Prenatal programing of motivated behaviors: can innate immunity prime behavior?
2022-06-29LarisaMontalvoMartnezGabrielaCruzCarrilloRogerMaldonadoRuizLuisTrujilloVillarrealEduardoGarzaVillarrealAlbertoCamachoMorales
Larisa Montalvo-Martínez , Gabriela Cruz-Carrillo , Roger Maldonado-Ruiz , Luis A. Trujillo-Villarreal , Eduardo A. Garza-Villarreal, Alberto Camacho-Morales ,
Abstract Prenatal programming during pregnancy sets physiological outcomes in the offspring by integrating external or internal stimuli. Accordingly, pregnancy is an important stage of physiological adaptations to the environment where the fetus becomes exposed and adapted to the maternal milieu. Maternal exposure to high-energy dense diets can affect motivated behavior in the offspring leading to addiction and impaired sociability. A high-energy dense exposure also increases the pro-inflammatory cytokines profile in plasma and brain and favors microglia activation in the offspring. While still under investigation, prenatal exposure to high-energy dense diets promotes structural abnormalities in selective brain regions regulating motivation and social behavior in the offspring. The current review addresses the role of energy-dense foods programming central and peripheral inflammatory profiles during embryonic development and its effect on motivated behavior in the offspring. We provide preclinical and clinical evidence that supports the contribution of prenatal programming in shaping immune profiles that favor structural and brain circuit disruption leading to aberrant motivated behaviors after birth. We hope this minireview encourages future research on novel insights into the mechanisms underlying maternal programming of motivated behavior by central immune networks.
Key Words: addiction; autism; behavior; cytokines; diet; maternal immune activation; prenatal programming; sociability; trained immunity; western-diets

Introduction 280 Prenatal Programing of Motivated Behaviors in the Offspring 280 High-Energy Dense Diets Activate Innate Immunity during Prenatal Programming 281 Defining the Contribution of Innate Immune Activation to Defective Motivated Behaviors 281 Conclusion and Future Perspectives 282
Introduction
Fetal programming or prenatal programming proposes that certain diseases in adulthood are primed during embryonic development (Scher, 2021). Prenatal programming integrates external or internal stimuli that modulate metabolic, hormonal, immune, and behavioral nodes during development, allowing healthy or aberrant outcomes to the infant after birth (Scher, 2021). Pregnancy is an important stage of physiological adaptations to the environment during which the fetus is exposed to the mother’s hormonal, metabolic, and immune profiles (Scher, 2021). During pregnancy and lactation, neuronal maturation, including axonal pruning, synaptic plasticity, and stable tract formation between brain structures, are selectively programmed during pregnancy and lactation (Montalvo-Martínez et al., 2018; Trujillo Villarreal et al., 2021a).
Consumption of high-sugar, high-fat, or high-sugar-high-fat diets during the prenatal stage define aberrant behavioral phenotypes in the offspring, which might be exacerbated during adulthood. While still under investigation, prenatal exposure to high-sugar-high-fat diets sets defective behaviors such as incentive-motivation behaviors leading to compulsive or mood disorders. It is believed that high-sugar-high-fat diets, defined as high-energy diets, become an aberrant stimulus that favors activation of the maternal innate immune system and alters proper fetal development. In this review, we will provide preclinical and clinical evidence that supports the contribution of prenatal programming by high-energy diets in shaping immune profiles that favor susceptibility to aberrant motivated behaviors in the offspring. We hope this review encourages future research on prenatal programming, including maternal factors such as diet and inflammatory immune profiles, as well as offspring factors such as brain imaging to measure motivated behavior for reinforcements, in order to provide better therapeutic and lifestyle interventions during prenatal stages.
Search Strategy and Selection Criteria
Peer-reviewed articles were searched using PubMed database initially with no limitation on publication date. We designed a two-step strategy, for the first step, we searched terms: “autism” and “prenatal programming”; “autism” and “maternal immune activation”; “social behavior” and “high-fat diet”; and “addiction” and “high-fat diet”. This first strategy allowed us to identify the role of prenatal and/or maternal programming by high-energy diets on motivated behavior including sociability or addiction. For the second step, we search terms: “western diet” and “trained immunity”; “maternal immune activation” and “cytokines”; “cytokines” and “western diet” and “behavior”; “cytokines” and “diet” and “behavior” and “addiction”; and “cytokines” and “diet” and “behavior” and “sociability”. The second step displayed reports supporting the role of high-energy diets on innate immunity, innate training and cytokines profiles and their effect on social and/or addiction-like behavior. All publications cited by the references identified in the PubMed database were selectively examined and screened for their relevance to the main topic “Prenatal programing of innate immunity on motivated behaviors after birth”.
We aimed to provide a compat proposal providing evidence of prenatal triggers modulating the establishment of brain circuitry and their effects of behavior after birth.
Prenatal Programing of Motivated Behaviors in the Offspring
In nature, certain stimuli are rewarding or pleasurable and become adopted by individuals through motivated behaviors. By definition, motivated behaviors are voluntary behaviors that constantly engage in activities that provide reward or pleasure, such as drinking, eating or sex, or some social experiences. In the brain, rewards activate two main dopaminergic pathways that arise from the ventral tegmental area: the mesolimbic pathway and the mesocortical pathway. The mesolimbic pathway integrates the nucleus accumbens, central amygdala, basolateral amygdala, bed nucleus of stria terminalis, lateral septal area, and lateral hypothalamus; whereas the mesocortical pathway, the orbitofrontal and prefrontal cortices.
Ourselves and others have reported that prenatal programming by exposure to high-energy diets in dams primes aberrant motivated behavior in the offspring, including addiction-like, depression-like, and autism-like behaviors (Peleg-Raibstein et al., 2016; Camacho et al., 2017; Winther et al., 2018; Cruz-Carrillo et al., 2020; Gawlińska et al., 2021; Maldonado-Ruiz et al., 2021; Trujillo-Villarreal et al., 2021a, b). In particular, exposure to high-energy diets during fetal development primes addiction-like behavior in the offspring of rodents, as was determined by major lever press responses for food during the behavioral test schedule for reinforcers (Cruz-Carrillo et al., 2020;Figure
1A
andB
). It seems that the offspring experience a high reward value for food that imitates the motivational and impulsive behavior for seeking synthetic reinforcers such as alcohol or drugs. On its own, clinical confirmation of prenatal programming by nutrition as a leading cause of motivated behavior for food intake in humans is limited and largely derived from observational studies, without a proof-of-concept validation. However, some clinical reports have provided advances in the understanding of prenatal programming of food “preferences” in the offspring (Spahn et al., 2019). For instance, a taste for flavors such as alcohol, anise, carrot, or garlic can be transferred from the mother to the offspring through the amniotic fluid, and these flavors can be accepted when re-exposed during infancy (Mennella et al., 1995, 2017; Spahn et al., 2019). While these reports do not confirm motivated behavior for food intake in the offspring, some data documented that a greater intake of red or processed meat, low-fat snacks and desserts, and low-calorie beverages were positively associated with motivated behaviors for food, whereas consumption of refined grains, sugar-sweetened beverages and fruits, vegetables, and legumes were inversely associated with these behaviors (Lemeshow et al., 2018). Some potential limitations to the lack of consistency, reproducibility, and validation of the concept of prenatal programming by high-energy diets in mothers are in part due to pregnant women displaying episodes of cravings for palatable foods, which disturbs the accuracy of the diagnosis (Orloff and Hormes, 2014; Padmanabhan et al., 2015; Blau et al., 2020). Also, there is a lack of selective diagnosis criteria for motivation behavior for food based on the current categorization of the Diagnostic and Statistical Manual of Mental Disorders, 5Edition, which includes eating disorders such as anorexia nervosa, bulimia nervosa, or BE disorder (American Psychiatric Publishing, 2016).The effects of exposure to high-energy diets exposure during prenatal programming on defective behavior were recently confirmed by our research group. We reported for the first time that mice programmed by high-energy diets experienced defects in social interaction with their peers (Maldonado-Ruiz et al., 2021;Figure 2A
). Some clinical reports have confirmed the detrimental effect of exposure to high-energy diets on sociability in the descendants. However, no clear evidence has precisely confirmed the effect of high-energy diets during fetal programming on social interaction in the newborn. Recent reports documented that children diagnosed with autism spectrum disorder (ASD) display higher consumption of high-energy foods than typically developing peers, suggesting, in part, motivational behavior for food preferences (Plaza-Diaz et al., 2021). Also, maternal obesity leads to 1.39% to 1.59% of cases of ASD and a greater likelihood of having a child with ASD compared with their lean counterparts (Wang et al., 2016). While this clinical evidence does not confirm a causal effect of fetal programming by high-energy diets on ASD susceptibility and defective sociability, some reports have confirmed that a positive energy balance such as happened during maternal obesity does prime defective motivational behavior in people within the ASD.High-Energy Dense Diets Activate Innate Immunity during Prenatal Programming
We have just started to decode how prenatal exposure to high-energy diets sets the physiological triggers of motivated behavior in the offspring. Randomized Controlled Clinical Trials collectively confirm that polyphenols and combinations of nutrients such as terpenoids, but not long-chain polyunsaturated fatty acids, vitamin D, specific proteins, or amino acids, improved cognitive performance in humans (Gutierrez et al., 2021). Conversely, exposure to high-energy diets is thought to disrupt the physiological configuration of the reward system in rodents, which is expected to take place as early as embryonic day 13. High-energy diets refer to selective food formulas made of high-sugar, high-fat, or high-sugar-high-fat that provide most of the calories from those nutrients. However, these diets typically have little resemblance to the human Western dietary pattern in terms of macro- and micronutrient content (Hintze et al., 2018). For instance, according to the National Health and Nutrition Examination Survey, the typical American diet formula contains 49% of its calories from carbohydrates, 35% from fat, and 16% from protein (Hintze et al., 2018). In preclinical studies, the term “Western” diet is reserved for those formulas that derive 60% to 70% of its calories from carbohydrates (high-sugar), or 32% to 60% from fat (high fat) or a combination of 54% carbohydrates and 34% fat (high-sugar-high-fat). The high-fat formula has been extensively reported as an adverse trigger of prenatal programming, affecting the establishment of brain circuitry in the offspring.
While still under investigation, prenatal programming by exposure to highenergy diets and their effects on the offspring behavior seems to be primed at very early stages of development. Reports confirm that mothers provide an innate priming stimulus showing interleukin (IL)-6 accumulation in plasma after ingesting a high-fat diet feeding (Bordeleau et al., 2020). In fact, increases in the mRNA levels of Il-6 have been found in the placenta of damns exposed to the high-fat diet, and it could potentially cross the placental barrier to modulate fetal development (Dahlgren et al., 2006). Notably, higher maternal IL-6 concentration during pregnancy was associated with defective brain connectivity and cognitive performance in the newborn at 2 years of age (Graham et al., 2018; Rudolph et al., 2018). Finally, peripheral administration of IL-6 could induce neurovascular remodeling leading to increased permeability of the blood-brain barrier and defective behavior in mice (Menard et al., 2017). This evidence supports a causal effect of IL-6 accumulation in dams exposed to high-energy diets during pregnancy affecting the establishment of brain circuits establishment at the fetus.
Mechanistically, the high-fat diet formula has been confirmed as a trigger of innate immune activation. We and others have confirmed that the saturated lipid, palmitic acid, found in great concentration in the high-fat diet formulas, activates the Toll-like receptor 4 in the brain to pro-inflammatory IL-1β, IL-6, and TNF-α cytokines gene expression and released in plasma (Milanski et al., 2009; Delint-Ramirez et al., 2015). The Toll-like receptor 4/MyD88 pathway and NF-κB translocation into the nucleus subsequent production of proinflammatory cytokines (Kleinridders et al., 2009), and has been also reported to enhance microglial responses (Beaulieu et al., 2021). For instance, palmitic acid impairs migration and phagocytosis of microglia in response to a proinflammatory stimulus (West et al., 2019). Finally, palmitic acid was found to accumulated in the cerebrospinal fluid of overweight and obese subjects diagnosed with amnestic mild cognitive impairment (Melo et al., 2020). These pieces of evidence suggest that food rich in fat and sugar is perceived as an aberrant stimulus during prenatal programming, setting pro-inflammatory profiles, activating microglia, and affecting neuronal function at early stages of development.
As stated, prenatal programming modulates metabolic, hormonal, immune, and behavioral nodes during development, all of which are targeted and negatively disrupted by high-energy diets. However, it is not totally clear how these nodes are mutually integrated and become responsive to high-energy diets during the prenatal stage, setting aberrant behaviors in the offspring, and this is a major future perspective for research.
Defining the Contribution of Innate Immune Activation to Defective Motivated Behaviors
We have focused our research on the hypothesis that exposing dams to high-energy diets favors the activation of the innate immune system during embryonic development, which affects the integration of brain circuits and leads to aberrant motivated behaviors in the offspring. Reports have shown that central and peripheral immune cells interact with each other and coordinate major pathways of synaptic refinement and proper connectome establishment during neurodevelopment. In the last decade, experimental evidence has confirmed that the central nervous system is not an “immunologic privileged” organ, but that peripheral immune cells infiltrate the brain and coordinate neurodevelopment by central-peripheral crosstalk (Stephenson et al., 2018). Compromising the central-peripheral innate crosstalk develops neurodegenerative susceptibility in murine models (Stephenson et al., 2018). Physiologically, the innate system includes macrophages, neutrophils, basophils, eosinophils, and natural killer cells, and also the microglia, the brain resident macrophages.
The most realistic evidence confirming the role of innate immune activation favoring aberrant motivated behavior in the offspring came from mothers exposed to infection during pregnancy. Substantial preclinical and clinical reports have documented that maternal innate immune activation increases susceptibility to neuropsychiatric disorders in the offspring, including schizophrenia, attention-deficit/hyperactivity disorder, Tourette syndrome, bipolar disorder, and ASD (Brown and Meyer, 2018). Preclinical studies have provided some advances for characterizing biological traits linked to maternal immune activation that codes for aberrant motivational behavior in the offspring. Prenatal exposure to pro-inflammatory agents (such as lipopolysaccharide or poly I:C) reduces social interaction in murine models and nonhuman primates. Notably, defects in sociability correlate with an increase of pro-inflammatory cytokines INF-γ, IL-6, IL-17a, and TNF-α in plasma, and high expression of IL-6, toll-like receptor-4, and monocyte chemoattractant protein-1 (MCP-1) in the fetal brain (Careaga et al., 2017), which has been also documented in ASD subjects. It seems that maternal immune activation by Poly I:C disrupts cortical microstructure in rodents showing social defects which depends on the IL-17a signaling (Shin Yim et al., 2017). In this context, we have reported that asocial offspring born from murine dams exposed to high energy-diets shows plasma pro-inflammatory profile that includes INF-γ, IL-6, IL-17a, IL-6, and MCP-1, and also, volume brain alterations and reactive microglia and gliosis (Maldonado-Ruiz et al., 2021; Trujillo-Villarreal et al., 2021a;Figure 2A–D
). Notably, we discovered that systemic MCP-1 inhibition by an MCP-1 antibody increases the brain volume of the primary somatosensory cortex whereas decreases the brain volume of lobe X of cerebellum in the offspring of control and cafeteria subjects (Figure 2C
andD
). Accordingly, microglia, as the major innate immune cell in the brain, is sensitive to high energy-diets and altered establishment of neuronal circuits establishment during development by affecting myelination (Bordeleau et al., 2021), synaptic pruning (Smith et al., 2020), and potentially by modulating blood flow and neurovascular coupling to brain function (Szalay et al., 2016; Shen et al., 2020) or neuronal activity (Badimon et al., 2020). These pieces of evidence open a new research field suggesting that systemic chemokines might modulate microglial function and brain volume changes assisting motivated behaviors.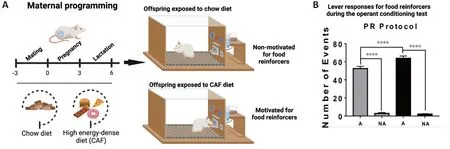
Figure 1|Maternal programming by high-energy dense diets (CAF) primes motivation behavior for food reinforcers. (A) Maternal programming model. Female Wistar rats were fed for 9 weeks including pre-pregnancy, pregnancy, and lactation with standard chow or CAF. Offspring was fed with control diet after weaning until 2 months of age and was trained in the operant training test protocol to diagnosed motivation behavior for food reinforcers. Offspring of dams exposed to CAF diet develops motivation behavior for food reinforcers. (B) Offspring of dams exposed to CAF diet shows major lever responses to work for food during the behavioral test. Adapted from Cruz-Carrillo et al. (2020) with permission. A: Motivated; NA: non-motivated; PR: progressive ratio. Created with BioRender.com.

Figure 2|Maternal programming by high-energy dense diets (CAF) primes defects in social behavior in the offspring. (A) Subjects born from mothers exposed to CAF develop major interaction with the inanimate object when compared with subjects exposed to chow diet. (B) Accumulation of MCP-1 chemokine in offspring of dams exposed to CAF during programming. (C) MRI imaging of primary somatosensory cortex (SSP) or lobe X of cerebellum (CbX) comparing volumetric changes in the offspring of control and CAF subjects followed MCP-1 antibody inoculation. Adapted from Maldonado-Ruiz et al. (2021) with permission. A: Motivated; CAF: cafeteria; CON: control; NA: non-motivated. Created with BioRender.com.
Together, exposure to high-energy diets activates the innate immune system during fetal development favoring pro-inflammatory profiles and structural brain abnormalities in murine models which are reminiscent of brain abnormalities found in ASD subjects.
Conclusion and Future Perspectives
Prenatal programming by high-energy diets highlights the in-utero environment and its critical role in prime susceptibility to aberrant motivated behaviors after birth. A particularly important point to consider is the fact that prenatal programming primes diverse behavioral outcomes coded by the activation of the reward circuit. Despite robust preclinical evidence supporting persistent long-term effects of prenatal programming on aberrant motivated behaviors, evidence in humans is limited and largely derived from observational studies thus, causality or proof of concept of selective physiological traits cannot be inferred.
A major perspective for future research focuses on cell type characterization of innate immune cell infiltration into the brain during physiological and pathological scenarios. In a remarkable report, Jonathan Kipnis and his group identified that a selective population of innate immune cells in the brain came from skull bone niches under homeostatic and pathological conditions (Cugurra et al., 2021). This provides a potential route of innate immune cells infiltration, which might be regulated during prenatal stages of development, modulating the establishment of brain circuitry. In any case, major and active direct-target technological approaches would improve and define how highenergy diets contribute to the infiltration of selective innate immune cells into the brain and the establishment of brain circuits that assist aberrant motivated behaviors after birth.
Acknowledgments:
The authors thank M.S. Alejandra Arreola-Triana (Facultad de Ciencias Biológicas, Universidad Autónoma de Nuevo León, San Nicolas de los Garza, México) for her support on editing this manuscript.
Author contributions:
LMM, GCC, RMR performed the experiments. LMM, GCC, RMR and ACM wrote the manuscript. LATV performed the experiments.EAGV wrote the original manuscript. All authors reviewed, edited, and approved the final manuscript.
Conflicts of interest:
The authors declare no conflicts of interest.
Open access statement:
This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
杂志排行
中国神经再生研究(英文版)的其它文章
- c-Abl kinase at the crossroads of healthy synaptic remodeling and synaptic dysfunction in neurodegenerative diseases
- The mechanism and relevant mediators associated with neuronal apoptosis and potential therapeutic targets in subarachnoid hemorrhage
- Microglia depletion as a therapeutic strategy: friend or foe in multiple sclerosis models?
- Brain and spinal cord trauma: what we know about the therapeutic potential of insulin growth factor 1 gene therapy
- Functions and mechanisms of cytosolic phospholipase A2 in central nervous system trauma
- Cre-recombinase systems for induction of neuronspecific knockout models: a guide for biomedical researchers
