and combinations)and synthetic polymers[such as polylactic acid,polyglycolic acid(PGA),and poly-DL-lactic-co-glycolic acid].Natural polymers,such as collagen,hyaluronic acid,chitosan,fibrin,and elastin,have been used alone or in combination with synthetic polymers or with ceramics[112].Alongside the osteoconductive and osteoinductive properties,biomaterial osteointegration is crucial for the stability of the engineered graft.Osteo integration is dependent on blood vessel colonization from the surrounding host tissue that allows for nutrient supply,waste removal and erasing of implant host interfaces that impede mechanical stability.Especially in the case of larger constructs,the biomaterial needs to be angioconductive and permissive to vascular in growth.Angioinduction,the ability of a biomaterial to actively induce and sustain the formation of new vessels,is another determinant of osteointegration accounting for adequate vascular supply and long-term stability of the engineered bone[113].
Advanced nanostructured materials with remarkable properties are promising for revolutionizing the field of bone engineering.Graphene,with its high surface area,high mechanical strength,and high functionalization potential,can induce ADSC differentiation even in the absence of osteogenic media.The feasibility of generating mechanically stable graphene-based implantable bone grafts and thein vivoosteoinductive capabilities of these implants need to be further tested[114].
Bioactive molecules largely employed for bone tissue engineering are osteoinductive growth factors from the BMP family.BMP-2 was clinically approved by the Food and Drug Administration for spine fusion,and BMP-7 was given a device exemption for the treatment of nonunions.As a result,many studies began investigating BMP as a modality to enhance ADSC-based osteogenesisin vivo,envisaging smoother clinical translation.However,since activation of the BMP pathway in ADSCs induces osteogenesis and adipogenesis,the use of BMP alone cannot always account for the desired fate decision.To shift the balance towards osteogenesis,switches such as the Wnt and extracellular signalregulated kinase pathways and the ratio between BMP receptors bone morphogenetic protein type IA receptor(BMPR-IA)/BMPR-IB are at play.Controlling the sequential cascade of growth factor availabilityin vivocan prove to be technically challenging.Several methods,such as controlled release,scaffold-mediated release,gene transfer technologies or stimulation of endogenous BMP activation,have been proposed[115].
Several growth factors relevant for osteogenesis,such as b-FGF or FGF-2,IGF-1,PDGF-BB,and VEGF,are contained in platelet-rich plasma(PRP),a blood-derived biologic that is easy to procure from autologous sources.PRP incorporated within composite hydrogel-ceramic scaffolds yielded increased osteogenic ADSC conversion in a rabbit calvarial model compared to non-PRP-treated implants[116].Different strategies of PRP coating of synthetic electrospun scaffolds appear promising,awaiting further tests forin vivovalidation of the procedure[117].Alternatively,overexpression of different transcription factors in ADSCs(RUNX2,VEGF,sonic hedgehog,and LIM mineralization protein)was shown to increase osteogenic differentiation and could prove an efficient strategy for inducing bone formationin vivo[118].
Another strategy of GF delivery could bein vitrocell preconditioning with osteoinductive molecules.FGF2-pretreated human ADSCs showed enhancedin vivoosteogenic potential in an ectopic bone model and increased osteoid formation in a dose-dependent manner[119].Exosomes are EVs of endosomal origin,ranging from 50-200 nm in diameter,that function as intracellular communication tools.MSCs,especially ADSC EVs,contain cell-specific proteins(cytoskeletal proteins,transmembrane proteins,and heat shock proteins),nucleic acids[DNA,mRNA,micro RNA(miRNA),long and short noncoding RNA],lipids,and enzymes.EVs are recognized as bioactive cargoes with importance for cell recruitment,migration,proliferation,andde novovascularization and have an important impact on tissue regeneration[120].ADSC-derived EVs have been investigated as potential tools for inducing osteogenic differentiation.The PLDA/PGA matrix slowly released EVs from osteogenic-induced ADSCs and was shown to promote osteogenesis of BMSCsin vitro.Furthermore,cell-free PLDA/PGAEV increased osteogenesis in a mouse calvarial model compared to the PLDA/PGA matrix only[121].EVs from osteogenic-induced ADSCs could promote osteogenesis in undifferentiated ADSCs.Remarkably,ADSCs could incorporate EVs faster than BMSCs(6 h compared to 48 h),which could be of importance for therapeutic applications.Even though the study was not validatedin vivo,the authors performed microarray gene expression and bioinformatics analyses,revealing that the differentially expressed exosomal miRNAs from osteogenic-induced ADSCs compared to undifferentiated ADSCs are involved in the osteogenetic process(the MAPK,Wnt,and TGF-β signalling pathways).The expression levels of miR-130a-3p,which blocks SIRT7,an antagonist of the Wnt pathway,were found to be significantly higher in EVs from osteogenic ADSCs.MiR-130a-p ultimately upregulates the Wnt pathway,possibly acting as the molecular mechanism of increased ADSC osteogenic induction by EVs[122].
Given their increasingly recognized role in modulating osteogenesis,miRNAs or inhibitors have been tested for inducing ADSC differentiation.Scaffold-mediated release to ADSCs or virus-transfected miR-148b,miR-26a,miR-135,or miR-130a-3p was found to increase bone formationin vitroandin vivo[123,124].Other miRNAs,such as miR146a,miR-17,miR-23a,and miR-31,were found to inhibit BMP2-induced osteogenesis,suppressing downstream factors in BMP-2-induced osteogenesis(such as RUNX2,Osterix,and SMAD1/4).Antisense inhibition of these miRNAs in ADSCs seeded on a βtricalcium phosphate scaffold was found to increase bone volume and BMD and to decrease scaffold residue persistence in critical size bone defects in rats[125].
Mechanical stimulation is crucial for obtaining bioengineered structures,especially in the case of musculoskeletal components.Functional tissue engineering is set to obtain robust bioequivalents that readily restore the morphology and load-bearing and motion capabilities of bone.A variety of mechanical loading procedures that apply cyclic hydrostatic pressure or tensile strain in dynamic culture conditions have been used to increase ADSC osteogenesis[126].Magnetomechanical stimulation using magnetic nanoparticles internalized by ADSCs and magnetic field exposure during the first phases of osteogenesis has been reported as a modality to deliver remote controlled and device-free mechanical stimulation[108](Figure 1).
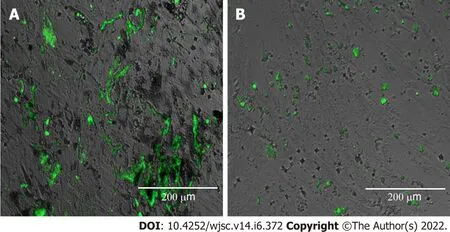
Figure 1 Adipose-derived mesenchymal stem cell osteogenesis under magnetomechanical stimulation.A: Osteogenesis of adipose-derived mesenchymal stem cells(ADSCs)loaded with micronutrient powders(MNPs)exposed to alternating MFs;B: Osteogenesis of ADSCs without MNPs exposed to MFs assessed with OsteoImage® Lonza showing green fluorescence for the deposited calcified extracellular matrix.
A consistent number of preclinical studies have reported the use of various combinations of supportive structures,bioactive molecules and/or functional loading for testing ADSC osteogenic capabilityin vivo.Reports about the successful use of ADSC-based tissue-engineered bone are abundant in the literature[56].Despite these encouraging results,translation to clinical settings has proven more difficult.The first report of clinical use was made in 2004 and involved ADSC use in a paediatric patient.A large calvarial posttraumatic bone defect was treated with autologous ADSCs and iliac crest cancellous bone autografts,fibrin glue and resorbable macroporous sheets[127].In the years to follow,several case reports emerged regarding the use of autologous ADSCs and clinically approved bone substitutes with or without BMPs for grafting of craniofacial bone defects(mandibular and maxillary bone)[128-130].The combination of autologous ADSCs expanded in good manufacturing practice facilities and ceramic bone substitutes resulted in uneventful healing of bone defects.Cranioplasty of large calvarial defects using autologous ADSCs and β-transmission control protocol was reported as a useful method to replace massive bone loss[131].Remarkably,all clinical reports regarding ADSC use involve the reconstruction of craniofacial bone defects.To our knowledge,recent years have not added to the reported clinical studies in this field.A list of current clinical trials registered on clinicaltrials.gov is available in Table 2.
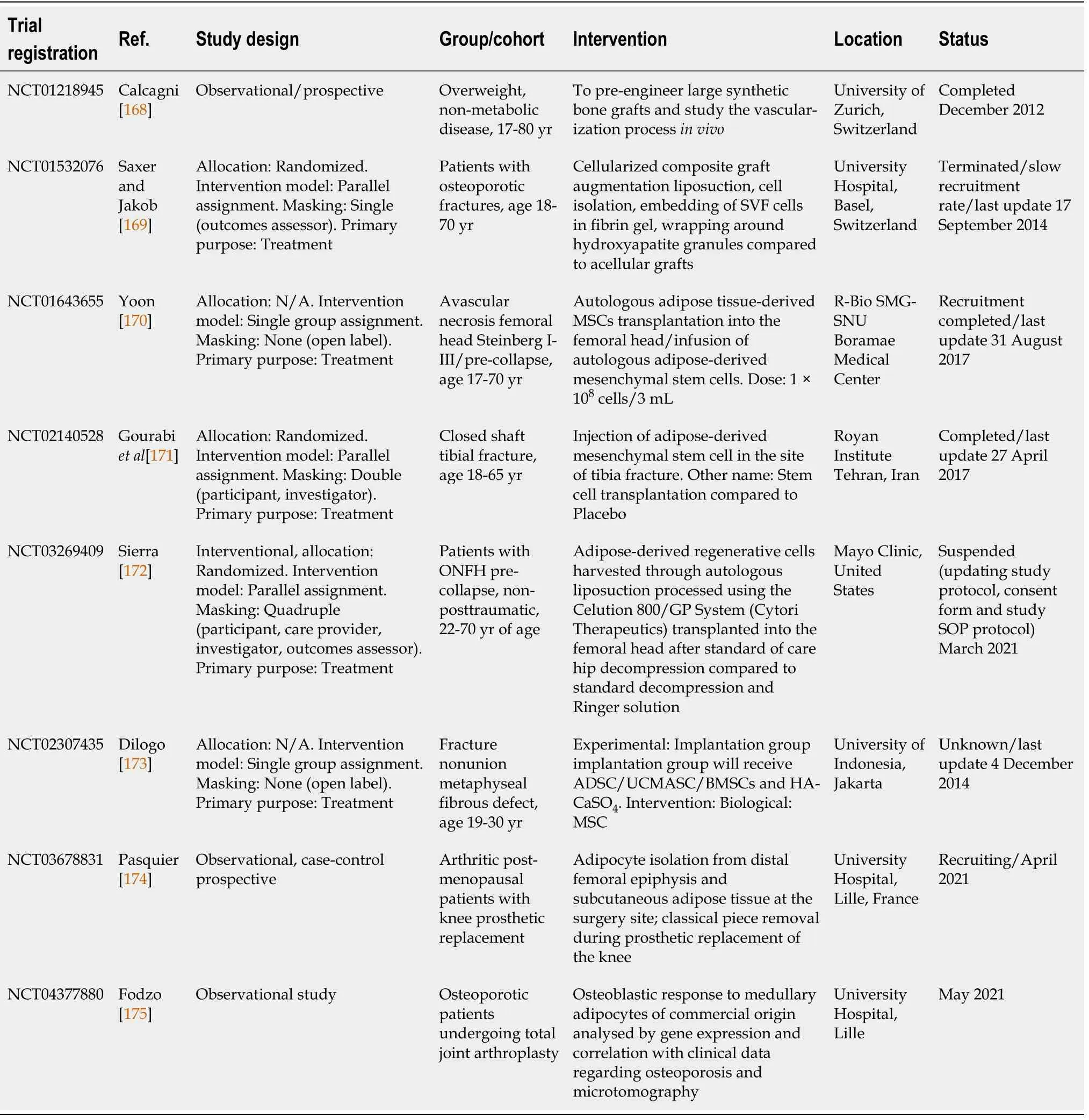
Table 2 Clinical trials using adipose-derived mesenchymal stem cells for bone regeneration registered on clinicaltrials.gov(June 2021)
THE SUPPORTIVE ROLE OF ADSC-CELL THERAPY FOR BONE DISEASES AND FOR AUGMENTING FRACTURE HEALING
The trophic role of ADSCs in tissue has been investigated for the treatment of metabolic bone diseases,such as osteoporosis.As a multifactorial disorder,osteoporosis has external and intrinsic determinants and is commonly associated with postmenopausal hormone depletion,ageing or long-term use of corticosteroid medication[132].Local or systemic delivery of ADSC suspensions as cell therapy is sought to modulate bone resorption,increase bone formation and enhance BMD.The procedure relies less on the capability of infused cells to differentiate into osteoblastic lineages but rather on cytokine and growth factor release.This paracrine activity is expected to increase osteoprogenitor cell recruitment,proliferation,differentiation,ECM formation and mineralization[133].Several preclinical studies report on the efficiency of autologous locally delivered ADSCs in improving bone strength in ovariectomized rats or in senescent mice[134,135].Systemic human ADSC delivery in ovariectomized nude mice was as effective as oestrogen therapy in protecting trabecular bone loss,without evidence of ADSC engraftment[136].
Osteonecrosis of the femoral head(ONFH)is considered to be produced by apoptosis of mature osteocytes mainly due to impaired blood supply.ONFH affects a younger population,leading to collapse of the femoral head,a situation that requires total joint replacement.Unlike other forms of cell therapy,in ONFH,the use of stem cells started in clinics with the use of bone marrow aspirate concentrate as a modality to deliver progenitor cells locally after core decompression[137].Most studies regarding the use of cultured MSCs for ONFH involve BMSCs;however,coculture with ADSCs was reported to have a synergistic effect mainly due to ADSC angiogenic potential[138].Stem cells are commonly delivered within a supportive structure,such as fibrin gel or bone substitute for retaining the cells,as well as a modality to support or to prevent the collapse of the femoral head.Implanted cell contribution is probably rather paracrine because the local environment is not favorable for cell survival and differentiation after transplantation.ADSCs have been tested as a modality to locally deliver angiogenic factors.VEGF-transfected ADSCs in coculture with BMSCs were effective in inducing osteogenesis and angiogenesisin vitroandin vivo;however,this role needs to be further tested in ONFH animal models[139].
Delayed or impaired fracture healing can complicate up to 10% of total fracture cases[140].Local risk factors can affect the quality and speed of bone healing,such as the severity of bone and soft tissue injury and the coexistence of multiple fractures or other associated trauma.Systemic factors,such as diabetes,obesity,malnutrition,smoking,and advanced age,are also known to represent a high risk for bone healing.ADSCs have been tested as a method for increasing the quality and decreasing the time of bone healing in animal models.Human ADSCs and their conditioned medium embedded in human blood plasma hydrogel were shown to increase fracture healing in surgically induced rat jaw fracture,demonstrating their paracrine effect in promoting bone union[141].Local ADSC injection in healthy and diabetic rat femoral nonunions induced significant bone healing,as assessed by histology,compared to nontreated groups independent of RANK,RANKL,or OPG gene expression[142].A combination of human ADSCs,cancellous bone grafts and chitosan gel consistently improved healing of the surgically induced nonunion of the femoral bone in rats,as confirmed by biomechanical and histological studies.ADSC presence was correlated with increased expression of VEGF and BMPs in the treated groups[143].Autologous ADSCs delivered by local injection in atrophic nonunions in rat tibia resulted in significantly increased callus and solid bone union[144].The report represents proof of concept of ADSC regenerative capabilities even in this difficult-to-treat variety of impaired fracture healing.The clinical use of ADSCs as a cell therapy for enhancing fracture healing has not yet been reported.
WAT-DERIVED PLURIPOTENT CELL POPULATIONS: MULTILINEAGE DIFFERENTIATING STRESS-ENDURING CELLS AND DEDIFFERENTIATED FAT
WAT is the source of two cell populations with tripoblastic differentiation potential and expression surface markers of pluripotency.Multilineage differentiating stress-enduring(MUSE)cells were initially obtained from dermal fibroblasts and BMSCs as stress-resistant populations[145].WAT was soon identified as a plentiful source of MUSE cells that could be obtained by means of positive immune separation for mesenchymal surface antigen CD105 and pluripotency marker stage-specific embryonic antigen 1(SSEA-1)[146].A remarkable characteristic of these cells is their ability to grow in adherent and suspension culture conditions.When MUSE cells are cultured in a single-cell suspension,they form so-called “M clusters” with morphological resemblance to ESC or induced pluripotent stem cell(IPSC)embryoid bodies formed from embryonic stem cells(ESCs)or IPSCs.Since MUSE cells do not generate tumors afterin vivoinjection into severe combined immunodeficient(SCID)mice,they are considered safer than ESCs or IPSCs.MUSE cells are a small percentage of tissue-derived MSCs and are considered to be responsive to the regenerative potential of these populations.Their ability to migrate to damaged tissue and to spontaneously differentiate into cells that pertain to damaged tissue is regarded as having important potential in RM since unlikely ESC or IPSC preinduction to the respective lineage is not necessary[147].MUSE cells have been tested in animal models for cardiovascular rescue(myocardial infarction)ischaemic stroke,lung injuries,kidney diseases and skin repair[148].Their use in bone regeneration has not yet been tested;however,good results obtained in treating experimental patellar osteochondral defects might indicate a possible future application[149].
Mature cell dedifferentiation has been reported as a source of a pluripotent-like cell population.Mature adipocytes from WAT dedifferentiatedin vitroby ceiling culture were found to revert to an undifferentiated phenotype and gain proliferative and differentiation capabilities[150].Dedifferentiated fat(DFAT)cells have triploblastic differentiation potentialin vitroand do not generate teratomas when injected into SCID mice[151].DFAT cells are more homogenous than ADSCs and display mesenchymal surface markers and SSEA-3.DFAT was found to differentiate multiple cell lineages,including adipogenic,osteogenic,chondrogenic,myogenic,angiogenic and neurogenic lineages,and was tested in preclinical models of spinal cord injury rehabilitation of cardiac tissue after infarction[152,153].DFAT cells were found to possess osteogenic capabilities when cocultured with periodontal ligament stem cells and might be a suitable cell source for periodontal regeneration[154].DFAT cells display better differentiation capabilities,including osteogenic capabilities,than ADSCs from the same source.WATderived pluripotent cell populations are more homogenous than ADSCs and possess multilineage differentiation potential.Their prospective use for bone regeneration strategies is appealing and warrants more investigation.
FAT GRAFTING AND BONE HEALING
Not only cells but also WAT as a whole have been used in plastic and cosmetic surgery for aesthetic reasons but also for supporting wound healing and skin support[155].WAT has only recently been tested for its possible effect in supporting bone healing.Fragmented autologous WAT was shown to significantly increase mineralized matrix deposition in calvarial defects in rabbits compared to blood clot-treated and nontreated controls[156].Fragmented WAT is investigated,as well as an autologous biomaterial that could be genetically modified to induce bone healing.In anin vitrostudy,genetically modified fragmented WAT overexpressing BMP-2 was shown to undergo mineralization in osteoinductive conditions[157].The same team reported that the homodimer BMP-2 induced increased mineralization at lower doses compared to heterodimer BMP-2/6 or BMP-2/7;however,these findings need to be confirmed byin vivostudies[158].WAT has been considered a modality to deliver microvascular grafts to healing bone defects to prevent atrophic nonunions.A thermoresponsive hydrogel(TRH)was used as a delivery system for WAT microfragments.However,local delivery of fragmented WAT-loaded TRH impaired bone formation in a murine model of bone defects,even though vascularization was improved.This undesirable outcome was thought to be produced by reduced VEGF expression in early phase bone healing,stressing the need for stage-specific delivery of bioactive factors[159].
CONCLUSION
Despite consistent research in recent decades,few clinical trials have tested the use of AT-or ATderived cells for bone regeneration.To date,no clinically approved engineered product or cell therapy exists for treating impaired fracture healing,osteoporosis or ONFH.Particular challenges regarding cell heterogeneity and the type of cell used for different bone regenerative strategies are adding to the general challenges encountered by the development and approval of any advanced therapeutic medicinal product(ATMP).ADSC stemness characteristics are donor-dependent.The age of the donor has been thought to influence the quantity and quality of mesenchymal progenitors derived from WAT;however,conflicting reports exist in this respect.A decreased yield of SVF and ADSC colony-forming units per tissue,increased mitochondrial ROS production and impaired migratory and differentiation potential were reported for elderly donors in some studies[160,161].Other studies,however,report similar characteristics of ADSCs derived from donors ranging from 8-62 years of age confirmed in a clinical case series where ADSCs were used for treating bone nonunions in combination with osteoconductive grafts[162].The differences might be explained by the fact that studies reporting impaired ADSC characteristics in elderly individuals do not elucidate their possible coexisting diseases(such as diabetes,metabolic syndrome,and obesity)ADSCs derived from T2D patients were found to possess reduced viability and proliferative potential,exhibiting mitochondrial dysfunction and a senescence phenotype due to excessive mitochondrial ROS accumulation[163].The T2D ADSC secretome was also modified with reduced VEGF,adiponectin,and chemokine(C-X-C motif)ligand-12 secretion and overproduction of leptin[164].ADSCs derived from obese donors displayed reduced proliferative and differentiation potential compared to ADSCs from normal BMI donors.Obese ADSCs were shown to induce a proinflammatory phenotype in murine Mcfs and microglia,increasing the expression of proinflammatory genes and nitric oxide pathway activity while impairing their phagocytosis and migration[165].Metabolic syndrome and T2D ADSCs have increased susceptibility to apoptosis and senescence with increased expression of senescence-associated β-galactosidase,a high level of antiapoptotic protein B cell lymphoma-2 and decreased expression of the marker of proliferation Ki-67.These changes result in decreased proliferation,morphological changes with enlarged cellular bodies and nuclei and increased apoptosis of ADSC factors that affect the stemness of ADSCs derived from these donors[166].WAT status obesity and weight loss,age and disease-related lipotrophy affect the quantity and quality of SVF and ADSCs that can be derived from autologous sources.
These findings underscore the need for thorough characterization of cells before their use for certain prospected clinical applications.Genomic and proteomic profiling of the ADSC phenotype,as well as their secretome,could identify biomarkers for selecting the appropriate cell source for a particular application in bone healing.This would result in possible test panels for determining whether autologous or allogenic cell sources are the best choice for the desired outcome.Modelling the desired profile for a specific application in bone healing(such as osteogenic potential and trophic and/or antiinflammatory effects)would help select the cell phenotype that is more suitable for bone tissue engineering or cell therapy for fracture healing or other bone-specific diseases.Cell profiling for a projected ATMP would positively impact product characterization,standardized manufacturing and quality control.
Expanding the use of pluripotent cells from WAT,MUSE and DFAT cells,which are less donordependent and have increased osteogenic potential,could increase the chance for successful bone engineering strategies.Given the capability of MUSE cells to traffic,home and differentiate at the site of injury,a combined acellular scaffold with systemic or local MUSE delivery could represent a convenient modality for bone grafting and fracture healing.An important gap of knowledge still exists regarding the mutual interrelation between different AT types and bone in its normal and pathological states.Not only AT but also bone metabolism,fracture and the modality of fracture treatment can influence AT locally and systemically.BMP-2 treatment of long bone fractures in high-and low-fat diet-fed mice was shown to display increased vessel parameters and femoral adipocyte numbers irrespective of diet.Local BMP-2 delivery was shown to exert a diet-dependent effect on lung endothelial and bone marrow endothelial cells,influencing gene expression andin vitrotube formation capabilities[167].These findings point out the necessity to investigate the complex interrelation between AT and bone from a systemic perspective.The role of BMAT in orchestrating local and systemic bone metabolism and bone healing and its interrelation with WAT and BAT need further investigation.Two recently registered clinical trials are salutary in this respect,as they are poised to compare WAT and BMAT characteristics in postmenopausal and osteoarthritic subjects(NCT03678831),as well as to model the complex interrelation between BMAT adipocytes and osteoblasts derived from osteoporotic patients(NCT04377880)(Table 2).
Multiple omics profiling of various cell populations and modelling their interactions in silico andin vitrowill increase the understanding of intricate factors that govern AT and bone balance.The increased availability of organoids and organs on chip technologies that enable high-throughput experiments will enable the validation of computer models.These models will derive improved therapeutic targets for treating bone diseases and impaired fracture healing,as well as methods for using preventive measures for maintaining health in both compartments.
ACKNOWLEDGEMENTS
The author wishes to thank Prof.Dr.Veronica Mocanu MD,PhD,for the insightful discussions that induced the concept of the manuscript.
FOOTNOTES
Author contributions:Labusca L drafted the manuscript,revised the literature and wrote the manuscript text.
Conflict-of-interest statement:All the authors report no relevant conflicts of interest for this article.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial(CC BYNC 4.0)license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is noncommercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:Romania
ORCID number:Luminita Labusca 0000-0001-9635-6893.
S-Editor:Wang JJ
L-Editor:A
P-Editor:Wang JJ
杂志排行
World Journal of Stem Cells的其它文章
- Disagreements in the therapeutic use of mesenchymal stem cellderived secretome
- Application and prospects of high-throughput screening for in vitro neurogenesis
- Role of stem cells-based in facial nerve reanimation:A meta-analysis of histological and neurophysiological outcomes
- Long noncoding RNAs in mesenchymal stromal/stem cells osteogenic differentiation:Implications in osteoarthritis pathogenesis
