Assessment of resting energy expenditure in patients with cirrhosis
2022-07-02ShaianeFerreiraCludioAugustoMarroniJessicaTainaSteinRobertaRaynAnaCristhinaHenzNatliaSchmidtRandhallCarteriSabrinaAlvesFernandes
Shaiane Ferreira, Cláudio Augusto Marroni, Jessica Taina Stein, Roberta Rayn, Ana Cristhina Henz, Natália P Schmidt, Randhall B Carteri, Sabrina Alves Fernandes
Shaiane Ferreira, Cláudio Augusto Marroni, Jessica Taina Stein, Roberta Rayn, Ana Cristhina Henz, Natália P Schmidt, Sabrina Alves Fernandes, Postgraduate Program in Hepatology, Federal University of Health Sciences of Porto Alegre (UFCSPA), Porto Alegre 90050-170, Brazil
Randhall B Carteri, Department of Nutrition, Centro Universitário Metodista - IPA, Porto Alegre 90420-060, Brazil
Randhall B Carteri, Department of Health and Behavior, Catholic University of Pelotas, Pelotas 96015-560, Brazil
Abstract BACKGROUND Malnutrition affects 20% to 50% of patients with cirrhosis. It may be associated with serious complications and has a direct impact on prognosis. Resting energy expenditure (REE) is an important parameter to guide the optimization of therapy and recovery of nutritional status in patients with cirrhosis. However, the REE of patients with cirrhosis is still unclear, casting doubt upon the optimal nutritional management approach.AIM To identify the best method that predicts the REE of cirrhotic patients, using indirect calorimetry (IC) as the gold standard.METHODS An observational study was performed on 90 patients with cirrhosis. REE was assessed by IC, bioelectrical impedance analysis (BIA), and predictive formulas, which were compared using Bland-Altman plots and the Student’s t-test.RESULTS REE values measured by IC (1607.72 ± 257.4 kcal) differed significantly from those determined by all other methods (BIA: 1790.48 ± 352.1 kcal; Harris & Benedict equation: 2373.54 ± 254.9 kcal; IOM equation: 1648.95 ± 185.6 kcal; Cunningham equation: 1764.29 ± 246.2 kcal), except the Food and Agriculture Organization of the United Nations, World Health Organization, and United Nations University (FAO/WHO/UNU) (1616.07 ± 214.6 kcal) and McArdle (1611.30 ± 241.8 kcal) equations. We found no significant association when comparing IC and 24-h dietary recall among different Child-Pugh classes of cirrhosis.CONCLUSION The IOM and FAO/WHO/UNU equations have the best agreement with the CI. These results indicate a possibility of different tools for the clinical practice on cirrhotic patients.
Key Words: Liver cirrhosis; Calorimetry; Indirect; Energy metabolism; Malnutrition
INTRODUCTION
The liver plays a key role in maintaining homeostasis and is the fundamental site of the metabolism of nutrients and other exogenous substances. Liver cirrhosis is the final stage of a chronic disease characterized by a process of disorganization in the lobular and vascular architecture of the liver, with fibrosis and diffuse nodular formation[1]. Importantly, it is estimated that there are 1.5 billion people diagnosed with chronic liver diseases, with an age-standardized incidence rate of 27.7/100000 for cirrhosis in these patients[2]. Patients with cirrhosis, regardless of etiology, commonly present malnutrition, resulting in a significant imbalance in energy metabolism that negatively impacts their prognosis and quality of life[3-5]. In this context, it is well established that cirrhotic patients benefit from improvements in dietary habits and nutritional interventions, and adequate dietary prescription depends on the precision of the protocols for energy requirement estimation.
The resting metabolic rate (RMR) reflects the energy required to maintain physiological processes, representing approximately 60% to 70% of the total daily energy requirement, whilst hepatic tissue metabolism accounts for almost 20% of the RMR in most patients[6,7]. RMR is influenced by different aspects of body composition, which could be drastically changed in the cirrhotic patient, due to hypercatabolism which is proportional to the disease progression[8]. Different studies show that protein degradation is measured by increased oxygen consumption through indirect calorimetry (IC), where an increase in resting energy expenditure (REE) is observed in 35% of people with cirrhosis compared to the healthy population[9-11]. This conflict could be explained by several confounding factors, such as the use of medication, the patient’s body composition, and the presence of comorbidities[12]. However, the current literature is still conflicting regarding the relationship between cirrhosis progression and RMR alterations. Some studies have reported an increase in REE compared to the healthy population[13,14] while others have reported a decrease in REE[15,16]. Therefore, since the nutritional prescription is crucial to mitigate the progression of liver malfunction and/or alleviate complications characteristic of cirrhosis, appropriate estimation of patients' energy requirements is vital.
IC is the most reliable method to estimate the RMR, but it is expensive and time-consuming, and requires trained personnel and specific apparatus[17]. Alternatively, several predictive equations were developed to estimate the REE using specific individual characteristics[18]. Although most of the equations were developed in different populations, their accuracy in clinical practice is widely variable. It is a feasible method for RMR estimation when the proper equation for the individual is applied[18].
Currently, there is still no predictive equation considered the most accurate for cirrhotic patients. So much so that in the meta-analysis by Eslamparastet al[19], when analyzing 17 articles on the estimation of RMR in cirrhotic patients, which compared IC with different predictive formulas, they observed that the RMR values are underestimated, especially in males and in the Western population. Furthermore, there are insufficient data regarding the value of RMR according to the severity of chronic liver disease.
Noteworthy, miscalculation of REE in patients with cirrhosis can lead to inaccurate or inappropriate therapeutic management and worsening symptoms such as anorexia, dysgeusia, early satiety, nausea, and vomiting (especially in the presence of hepatic encephalopathy), and may potentiate adverse drug reactions[20,21]. In this context, the objective of the present study was to determine the REE of patients with cirrhosis by IC and compare the values thus obtained to those estimated by bioelectrical impedance analysis (BIA) and common predictive equations, in order to identify a reliable method for calculating energy expenditure applicable in clinical practice.
MATERIALS AND METHODS
This was an observational study. We included 90 patients who were receiving clinical management of liver cirrhosis at the Outpatient Gastroenterology and Liver Transplantation Clinics of Santa Casa de Misericórdia de Porto Alegre, Rio Grande do Sul, Brazil from March 2017 to July 2018. All patients included in this study agreed to participate and provided written informed consent. The study protocol was approved by the Research Ethics Committees of Santa Casa de Misericórdia de Porto Alegre (No. 2.387.800). Sample size calculation was based on a previous study by Teramotoet al[22] which compared measured and predicted energy expenditure in patients with cirrhosis. Considering a statistical power of 80% and a significance level of 5%, the minimum sample size was estimated at 90 patients.
Adult patients (age 18 years or older) of both sexes with cirrhosis of the liver were eligible for inclusion. Patients on enteral feeding were excluded, as were those with amputation of any limb and those unable to complete the proposed evaluations (e.g., those who reported discomfort during IC, who could not remain in position, or who had a pacemaker which precluded BIA). Data from the electronic medical records of the patients, related to the diagnosis, staging by the Child-Pugh score, age, and sex of the participants, were collected. The diagnosis of cirrhosis was made by clinical, laboratory, imaging, and/or, eventually, liver biopsy in accordance with the hospital liver transplant group standards[12].
Current body weight was measured on a calibrated Filizola anthropometric scale (precision 0.1 kg). Height was measured with a wall-mounted stadiometer, with the patient standing upright and barefoot. Body mass index (BMI) was calculated as {BMI = weight (kg)/[height (cm)]2} and classified according to the World Health Organization curves[23].
BIA was performed as described elsewhere using a Biodynamics model 450 BIA device (current 800 µA, frequency 50 kHz), with electrodes placed on the hand/wrist and foot/ankle.
IC was performed by the same investigator, using a Korr MetaCheck calorimeter. The assessment was begun after a minimum of 4 h and a 30-min rest. Measurement was performed with the patient perfectly still in the supine position, for 10 to 30 min, wearing a rigid face mask. The formula described by Weir (14) was used to calculate REE during the most stable period of analysis, based on O2consumption (VO2), CO2output (VCO2), and urine urea nitrogen, as follows: REE = [3.9 (VO2)] + [1.1 (VCO2)][24].
Table 1 describes the energy expenditure predictive equations used in the study: BIA - Based on Grande & Keys[25]; Cunningham[26]; Harris and Bennedict[27]; Food and Agriculture Organization of the United Nations, World Health Organization and United Nations University [Food and Agriculture Organization of the United Nations, World Health Organization and United Nations University (FAO/WHO/UNU)][23]; Institute of Medicine[28]; McArdle[29]; and Mifflin[30].

Table 1 Predictive equations for derivation of energy expenditure, all values obtained in kilocalories
Statistical analysis
Quantitative variables are expressed as the mean and standard deviation, and categorical variables, as absolute and relative frequencies. The equations were compared with IC using the Bland-Altman method[31], and also the Student’st-test for paired samples. The Student’st-test for paired samples was also used for comparison between IC and 24 h dietary recall findings. The correlation between BMI and IC was assessed by Pearson’s correlation coefficient. Analysis of variance (ANOVA) with Tukey’s posthoc test was used for comparison of mean 24-h dietary recall and REE-IC according to Child-Pugh class. The significance level was set at 5% (P< 0.05). All analyses were performed with PASW Statistics, Version 18.0.
RESULTS
Ninety patients, with a mean age of 57.1 (± 9.3) years, were assessed. Of these, 52 (57.8%) were male. The clinical profile of the sample is described in Table 2.

Table 2 Sample characteristics
Table 3 shows the values of REE in kilocalories, measured by IC and predictive methods. The mean REE measured by IC was 1607.72 ± 257.4. A correlation between REE measured by IC and muscle mass in kilograms (R2= 0.353,P= 0.001) was found. Also, the IC values were not different between patients classified in groups in accordance with their Child-Pugh scores (P= 0.885). Although the IC values showed a positive correlation with predictive methods, the IC values were significantly different when compared to predictive methods, except for the McArdle and FAO/WHO/UNU predictive equations.
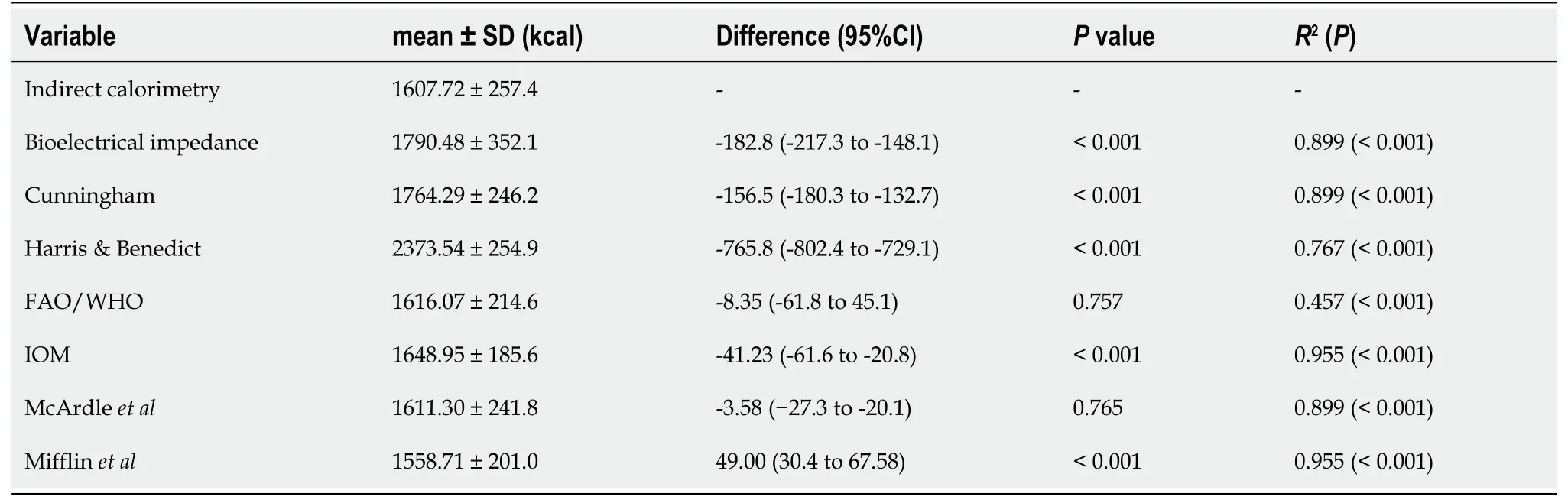
Table 3 Comparisons and correlations between resting energy expenditure measured by indirect calorimetry and different predictive methods
As shown in Figure 1, we found differences in agreement between IC and the predictive methods. The best agreement was found between IC and the IOM equation, followed by FAO/WHO/UNU and McArdle equations. The agreement between IC and BIA was below 10% of the mean difference. The Harris and Benedict and the Mifflin equations showed less agreement with the IC values. The ANOVA analysis showed no differences of IC or REE estimated by different methods when patients were grouped by their Child-Pugh scores (data not showed).

Figure 1 Bland-Altman plots comparing indirect calorimetry with predictive methods. A: Mean of indirect calorimetry (IC) and bioelectrical impedance analysis (BIA); B: Mean of IC and Harris & Bennedict; C: Mean of IC and IOM; D: Mean of IC and FAO/WHO; E: Mean of IC and McArdle; F: Mean of IC and Cunningham; G: Mean of IC and Mifflin. BIA: Bioelectrical impedance analysis; FAO: Food and Agriculture indirect calorimetry Organization of the United Nations; IC: Indirect calorimetry; WHO: World Health Organization.
DISCUSSION
The present study aimed to determine the REE of patients with cirrhosis by IC and compare the values thus obtained to those estimated by BIA and common predictive equations. The IOM and FAO/WHO/UNU equations showed the best agreement with IC, whilst the McArdle equation and BIA could also be considered appropriate for REE estimation.
The present study evaluated 90 patients, with a mean age of 57 (± 9.3) years, which is close to that previously reported[32] whilst the male predominance of the sample is also consistent with prior work by Tajikaet al[5] and Wilkens Knudsenet al[33]. Regarding Child-Pugh classification, our sample was homogeneous, with 33 patients in class A, 36 in class B, and 21 identified as having class C; this proportion differs from that reported by Qing-Hua Meng, where 60% of patients had Child-Pugh A and only eight had class C[34]. Regarding nutritional status, the mean BMI of patients in our study was 28.6 ± 5.6 kg/m2, which would classify them as overweight[23]. This result is in line with Brazilian studies of patients with cirrhosis which confirmed the same classification[3,35]. Like Fernandeset al[3], we did not find BMI to be a reliable method of estimating nutritional status in this population, due to the distortion of body weight inherent to the underlying disorder. Strikingly, we did not identify a correlation between BMI and REE, albeit we report a correlation between REE and muscle mass (in kilograms).
IC is considered by many researchers as the gold standard for measuring REE. It is a non-invasive method, capable of measuring basal energy expenditure by means of gas exchange, thus ensuring greater precision in measurement[35-37]. In our study, the average REE-IC was 1522 ± 271 kcal, very close to the result reported by Pintoet al[36] of 1534 ± 300 kcal in a sample of 45 patients waitlisted for liver transplantation, which corroborates the expectation of accuracy of caloric prediction by this method.
Comparison of REE-IC values with those calculated by the Harris and Benedict (HB) equation revealed super estimated values. Consistent with other studies[22,34,35], our findings suggest that common predictive equations for estimation of REE could be clinically inaccurate in cirrhotic patients, since they are usually based on body weight, a parameter that can be altered by several factors-such as ascites and fluid retention-and thus directly affect the energy expenditure estimated by the equation[33]. Thus, even considering their low cost and applicability, using predictive equation should consider the aforementioned aspects, since overestimation of REE has been reported in many previously published comparisons[22,34,35]. Corroborating our line of thought, Menget al[34] found a reduced REE in 53% of their sample of 153 patients with liver cirrhosis when REE measured by IC as compared to REE estimated by the HB equation. Likewise, Teramotoet al[22] evaluated 488 patients and found that the estimated REE was 1256 kcal by ICvs1279 kcal by the HB formula.
Boullataet al[38] aimed to compare the accuracy of seven predictive equations, including the Harris-Benedict and the Mifflin equations, against measured REE in hospitalized patients, including patients with obesity and critical illness. The authors concluded that no predictive method was accurate when considering accuracy as 90% to 110% of the value obtained by IC. In our study, most of the evaluated predictive methods resulted in an error below 10%. Further, based on our findings, in circumstances where IC is not available, the FAO/WHO/UNU or McArdleet al[29] equations can be used to accurately estimate REE, since they may yield values closer to those of IC in patients with cirrhosis. Also, the IOM equation could be used, since it also showed good agreement in the Bland-Altman analysis, albeit it was significantly different in thet-test. Noteworthy, a previous study including patients with portal hypertension reported that the Zanellaet al[39] equation was one of the predictive methods that differed most in REE estimates in the study population. As in our study, IC yielded a higher value than all other methods. The authors noted that all other methods underestimated the predicted REE by more than 200 kcal when compared to IC, except Cunningham’s predictive equation. Therefore, it bears stressing that the same method of assessment in different populations can present different correlations with the available predictive equations.
Although we have not found prior publications supporting the use of BIA to determine REE as a means of extrapolating energy expenditure in patients with cirrhosis, this method was used in a study by Strainet al[40] of morbidly obese patients. There was no significant difference between the value predicted by BIA (which was based on the HB equation) and IC, which could support the indication of BIA as a good predictor of energy expenditure in this population[40]. Our study also found that the BIA equipment was able to predict REE, albeit different BIA equipment applies different equations to predict REE using body composition parameters, and users should observe which equation is being applied.
We found no significant differences in IC between patients classified as Child-Pugh A and those classified as Child-Pugh B. In this respect, our findings corroborate those of Teramotoet al[22] and Menget al[34], who found no statistically significant difference in IC when comparing the three Child-Pugh prognostic classes. Moreover, Belarminoet al[32] reported that the dietary intake of patients with cirrhosis in their sample was 1.4 times greater than that predicted by IC, while in our study, it was 1.14 times greater. Teramotoet al[22] found that most patients in their sample had adequate dietary intake and there was no statistically significant difference between Child-Pugh classes, corroborating the findings in the present study. Menget al[34] highlighted that dietary intake can be impaired by factors such as anorexia, weakness, fatigue, low-grade encephalopathy, and restrictions on sodium, protein, and fluid intake. These data, in addition to the insufficient energy intake in 48% of the patients studied by Nuneset al[41], who evaluated a sample of 25 cirrhotic patients and found an average of 2012 ± 720 kcal, highlight the importance of adequate estimation of REE in these patients, to prevent malnutrition and improve prognosis and outcomes.
Limitations of the present study include the absence of a healthy control group for comparison and the possibility of recall bias interfering with the 24 h dietary recall, despite this being a validated method.
CONCLUSION
The present study aimed to determine the REE of patients with cirrhosis by IC and compare the values thus obtained to those estimated by BIA and common predictive equations. The McArdle and FAO/WHO/UNU equations showed the best agreement with IC, whilst the IOM equation and BIA could also be considered appropriate for REE estimation. Further studies in different populations of patients with cirrhosis, including different severity profiles, are needed to determine the best methods for REE estimation in clinical practice.
ARTICLE HIGHLIGHTS
Research background
Patients with cirrhosis commonly present malnutrition, resulting in a significant imbalance in energy metabolism that negatively impacts their prognosis and quality of life. However, adequate dietary prescription depends on the precision of the protocols for energy requirement estimation, and the current literature is still conflicting regarding the relationship between cirrhosis progression and resting metabolic rate alterations.
Research motivation
Reliable calculation of resting energy expenditure (REE) in patients with cirrhosis is pivotal to appropriate therapeutic management. However, there is still a need to evaluate which of the predictive equations is more effective in the clinical setting.
Research objectives
The objective of the present study was to determine the REE of patients with cirrhosis by indirect calorimetry (IC) and compare the values thus obtained to those estimated by bioelectrical impedance analysis (BIA) and common predictive equations.
Research methods
This was an observational study performed at the Outpatient Gastroenterology and Liver Transplantation Clinics of Santa Casa de Misericórdia de Porto Alegre, Rio Grande do Sul, Brazil. Data from the electronic medical records of the patients, related to the diagnosis, staging by the Child-Pugh score, age, and sex of the participants, were collected. The diagnosis of cirrhosis was made by clinical,laboratory, imaging, and/or, eventually, liver biopsy in accordance with the hospital liver transplant group standards. BIA and IC were performed and the results were compared to energy expenditure predictive equations using the Bland-Altman method, and also the Student’s t-test for paired samples.
Research results
Ninety patients, with a mean age of 57.1 years, were assessed. The mean REE measured by IC was 1607.72 and there were no differences in REE when comparing groups with different Child-Pugh scores.The IC values were significantly different when compared to predictive methods, except for the McArdle and Food and Agriculture Organization of the United Nations, World Health Organization and United Nations University (FAO/WHO/UNU) predictive equations. The best agreement was found between IC and the IOM equation, followed by the FAO/WHO/UNU and McArdle equations.The agreement between IC and BIA was below 10% of the mean difference. The Harris and Benedict and the Mifflin equations showed less agreement with the IC values.
Research conclusions
The present study determined the REE of patients with cirrhosis, indicating that the McArdle and FAO/WHO/UNU equations showed the best agreement with IC, whilst the IOM and BIA could also be considered appropriate for REE estimation.
Research perspectives
Further studies in different populations of patients with cirrhosis, including different severity profiles,are needed to determine the best methods for REE estimation in clinical practice.
FOOTNOTES
Author contributions:Ferreira S contributed to the conception and design of the study, data collection, statistical analysis and writing of the manuscript; Marroni CA contributed to the conception and design of the study and writing of the manuscript; Stein JT, Henz AC and Rayn RG collected the data; Schmidt NP contributed to the conception and design of the study, data collection; Carteri RB statistical analysis and manuscript writing; Fernandes SA manuscript writing and critical review.
Institutional review board statement:This study was approved by the Research Ethics Committee of Irmandade Santa Casa de Misericórdia de Porto Alegre (No. 2.387.800).
Informed consent statement:Patients who agreed to participate in the study signed the Free and Informed Consent Form.
Conflict-of-interest statement:All authors declare that there are no conflicts of interest related to this article.
Data sharing statement:No additional data is available for sharing.
STROBE statement:The authors have read the STROBE Statement-checklist of items, and the manuscript was prepared and revised according to the STROBE Statement-checklist of items.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:Brazil
ORCID number:Shaiane Ferreira 0000-0002-8131-6773; Cláudio Augusto Marroni 0000-0002-1718-6548; Jessica Taina Stein 0000-0001-9151-4303; Roberta Rayn 0000-0002-8492-8804; Ana Cristhina Henz 0000-0002-4260-2881; Natália P Schmidt 0000-0002-1084-7147; Randhall B Carteri 0000-0003-4124-9470; Sabrina Alves Fernandes 0000-0001-8504-603X.
S-Editor:Liu JH
L-Editor:Wang TQ
P-Editor:Liu JH
杂志排行
World Journal of Hepatology的其它文章
- Revolution in the diagnosis and management of hepatitis C virus infection in current era
- Evidence-based approach to management of hepatic encephalopathy in adults
- Direct oral anticoagulant administration in cirrhotic patients with portal vein thrombosis: What is the evidence?
- Noninvasive diagnosis of periportal fibrosis in schistosomiasis mansoni: A comprehensive review
- Review on hepatitis B virus precore/core promoter mutations and their correlation with genotypes and liver disease severity
- Assessment of periportal fibrosis in Schistosomiasis mansoni patients by proton nuclear magnetic resonance-based metabonomics models
