Evidence-based approach to management of hepatic encephalopathy in adults
2022-07-02GillesJaddHoilatFathimaKeshiaSuhailTalalAdhamiSavioJohn
Gilles Jadd Hoilat, Fathima Keshia Suhail, Talal Adhami, Savio John
Gilles Jadd Hoilat, Fathima Keshia Suhail, Department of Medicine, SUNY Upstate Medical University, Syracuse, NY 13210, United States
Talal Adhami, Department of Gastroenterology, Cleveland Clinic Foundation, Cleveland, OH 44195, United States
Savio John, Department of Gastroenterology, SUNY Upstate Medical University, Syracuse, NY 13210, United States
Abstract Hepatic encephalopathy (HE) is a reversible syndrome of impaired brain function and represents one of the many complications of portal hypertension and decompensated liver disease. Although ammonia is clearly implicated in the pathogenesis of HE, the pathogenesis of HE is multifactorial with numerous mechanisms that results in functional impairment of neuronal cells. The initial management of HE focuses on supportive care and stabilization which includes providing appropriate nutritional support. Thereafter, focus should be on identifying and treating the precipitating factors. There are many therapeutic agents available for the management of HE, most of which are directed towards lowering the gut nitrogen load and thus the serum ammonia level. This review aims to provide an update on the conventional and emerging treatment options for HE.
Key Words: Hepatic encephalopathy; Lactulose; Rifaximin; Fecal microbiota transplant; Zinc; L-ornithine L-aspartate
INTRODUCTION
Hepatic encephalopathy (HE) is a reversible syndrome of impaired brain function and represents one of the many complications of portal hypertension and decompensated liver disease. It is estimated to be present in 50% to 70% of patients with liver cirrhosis[1]. It is a debilitating disease that affects the quality of life of both the patients and their caregivers and contributes to significant health care resource utilization making it an economic burden on health care facilities[2]. In 2009, 23000 patients were admitted for HE, with an average length of stay of 8.5 d exhausting $ 63108 per case[2]. The pathogenesis of HE is multifactorial with numerous mechanisms that results in functional impairment of neuronal cells, none of which are clearly understood[3].
Ammonia, which is a gut-derived nitrogenous toxin produced by the bacterial metabolism of urea from dietary proteins, has been considered the primary pathophysiologic mechanism of HE. It is normally metabolized by the liver and cleared mostly by the kidney and to a lesser extent in the muscle. In patients with cirrhosis and portal hypertension, the hepatic metabolism of ammonia is impaired and there is shunting of ammonia-rich portal blood to the systemic circulation without detoxification. In the brain, ammonia crosses the blood-brain barrier and is metabolized in the astrocytes by glutamine synthetase, which converts ammonia and glutamate to glutamine. Accumulation of glutamine in astrocytes creates an osmotic gradient, resulting in astrocyte swelling and generation of reactive oxygen species, thereby contributing to the cerebral dysfunction. Ammonia also binds to gamma-aminobutyric acid (GABA) receptors on astrocytes, leads to neurosteroids activation, which further contribute to the occurrence of HE. Moreover, the dysbiosis and increased gut permeability seen in cirrhotic patients causes an increase in the production of multiple inflammatory cytokines, which leads to increased blood-brain barrier permeability and cerebral edema[3-5].
Although ammonia is clearly implicated in the pathogenesis of HE, additional factors include inhibition of neurotransmission in the central nervous systemviaGABA receptors and alteration in other CNS neurotransmitters and circulating amino acids .The precipitating factors for HE include liver failure causing decreased metabolism of ammonia, hypoxia and increased ammonia load due to gastrointestinal bleeding, sepsis, alterations in gut flora, hepatocyte necrosis, neuroinflammation, and structural and functional changes in the brain due to other disease process, presence of spontaneous or iatrogenic portosystemic shunt, and other conditions such as hypokalemia, hyponatremia and use of sedatives.
HE has varying degrees of severity and is commonly divided according to the West Haven criteria into covert HE (CHE) and overt HE (OHE). CHE can be either minimal HE (MHE) or grade I HE, while OHE includes grade II-IV[3,6]. OHE is a spectrum of neuropsychological abnormalities that can usually be detected by bedside clinical tests in contrast to CHE, where specific psychometric tests are needed to discern them because of quasi-normal mental status of the patient at bedside. OHE is present in 30%-45% of patients, with a yearly cumulative risk of development in 20% of patients with cirrhosis. Around 60%-80% of patients diagnosed with liver cirrhosis have evidence of cognitive dysfunction or MHE[7].
The 2014 American Association for the Study of Liver Diseases (AASLD) and the European Association for the Study of the Liver clinical practice guidelines for HE management[8] recommend classifying HE based on 4 factors detailed in Figure 1: (1) Underlying disease (Type A: HE due to acute liver failure, type B: HE due to portosystemic shunts and type C: HE due to cirrhosis); (2) Severity; (3) Time course; and (4) Precipitating factors.
The initial management of HE focuses on supportive care and stabilization which includes providing appropriate nutritional support to maintain an energy intake of 35-40 kcal/kg/d, with a protein intake of 1.2-1.5 g/kg/d, correction of precipitating causes which include dehydration and electrolyte abnormalities such as hypokalemia and metabolic alkalosis[9,10]. Thereafter, focus should be on identifying and treating the precipitating factors underlined in Figure 1. There are many therapeutic agents available for the management of HE, most of them are directed towards lowering the gut nitrogen load and thus the serum ammonia levels. This review aims to provide an update on the conventional and emerging treatment options for HE.
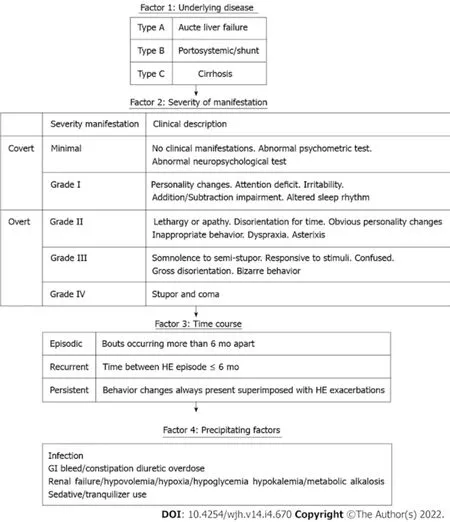
Figure 1 Classification of hepatic encephalopathy based on 4 factors. Portions of this figure are adapted from the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver clinical practice guidelines for hepatic encephalopathy management.
GUT FLORA MODIFYING AGENTS
Nonabsorbable disaccharides
The nonabsorbable disaccharides lactulose and lactitol are considered the first-line therapeutic agents for treating HE. They are shown to significantly improve cognition and quality of life in patients with MHE, with lactitol having fewer side effects compared to lactulose[11,12].
It reduces intestinal ammonia production and absorption by four main mechanisms[13-15]: (1) The catabolism of lactulose by bacterial flora in the colon decreases the pH. The acidic pH leads to the formation of nonabsorbable NH4+from NH3. The trapping of NH4+(impermeable to membranes) in the colon reduces the plasma ammonia concentration; (2) It causes a laxative effect by increasing intraluminal osmolality as well as gas formation leading to a reduction in gastrointestinal transit time; thus, reducing the time for ammonia absorption and increasing the fecal nitrogen excretion; (3) It promotes an increase in ammonia uptake by colonic bacteria as a nitrogen source for protein synthesis and decrease in the formation of potentially toxic short-chain fatty acids; and (4) The acidic pH caused by lactulose modifies the colonic flora by displacing the urease- producing bacteria involved in ammonia synthesis with non-urease-producing Lactobacillus.
A systematic review published by the Cochrane collaboration in 2016 included 38 randomized controlled trials (RCTs) with a total of 1826 patients and compared lactulosevsplacebo. Results were promising and showed a positive effect of non-absorbable disaccharides on mortality [relative risk (RR) = 0.59, 95% confidence interval (CI): 0.40-0.87] and HE (RR = 0.58, 95%CI: 0.50-0.69)[16].
Lactulose (or lactitol in some countries) is often used as the first-line treatment for OHE, at a dose of 30-45 mL (20-30 g) of lactulose syrup every 1-2 h until at least two soft bowel movements are produced. Subsequently, the dosing is of lactulose is titrated to two to four times a day to achieve and maintain two to three bowel movements per day[8]. An approximately equivalent dose of lactitol is 67-100 g lactitol powder diluted in 100 mL of water. Lactulose and lactitol may be given as enemas, if patients are unable to take them orally, as 1-3 L of a 20 percent solution.
Antibiotics
Antibiotics with activity targeting urease producing gut bacteria have an ammonia lowering effect. These antibiotics include rifaximin, neomycin, vancomycin and metronidazole.
Rifaximin:Rifaximin has low systemic absorption, wide antimicrobial spectrum, and low occurrence of side effects[17]. The dose of rifaximin is 550 mg orally twice daily or 400 mg orally three times daily. It is typically used as a combination therapy with lactulose to treat acute encephalopathy and prevent recurrent HE when response to lactulose monotherapy is not inadequate. A randomized trial where 299 patients with cirrhosis and documented HE who were in remission at the start of the trial were administered 550 mg of rifaximin twice dailyvsplacebo for a total of 6 mo found that rifaximin was more effective than placebo in preventing recurrent episodes of HE[18]. Another randomized trial comparing the combination of rifaximin and lactulose with lactulose alone in 120 patients hospitalized with OHE showed that patients who received the combination therapy were more likely to have complete resolution of HE and lower mortality[19].
A recent systematic review of five randomized and five observational studies involving 2276 patients by Wanget al[20] comparing combination therapy (rifaximin + lactulose)vslactulose alone showed that combination therapy significantly increased clinical efficacy compared with lactulose alone in HE patients [risk difference (RD) = 0.19, 95%CI: 0.09-0.29,P= 0.0002] with a number needed to treat (NNT) of 5 in primary analysis. Combination therapy also significantly reduced the mortality in HE patients compared with lactulose alone (RD = 0.11, 95%CI: -0.19 to -0.03,P= 0.009) with an NNT of 9 in primary analysis. Rifaximin has a place mostly in prevention of recurrence of HE when lactulose alone fails; however, recent studies showed that combination therapy with lactulose might be more beneficial[20].
Neomycin:Neomycin acts by inhibiting the activity of glutaminase, consequently decreasing ammonia production from glutamine in the intestinal mucosa. Although widely used for the treatment of HE in the past, neomycin has a significant side effect profile including ototoxicity, nephrotoxicity, and enterocolitis. The AASLD guidelines[8] recommend neomycin as an alternative for the treatment of OHE[21].
Vancomycin and metronidazole:Vancomycin and metronidazole have also been studied alone or in conjunction with lactulose. In one study[22] involving 12 patients with cirrhosis and encephalopathy who were given 2 g of vancomycin, all 12 patients showed a remarkable clinical improvement after treatment. Another study[23] showed that 19 patients with varying grades of encephalopathy that were treated with 1 wk of metronidazole had significant improvement in mental status scores and asterixis, similar to neomycin. Therefore, the authors concluded that metronidazole may be as effective as neomycin. The serious side effect of metronidazole (neurotoxicity) and vancomycin (bacterial resistance) has limited the use of these agents. Hence, long term treatment with these agents is not recommended.
Probiotics
Probiotics are formulations of microorganisms that modify gut flora to acid resistant, non-urease producing flora, resulting in diminished ammonia production and absorption. Prebiotics include compounds such as lactulose and fermentable fiber which promote the growth of beneficial gut flora. The most beneficial species of gut flora in the treatment of HE appears to be Lactobacilli and bifidobacterial[24]. Most commercial probiotic products are derived from food sources, especially cultured milk products. The Cochrane review of 21 trials with 1420 participants that compared probiotics in any dosage with placebo, or with any other treatment in people with HE, concluded that compared with placebo, probiotics probably improve recovery and may reduce recurrences of OHE, quality of life, and plasma ammonia concentrations. Probiotics, however, have no effect on mortality or significant clinical outcomes when probiotics were compared with lactulose[25]. Probiotic groups had reduced plasma ammonia concentrations compared with the placebo/no intervention groups, but not when compared with lactulose groups. Additional studies are needed before probiotics can routinely be recommended for the treatment or prevention of HE.
Fecal microbiota transplant
Cirrhosis is a leading cause of HE. Compared to healthy individual, the fecal microbiome of cirrhotic patients has prevalent pathogenic bacteria such asEnterobacteriaceaeandStreptococcaceaeand reduced beneficial bacteria such asLachnospiraceaeandRuminococcaceae[26]. When the human gut microbiome was compared in patients before and after developing a HE episode, it was found that that there was a significant change in microbial abundance[27]. Subsequent studies conducted on this topic included investigating the fecal microbiome in this subgroup of patients and evaluating if fecal microbiota transplant (FMT) in patients with HE might treat or prevent further episodes of HE and improve cognitive outcomes.
A summary of the pertinent published studies and abstracts investigating the use of FMT in patients with HE till this date is shown in Tables 1 and 2. There was a total of 4 RCTs,1 case series and 1 case report included in this analysis.
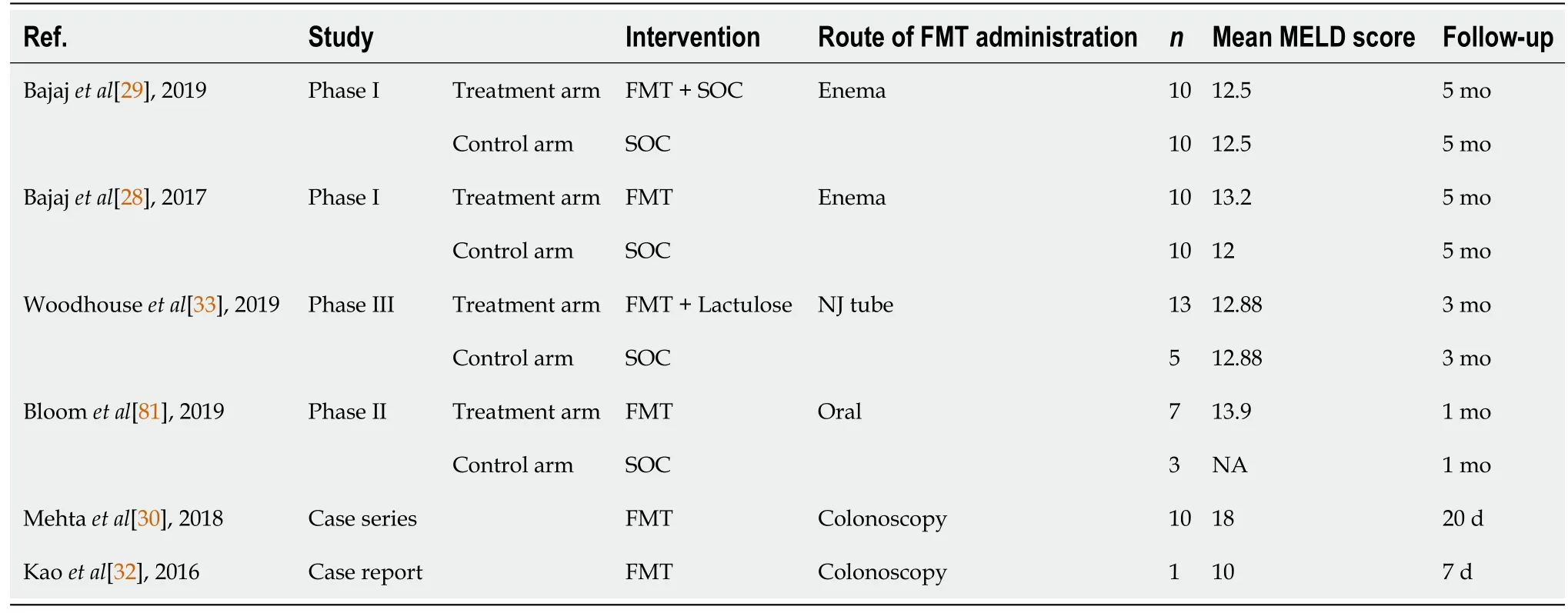
Table 1 Table summarizing characteristics of all studies involving fecal microbiota transplant in cirrhotic patients

Table 2 Table summarizing the findings of all studies involving fecal microbiota transplant in cirrhotic patients
In the phase I study conducted in 2017[28] and subsequently in 2019[29], the number of hospitalizations as well as HE episodes was significantly lower in the patients that underwent FMT. Another study[30] showed that 2/10 patients required hospitalization and 3/10 developed an encephalopathy episode after FMT at 20 wk. FMT also appeared to be safe and well tolerated[31].
Cognition was assessed using EncephalApp Stroop test in 4 studies[28,29,32]. Psychometric Hepatic Encephalopathy Score (PHES) was utilized in three studies[28,29]. Improvement in the time taken to complete the EncephalApp Stroop test and improvement in the PHES score was demonstrated in these studies. There was a remarkable improvement in PHES total score (P= 0.003) and EncephalApp Stroop (P= 0.01) in the FMT group compared to baseline[28], possibly indicating that FMT might also reverse cognitive impairment in patients with HE.
The PHES and EncephalApp-Stroop test are validated tests for HE-related cognitive function tests and improvement in these tests in patients who underwent FMT is promising. FMT has also been shown to influence lowering serum ammonia levels in three studies[30,32,33]. Although FMT looks promising, additional larger RCTs are needed to validate the results.
NUTRITION, DIETARY MODIFICATION AND SUPPLEMENTATION
Zinc
It has been demonstrated that zinc deficiency is common in patients with liver cirrhosis[34] and lower serum zinc level has also been a precipitating factor for HE[35]. Zinc deficiency results in decreased activity of muscle glutamine synthetase, an important enzyme in reducing serum ammonia levels making zinc an important factor in ammonia detoxification[36].
Chavez-Tapiaet al[37] published a metanalysis that included four RCTs of 233 patients evaluating the effect of oral zinc supplementationvsplacebo or standard therapy over HE. Three studies showed an improvement in performance on number connection test in the zinc group compared to placebo or standard therapy. This improvement suggests a beneficial effect of oral zinc in encephalopathic patients. However, there was no beneficial effect on HE recurrence. Shenet al[38] also published a metanalysis that included four RCTs of 247 patient’s and concluded that a combination treatment of zinc and lactulose over 3-6 mo significantly improved performance in the number connection test compared to lactulose alone. The effect of short-term (10 d) oral zinc supplementation (zinc sulfate 600 mg/d) on HE, was assessed in a double-blind, crossover trial involving fifteen cirrhotic patients with stable, chronic HE. Serum zinc was significantly raised after oral zinc administration and reached the levels observed in cirrhotics without HE. Despite this, the study failed to confirm that short-term oral zinc supplementation improves chronic HE[39]. Zinc supplementation cannot be recommended for treatment of HE in the absence of larger sample size study.
Branched-chain amino acids
In cirrhotic patients, it has been clarified that there is an imbalance between aromatic amino acids (AAA) and branch-chain amino acids (BCAA) where serum concentrations of AAA are increased and BCAA are decreased. These alterations are thought to increase brain levels of aromatic amino acid precursors for monoamine neurotransmitters which contribute to altered neuronal excitability and development of HE[40].
In 2017, the Cochrane collaboration published a systemic review of 827 patients in 16 RCTs in which use of oral (eight trials) or intravenous BCAAs (seven trials) was compared with placebo, diet, lactulose, or neomycin. BCAAs were found to have a beneficial effect on manifestations of HE. More specifically, when excluding trials on lactulose or neomycin, BCAA had a beneficial effect on HE. However, when analyzing trials with a lactulose or neomycin control, it was found that there was no statistically significant benefit of BCAA over lactulose or neomycin. Gastrointestinal discomfort was the main adverse reaction observed while using BCAA with no serious adverse events reported and this intervention did not seem to influence mortality and quality of life[41]. In summary, it was found that oral, but not intravenous, BCAAs may have beneficial effects. BCAA supplementation may be considered in severely protein-intolerant patients as no benefit was observed in protein-tolerant patients.
Acetyl-L-carnitine
Carnitine is a metabolite in the degradation pathway of the essential amino acid lysine and is a substance natural to the body. Acetyl-L-carnitine (ALC) is readily formed in cells by the enzymatic addition of an acetyl group to carnitine. The major difference between ALC and carnitine is that ALC is more easily absorbed from the gut, and more readily crosses the blood-brain barrier. Carnitine is a carrier for short chain fatty acids across the mitochondrial membrane and is thought to have neuroprotective properties. A systematic review[42] concluded that ALC was effective in improving serum ammonia level (weighted mean difference 25.90, 95%CI: 20.89-30.91,P< 0.05) and number connection test completion time (weighted mean difference: 16.62, 95%CI: 9.88-23.36,P< 0.05), and thus a promising treatment for HE. However, in 2019, the Cochrane collaboration published a systemic review of 398 patients in 5 clinical trials that compared ALC plus standard care (e.g.,antibiotics, lactulose)vsplacebo or standard care in participants with cirrhosis with covert or OHE. The review showed that ALC reduces serum ammonium levels compared with placebo however no information was found about all-cause mortality, serious adverse events, or days of hospitalization. No clear differences were found between ALC and placebo regarding quality of life, fatigue, and non-serious adverse event[43]. In summary, further RCTs are needed to assess ALCvsplacebo.
CENTRAL NERVOUS SYSTEM ACTING AGENTS
Flumazenil
Flumazenil is a short acting benzodiazepine receptor antagonist that was described in multiple trials to benefit patients with HE by antagonizing and eliciting a negative allosteric modulatory effect on the central benzodiazepine receptors[44-46]. The Cochrane collaboration published in 2017 a systematic review[47] of 14 RCT that included 867 patients comparing flumazenilvsplacebo. The duration of follow-up was less than 1 d in the majority of the RCT and it was shown that flumazenil was associated with a beneficial effect on HE but with no beneficial effect on mortality, serious adverse events, or health-related quality of life. Although flumazenil yielded short term improvement, all except one of the RCT were described as having high-risk bias. Flumazenil is not recommended for routine clinical use, though it may be considered for select patients who have received benzodiazepine therapy.
Dopamine agonists
Bromocriptine, a dopamine receptor agonist, has been studied as a potential treatment for HE. In one study, 6 patients with cirrhosis and severe HE unresponsive to standard therapy were given oral bromocriptine up to 15 mg daily. All patients showed significant improvement clinically as well as improvement in cerebral blood flow and cerebral glucose consumption which led the authors to conclude that bromocriptine is a useful treatment for chronic HE when conventional therapy fails[48]. A recent metanalysis of 5 trials including levodopa and bromocriptine reported no beneficial effects on HE and mortality[49]. The available clinical data are insufficient to assess the benefit of dopamine agonists; however, they might be useful in patients not responding to first line therapies[48,50].
AMMONIA SCAVENGING AGENTS
L-ornithine L-aspartate
L-ornithine L-aspartate (LOLA) is a combination of two endogenous amino acids. In patients with cirrhosis, the activities of carbamoyl phosphate synthetase and glutamine synthetase are impaired. Ornithine activates both the enzymes, ornithine and aspartate increase ammonia removalviastimulation of glutamine synthesis. LOLA has thus been shown to have ammonia lowering actionsviastimulation of urea synthesis by residual periportal hepatocytes and ammonia removal by glutamine synthesis in skeletal muscle[51].
The first published analysis considered[52] five double-blind, placebo-controlled RCTs in 246 patients with cirrhosis (Child-Pugh status A or B) and compared intravenous infusions of 20-40 mg of LOLA over 4-8 h for a 7-d period to placebo. LOLA treatment showed a 3.22-fold greater chance of resolution of OHE and significant reduction of post-prandial serum ammonia after 7 d of therapy compared to placebo. Subsequently, a high quality meta-analysis of three randomized trials that included 212 patients with cirrhosis[53], a meta-analysis of 8 RCTs with 646 patients with cirrhosis[54], and a metaanalysis of 15 RCTs with 1023 patients[55] showed that LOLA was significantly more effective than placebo in patient with OHE. In another met analysis[56], LOLA was shown to significantly reduce serum ammonia level (MD = 17.50, 95%CI: -27.73 to -7.26), regardless of its formulation, compared to placebo. When compared to other ammonia lower agents, LOLA was noted to cause decreases in serum ammonia levels compared to lactulose[57], and improvement of psychometric test scores compared to rifaximin and probiotics[58,59].
A very recent metanalysis published by the Cochrane collaboration[55] in 2018 of 36 RCTs encompassing 2377 patients showed that LOLA had beneficial effect on HE compared with placebo. In patients with MHE, LOLA was found to be comparable to lactulose and rifaximin for both reversal of deficits in psychometric test scores and for slowing of progression from MHE to OHE[51]. Treatment with LOLA is beneficial compared with placebo, but trials comparing LOLA with lactulose are needed. Overall, LOLA has been evaluated to be a safe, effective, and well-tolerated. It is routinely given to patients with HE outside of the United States.
Ornithine phenylacetate, phenylbutyrate and sodium benzoate
L-ornithine combined with phenylacetate as L-ornithine phenylacetate (OPA) lowers ammonia level by L ornithine stimulating synthesis of glutamine from ammonia in skeletal muscle and phenylacetate binding to glutamine and excreting the compound as phenylacetylglutamine through the kidneys in the urine[60]. A study performed by Stravitzet al[61] included 47 patients with acute liver injury/acute liver failure and ammonia level above 60 μM showed that this therapy is safe and well-tolerated in patients with acute liver failure. A metanalysis published by the Cochrane database in 2019[62] failed to show beneficial or harmful effects of OPAvsplacebo. Up to this date, there is no clear clinical evidence linking OPA to HE.
Phenylbutyrate (PB), a prodrug of phenylacetate is rapidly oxidized to phenylacetate and acts in the same way[63]. A phase II trial of 178 patients[64] compared glycerol PB (GPB) to placebo and concluded that GPB decreased the frequency of HE events as well as the ammonia levels in patients with cirrhosis and HE and had a comparable safety profile to placebo. However, larger RCTs are needed to confirm these results.
Sodium benzoate is generally used in food and beverage preservative. It conjugates with glycine in the liver and the kidney to form hippuric acid which carries waste nitrogen and is then excreted by the kidneys[13,65]. This low-priced adjunctive agent has shown promising results but cannot be used as first-line therapy until additional randomized trials are conducted. It can be considered as an alternative in patients with good creatinine clearance who are unable to tolerate standard of care regimen or have failed to improve despite the standard regimen[65,66]. A small RCT performed in 1992 by Sushmaet al[67], however, compared sodium benzoate to lactulose and concluded that that sodium benzoate is as safe and effective as lactulose.
Spherical carbon microsphere adsorbent (AST-120)
This is an orally administered, engineered carbon microsphere. Compared to activated charcoal, it possesses a better adsorptive capacity for certain organic compounds. It binds to ammonia in the gastrointestinal lumen and facilitate its excretion[62,68]. In rats, it has shown to lower serum ammonia levels and normalize brain water content[68].
Polyethylene glycol 3350-electrolyte solution
Prior to the introduction of lactulose as a therapeutic option for HE, laxative agents were used, suggesting that catharsis might be an effective treatment of HE. However, since the adoption of lactulose for the treatment of HE, studies of cathartic methods have largely been abandoned until recently when a growing interest has developed over a safe, commonly used, and highly effective laxative: Polyethylene glycol 3350-electrolyte solution (PEG)[69]. Multiple RCTs[69-71] studied the effect of PEGvslactulose for the treatment of HE. These studies showed that PEG led to a more rapid improvement of the HE scoring algorithm score in 24 h and shortened the hospital say. Larger trials are needed to confirm these results before recommending PEG as a routine treatment for patients with cirrhosis and encephalopathy.
Molecular absorbent recirculating system
Molecular absorbent recirculating system (MARS) is an extracorporeal hepatic support system that integrates the mechanisms of dialysis, ultrafiltration, and adsorption. It utilizes an albumin dialysate across a semi-permeable high-fluid membrane to remove protein-bound and water-soluble toxins[72]. MARS was first approved by the United States Food and Drug Administration in 2005 for use in drug overdoses and an additional approval was granted in 2012 for use in HE due to decompensated chronic liver disease[73].
The RELIEF trial that compared standard therapyvsMARS in 189 patients with acute on chronic liver failure showed that the patients treated with MARS had a significant improvement in symptoms of HE (38.2%vs62.5%, respectively). Specifically, patients with HE treated with MARS improved from grade III-IV to grade 0-I. The study was statistically significant (P= 0.07)[74].
MARS is an expensive therapy necessitating specific skill set and expertise to operate. It is offered only in a few institutions in the United States and Europe. Survival benefit has not been demonstrated. Larger RCTs are essential to rationalize its usage at a greater scale.
VASCULAR/INTERVENTIONAL MANAGEMENT
Embolization of portosystemic shunts
Patients who fail medical management are referred to as having refractory HE. These patients may harbor large spontaneous portosystemic shunts (SPSS), mainly splenorenal shunt, leading to sustained HE episodes. A few studies have investigated embolization of these shunts in selected patients and have noticed beneficial results in the treatment of HE episodes. In a multicenter study of 37 patients with diagnosed refractory HE and SPSS, 18 patients managed to remain free of HE for about 2 years, there was also an overall improvement in autonomy and a decrease in the number of hospitalizations[75]. In another retrospective study[76] involving 20 patients who were eligible for embolization of SPSS, all the patients had immediate improvement by day 7, and 67% of the patients were free from HE related hospitalization over 1 year. Therefore, SPSS embolization may be a treatment option in a select group of patients with refractory HE.
Although HE is not an indication for liver transplant, liver transplantation remains the definitive treatment for reversal of the complications related to cirrhosis. Studies have shown that patients become free of HE following transplantation; follow up studies have also shown that HE may become irreversible despite liver transplant[77-80].
CONCLUSION
Management of HE, since its initial description, has seen great advancement. However, there still exists a wide discrepancy in delivery of care and patient outcomes. Our understanding of the underlying pathophysiologic mechanisms is still limited. Further research into the pathogenesis of the disease may lead to development of more definitive as well as targeted treatment options.
FOOTNOTES
Author contributions:Hoilat GJ contributed to the manuscript conception and design, literature review, drafting of the manuscript, and submission of the manuscript; Hoilat GJ, Suhail FK, Adhami T, and John S contributed to the critical revision of the manuscript for important intellectual content; Adhami T and John S contributed to the study supervision; John S is responsible for the overall work as a guarantor.
Conflict-of-interest statement:We have no affiliations with any organization or entity with any financial or nonfinancial interest in the subject matter pertaining to this manuscript.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:United States
ORCID number:Gilles Jadd Hoilat 0000-0003-4493-9629; Fathima Keshia Suhail 0000-0001-6420-7172; Talal Adhami 0000-0002-4647-9956; Savio John 0000-0002-6665-6905.
S-Editor:Wang JJ
L-Editor:A
P-Editor:Wang JJ
杂志排行
World Journal of Hepatology的其它文章
- Revolution in the diagnosis and management of hepatitis C virus infection in current era
- Direct oral anticoagulant administration in cirrhotic patients with portal vein thrombosis: What is the evidence?
- Noninvasive diagnosis of periportal fibrosis in schistosomiasis mansoni: A comprehensive review
- Review on hepatitis B virus precore/core promoter mutations and their correlation with genotypes and liver disease severity
- Assessment of periportal fibrosis in Schistosomiasis mansoni patients by proton nuclear magnetic resonance-based metabonomics models
- Baicalin provides protection against fluoxetine-induced hepatotoxicity by modulation of oxidative stress and inflammation
