Cre-recombinase systems for induction of neuronspecific knockout models: a guide for biomedical researchers
2022-06-29TetianaShcholokEftekharEftekharpour
Tetiana Shcholok, Eftekhar Eftekharpour
Abstract Gene deletion has been a valuable tool for unraveling the mysteries of molecular biology. Early approaches included gene trapping and gene targetting to disrupt or delete a gene randomly or at a specific location, respectively. Using these technologies in mouse embryos led to the generation of mouse knockout models and many scientific discoveries. The efficacy and specificity of these approaches have significantly increased with the advent of new technology such as clustered regularly interspaced short palindromic repeats for targetted gene deletion. However, several limitations including unwanted off-target gene deletion have hindered their widespread use in the field. Crerecombinase technology has provided additional capacity for cell-specific gene deletion. In this review, we provide a summary of currently available literature on the application of this system for targetted deletion of neuronal genes. This article has been constructed to provide some background information for the new trainees on the mechanism and to provide necessary information for the design, and application of the Cre-recombinase system through reviewing the most frequent promoters that are currently available for genetic manipulation of neurons. We additionally will provide a summary of the latest technological developments that can be used for targeting neurons. This may also serve as a general guide for the selection of appropriate models for biomedical research.
Key Words: central nervous system; cerebellum; Cre/LoxP system; Cre-recombinase transduction; gene deletion; gene delivery; hippocampus; in vivo genome editing; stereotaxic injection

Introduction 273 The Concept of Tissue-Specific and/or Time-Specific Cre-Mediated Recombination or Conditional Knock-Out 273 Cre Promoters for Pan-Neuronal Downregulation of Target Proteins 275 Cre Promoters That Selectively Target Major Neuronal Subtypes 275 Cre-Containing Strains That Originate from Different Founder Lines Display Differences in Cre/LoxP Recombination Patterns 276 Cre Promoters That Selectively Target Small Neuronal Populations 276 Brain Region-Specific Gene Downregulation 276 Cre-Recombinase Delivery Methods 277 Conclusions 277
Introduction
In vitro
andin vivo
gene knockout models are essential tools to study the gene function in physiology and pathophysiology. Owing to the complexity of neuronal networks in the brain, functional genomic research is a hot topic in neuroscience and therefore a neuron-specific gene knockout model would be an important tool for studying neuronal biology and development, as well as in disease conditions. Additionally, such models might be useful in the examination of glial-neuronal interaction in the aforementioned conditions. The contribution of these models has been best demonstrated toward the formation of our knowledge of neurodegenerative disorders. Several approaches have been developed for these purposes; however, Cre-recombinase technology is often considered the gold standard and the method of choice.The discovery of Cre-recombinase by late Nat L. Sternberg (Yarmolinsky and Hoess, 2015) has led to the generation of cell-specific knockout models in eukaryotes. Despite the ingenuity of this technique, the efficacy of different vector constructs for neuronal transfection and transcription remains a critical factor. In this article, we aim to review the available literature on Crerecombinase technology to help the investigators in designing their study and choosing the most suitable animal model for their research. We will also provide a summary of delivery routes for gene targeting for the central nervous system (CNS).only Cre-systems with exclusive expression in neurons were included (529 recombinase-containing alleles). We further narrowed the list to include the current commercially available mouse models and selected only one entry for each genotype when alternative strains were available. A total of 190 unique Cre-promoters with selective expression in the nervous system were further screened by excluding those Cre-promoters targeting neuroendocrine cells, retinal neuroepithelium, peripheral neural structures (e.g., peripheral nerves, plexuses, etc.), glial or those expressed only in small neuronal subpopulations. At this stage, we selected 37 Cre-promoters that satisfied these criteria. We further reviewed the most frequently used commercially available Cre lines that were reported in publications within the last five years (January 2016 – December 2021) and included the original literature characterizing these models. An important inclusion condition for the final list was the characterization of the model by the investigators and independently from the supplier’s available information to confirm the accuracy of descriptions provided by the suppliers. Therefore, this review provides an overview of the current commercially available mouse models that are most frequently used for the generation of neuron-specific gene manipulations in mice (Figure 1
).
Figure 1|Visual representation for the literature review strategy applied in the current work.
The Concept of Tissue-Specific and/or Time-Specific Cre-Mediated Recombination or Conditional Knock-Out
Literature Search Criteria
The literature search was performed on data permanently archived at the Mouse Genome Informatics Cre Portal (http://www.Creportal.org) in this narrative review. The Mouse Genome Informatics database included 3720 recombinase-containing alleles at the time of our initial search (Oct 2021). Entries were selected based on the predicted tissue-specificity, and therefore,
Genetic manipulations enable the examination of selected gene functions forin vitro
andin vivo
investigations. Although conventional embryonic gene knockout models are used widely in molecular biology, these models can be associated with developmental defects that affect the survival rate and interfere with the normal physiology in adult animals (Wu et al., 2019). Additionally, general non-specific effects of gene knockout complicate the task of distinguishing direct and indirect effects on specific tissues or cell types.Therefore, tissue-specific (conditional) gene knockouts appear as a relatively simple and powerful tool that can potentially avoid perinatal loss and at the same time allow spatial and temporal control of genetic manipulations. Despite the availability of other alternative approaches for generating knockout models such as homologous recombination, zinc-finger nucleases (Kim et al., 1996), transcription activation-like effector nucleases (Miller et al., 2011), and clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated (Cas) nucleases (Jinek et al., 2012), Cre/LoxP principle is the most common approach in biomedical sciences.
The Cre/LoxP technique requires two components: 1) Cells or animals harboring the LoxP sites, a specific 34-base pair bp sequence, which is the site of recombination, and two flanking 13-bp inverted repeats. 2) Crerecombinase activity (an enzyme that catalyzes recombination between two LoxP sites) (Nagy, 2000). The Cre-recombinase expression is regulated by a tissue/cell-type-specific gene promoter, a DNA sequence encoding protein that is selectively expressed in the cell/tissue of interest (e.g., neural, endocrine, etc.). It drives the Cre-recombinase expression strictly in the targeted tissue/cell type (Utomo et al., 1999). When expressed/activated, Cre protein catalyzes recombination between the two LoxP sequences, enabling deletion (Cox et al., 2012; Chen et al., 2019), inversion (Oberdoerffer, 2003), translocation (Smith et al., 2000), or insertion of the targeted DNA sequence (Michel et al., 2010;Figure 2
). Despite being a powerful and trusted tool of cell-specific DNA editing, Crerecombinase expression driven by a single gene-promoter is sometimes not sufficient to reach the desired recombination specificity (Luo et al., 2008). The split-Cre system is a novel Cre-recombination method for increased cell specificity. The novelty of this method is based on the expression of two fragments of Cre-recombinase protein that are regulated by two different promoters. In this case, Cre/LoxP recombination occurs only when the two fragments of Cre-protein fuse inside the cell expressing both of the promoter genes (Hirrlinger et al., 2009;Figure 3
).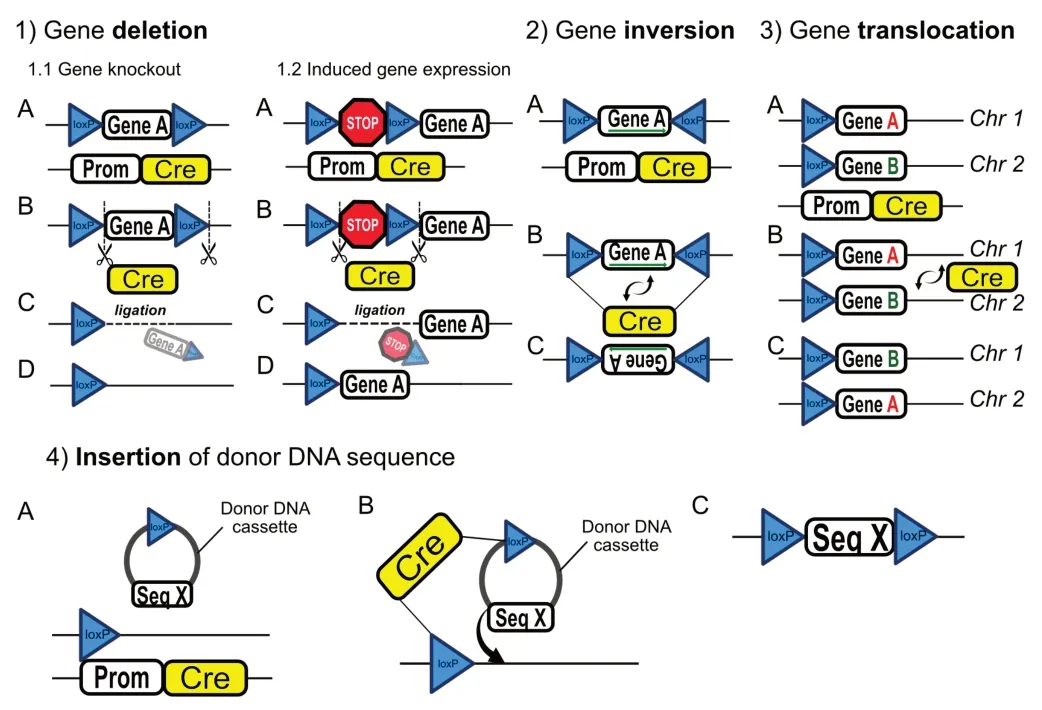
Figure 2|Different application mechanisms of the Cre-recombinase system.The Cre-recombinase technology enables genetic manipulation with high efficacy in a cell type or time-specific manner. Schematic diagrams describe different applications of this system. 1) Gene deletion may be applied either for gene knockout or induced gene expression purposes. When used for gene knockout, two LoxP sequences are placed with similar orientations on either side of a targeted DNA sequence (Gene A) (1.1 A). In this format, Cre-recombinase expression driven by a cell-type-specific genepromoter (Prom) (1.1 B) results in the removal of Gene A and one LoxP site, followed by DNA ligation (1.1 C and D). For induced gene expression, a stop codon is floxed with two identical LoxP sequences (1.2 A and B). Activation of Cre-recombinase under the control of a cell-specific promoter will result in the removal of the transcription stop codon, leading to induction of Gene A expression (1.2 C and D). 2) Gene inversion is achieved via floxing the targeted gene sequence (Gene A) with two LoxP sites that have an opposite orientation (2 A and B). Cre/LoxP recombination results in inversion of nucleotide sequence in Gene A (2 C). 3) Gene translocation can occur when two identically oriented LoxP sites are located on two separate chromosomes (3 A). Cre/LoxP recombination mediates translocation of the targeted DNA sequences (Gene A and Gene B) between two chromosomes (Chr 1 and Chr 2) (3 B, C). 4) Insertion of donor DNA sequence occurs upon introduction of donor DNA cassette that consists of LoxP sequence and the donor sequence (Seq X). The site for cassette insertion is marked by LoxP sequence located on the targeted chromosome (4 A). Finally, Cre/LoxP recombination results in the insertion of Seq X into the host DNA sequence (4 B and C).

Figure 3|Split-Cre recombinase allows more specific targeting of neuronal subpopulations.The Split-Cre system is designed to prevent leaky expression of Cre-recombinase and therefore enable more specific targeting of neuronal subpopulations. For this purpose, Cre protein is divided into two fragments (N-terminal and C-terminal respectively) associated with two different cell-type-specific promoters (Prom1 and Prom2) (3 A). Thus, only when both promoters are expressed by a cell, the two fragments of Cre-recombinase are synthesized, fused (3 B), and capable of performing Cre/LoxP recombination at floxed DNA sites (3 C and D).
In addition to cell specificity, Cre-recombinase can also be used for temporal control of the desired effect. This effect can be induced using CreER, a variant of Cre-recombinase that contains an estrogen receptor domain (Walrath et al., 2010). Once expressed in the cell, CreERremains in the cytoplasm with no biological function. The recombinase activity will be activated at the desired time point following the administration of Tamoxifen, a breast cancer treatment drug. Interaction of CreERwith 4-hydroxytamoxifen, the metabolite of Tamoxifen, reactivates the recombinase activity and induces a specific effect, e.g., gene expression (Kristianto et al., 2017;Figure 4
).
Figure 4|Temporal control of Cre/LoxP recombination. Inducible Cre/LoxP recombination can be achieved via the implementation of CreERT2, a variant of Cre-recombinase which is a fusion of a mutated ligand-binding domain of the estrogen receptor (ER) and Cre-recombinase. Upon expression, CreERT2 has no recombination activity and is transported from the nucleus to the cytoplasm where it remains shuttled until Tamoxifen injection. Once injected, Tamoxifen undergoes intracellular metabolic conversions (mainly performed by CYP2D6) that result in the generation of the active metabolite, 4-hydroxytamoxifen (4OH-Tamoxifen). The latter acts as a specific activator of ER and binds to CreERT2 shuttered in the cytoplasm. Upon this event, CreERT2 gains its activity and is transported back to the nucleus to perform Cre/LoxP recombination.
Forin vivo
investigations, the Cre/LoxP recombination is achieved by crossing two pre-designed mouse strains, one bearing the LoxP (floxed) sequences and the second one containing the Cre-recombinase system. The offspring mice harboring both the floxed alleles and Cre-recombinase will be subjected to Crerecombination and generation of the mutant of interest (mutant) (Utomo et al., 1999;Figure 5
). The efficacy of the system is often less than expected and does not always follow the pattern of gene-promoter expression, as an ectopic or suboptimal expression of Cre recombination has been reported (Kemshead et al., 1982). Additionally, different promoters have varying degrees of efficacy, and therefore rigorous characterization of the available Cre strains in terms of their tissue/cell-type specificity, effectiveness, or timeline must be considered for research. The International Knockout Mouse Consortium and Mouse Genome Informatics Cre Portal are valuable open resources that are the result of collaborative approaches aimed at gathering this information. These online databases include available up-to-date information on existing Cre strains from across the world, although this resource has not been completed yet. Examination of available literature indicates that selecting a Cre promoter forin vivo
study of gene function remains a major challenge for investigators. A review of available promoters that induce pan-neuronal or specific groups/types of neurons is provided here.
Figure 5|Breeding strategy for generation of Cre/LoxP mediated neuron-specific gene knockout. Generation of neuron-specific gene knockout colony begins with crossing a Cre-negative male with a double-floxed targeted DNA sequence (Gene A) and a Cre-positive nonfloxed female (A). The offspring (Generation F1) will include Cre-positive single-floxed female. In this animal, Cre-recombinase driven by neuron-specific gene-promoter (Prom) will delete Gene A sequence from one chromosome, while it will remain intact on the homologous chromosome. This female is then backcrossed with a Cre-negative doublefloxed male (B). Generation F2 will then include double-floxed Cre-positive animals that are considered to be complete Gene A knockout (C). In these mice, cell-type-specific gene-promoter (Prom) will mediate Cre/LoxP recombination selectively in neurons. While in other cell types (e.g., astrocytes) expression of Gene A will not be interrupted. Using Cre-positive males for breeding purposes is not recommended because of the risk of Cre recombination in germ cells that impacts cell-specificity of gene knockout in offspring.
Cre Promoters for Pan-Neuronal Downregulation of Target Proteins
Synapsin 1-Cre
Synapsin 1 (Syn-1) is one of the most extensively used neuron-specific transcriptional promoters for generating pan-neuronal knockouts. Syn-1 is associated with the cytoplasmic surface of synaptic vesicles in both peripheral and CNS (1983). Successful Syn-1 mediated Cre recombination is predicted to be limited to mature differentiated neurons but spares non-neuronal cells and pre-mature neurons. The neuronal specificity of Syn-1 has been confirmed using immunohistochemistry and western blotting (Petr et al., 2015), however, evidence of its expression variability in different regions of CNS has been documented. This includes a mosaic recombination pattern of Syn-1-Cre recombination, which questions its reliability as a pan-neuronal knockout tool. Denroche et al. (2016) using quantitative PCR and immunohistology, identified robust efficacy of Syn-1 mediated Cre recombination in the brainstem, cortex, hypothalamus, and spinal cord, while the expression level of target protein was not significant in the hippocampus and olfactory bulb. In another study, Trosclair et al. (2020) compared the efficacy of Syn-1-Cre mediated knockout between the dentate gyrus of the hippocampus with cerebellum. Using both western blotting and immunohistology, they identified significantly higher residual expression of the targeted protein (low efficacy) in the cerebellum compared to the granular layer of DG. A more detailed report on hippocampal expression was provided by Zhang et al. (2015). They first confirmed the neuron-specificity of Syn-1-Cre expression by co-staining with neuronal marker NeuN. According to this report, the Cre-recombinase approach effectively knocked out the target protein in NeuN positive DG granular neurons at postnatal day 7 or in DG and CA3 regions of the 14, and 21-day old mice. Interestingly, the authors showed that Cre-recombination in hippocampal CA1 neurons was not effective as a comparable expression of target protein was detected between the control and experimental samples. These results were additionally confirmed in another study by performing western blotting after microdissection of different hippocampal regions (He et al., 2004). CA1 tissue lysate showed no change in the expression of target protein after Cre-recombination as compared with CA3 and dentate gyrus. The differential efficacy of Cre-recombinase activity in different regions of the hippocampus is specifically important when examining the target protein involvement in neurodegenerative diseases. Unpublished data from our group show little to no change in target protein in the CA1 region.
Myosin light chain-Cre (CRE3
)Myosin light chain (Mylpf) protein is a subunit of myosin with structural and functional properties different from the myosin heavy chain protein that is widely expressed in muscle tissue (Schiaffino et al., 2015). Mylpf belongs to a large and diverse family of Cabinding proteins and is primarily studied in the cardiovascular system (Szczesna-Cordary and de Tombe, 2016). While attempting to use a Cre-recombinase system for deletion of Mylpf II in cardiac cells, Banares et al. (2005) discovered that when driven by Mylpf II promoter, Cre-recombination showed very little or no successful gene deletion in cardiac cells, as confirmed by crossing the Cre-recombinase mouse with a lacZ indicator line (ROSA26). They demonstrated robust Cre activity in a neuron-specific manner throughout the brain gray matter, cerebellum, spinal cord, retina, dorsal, and sympathetic ganglia. The Mylpf-Cre mediated activity was detected as early as E11.5 gestation day and was restricted to neurons (Banares et al., 2005). In an attempt to validate the tissue-specificity of Mylpf-Cre recombination, Krajewska et al. (2011) compared the genomic DNA from different tissues and organs. Recombination was shown to take place in different areas of the brain (brain stem, thalamus, basal ganglia, cerebellum, and cortex) but not in the liver, kidneys, or heart (Krajewska et al., 2011). There is currently no evidence of non-neuronal expression of Mylpf-Cre recombination, suggesting the suitability of this system for pan-neuronal protein downregulation. However, it is recommended to test the efficacy of protein downregulation by immunohistochemistry and western blotting on micro-dissected tissues of interest.
Actin-like protein 6B-Cre
Actin-like protein 6B (Actl6b) also known as BAF53b is another promoter used for pan-neuronal gene deletion. Actl6b is a subunit of a neuron-specific chromatin remodeling BAF (BRG1/brm-associated factor) (Zhan et al., 2015). This gene is involved in gene expression regulation by structural modification of chromatin, specifically in the brain. BAF53b-mediated Cre expression is reported to start at embryonic day 13.5 both in CNS and peripheral nervous system (dorsal root ganglion, sympathetic ganglia, and nerve tracts) in all neuronal subtypes, regardless of their maturation level (Wu et al., 2007). The BAF53b-mediated Cre expression persists in hippocampal neuronal cultures from E16.5 mice as confirmed using a reporter assay (Zhan et al., 2015). This indicates the potential usefulness of this system for primary neuronal cultures, however, due to high lethality at birth caused by respiration deficiency, these mice cannot be used for studying the neurobiology of adult animals and therefore is a significant limitation forin vivo
studies.Cre Promoters That Selectively Target Major Neuronal Subtypes
Calcium/calmodulin-dependent protein kinase type II subunit alpha-Cre
Calcium/calmodulin-dependent protein kinase type II subunit alpha (CAMKIIα) is a member of the serine/threonine-specific protein kinase family and has a significant role in synaptic plasticity, specifically at glutamatergic synapses (Kool et al., 2019). Using CAMKIIα is a common strategy for the generation of neuron-specific knockouts in the neocortex and hippocampus. Early reports describing the use of CAMKIIα as a promoter for Cre recombinase, showed its exclusive activity in the forebrain neurons (Mayford et al., 1996). The application of this approach was associated with an agedependent increase in target knockout efficacy (Liu et al., 2010). Examining the cerebral cortex at 3, 6, 9, 12, 15, 18, 21, and 24 months by western blotting, authors reported a significant downregulation of the target protein as early as 6 months of age and reaching its lowest level at 12 months, after which no significant changes were detected for up to the age of 24 months. The target protein decrease was evident in the cortex, hippocampus, striatum, hypothalamus, thalamus, or amygdala, but not in the cerebellum or pituitary gland (Liu et al., 2010). While this study indicated the need for detailed characterization of both temporal and spatial distribution of the Cre-recombinase activity in animal models, it also showed the regional variation for the Cre-mediated gene deletion in these mice (Liu et al., 2010). Low expression of Cre-recombinase activity in the cerebellum in the CAMKIIa model was independently confirmed by another group (Kunze et al., 2012). Kunze et al. (2012) showed that despite relatively faint expression of CAMKIIα-Cre recombinase in the cerebellum, the Purkinje cells stained positive for Cre. Considering the contribution of Purkinje cells to fine-tuning sensory-motor movements (Medina, 2011), this study raises the possibility of an unwanted gene-knockout effect in the Purkinje cells, which may affect animals’ ability to perform a motor task. To further characterize this model, Lu et al. (2019) investigated CAMKIIα-Cre expression in a subregion-specific manner. They reported that 65–70% of neurons in the cortex and CA1 of the hippocampus responded to CAMKIIα-Cre mediated recombination but the inhibitory neurons that do not express CAMKIIα, maintained normal levels of the target protein in this model.
Empty spiracles homeobox 1-Cre
Empty spiracles homeobox 1 (Emx1) protein is an abundantly expressed transcription factor in developing telencephalon (Cecchi and Boncinelli, 2000). It is known to play a role in the proliferation of neural stem cells, differentiation of layer-specific neuronal phenotypes, and glial/neuronal fate specification (Schuurmans and Guillemot, 2002). Emx1 is constitutively expressed in all subgroups of cortical neurons during proliferation, migration, differentiation, and maturation stages. Interestingly, despite this fact, the level of Emx1 mRNA expression is a subject of significant variation between different neuronal subtypes (Schuurmans and Guillemot, 2002). Emx1 expression is a dynamic process that involves various brain regions (Cecchi and Boncinelli, 2000). Examination of its mRNA levels indicates an early appearance at E9.5-E11.5 followed by a subsequent increase in the ventricular zone for up to E17.5 days (Cecchi and Boncinelli, 2000). This increase continues during the early postnatal life in layers V and VI of the cerebral cortex (Cecchi and Boncinelli, 2000). The Emx1 mediated Crerecombination is reported to be specifically effective when targeting the hippocampal dentate gyrus. The group reported significant downregulation of the targeted floxed gene in mice hippocampal tissue (P4) as illustrated by q-RT-PCR and western blotting (Xu et al., 2019).
Gorski et al. (2002) have also examined the expression of Emx1-Cre expression within various neuronal and non-neuronal cell populations. In adult mice, abundant recombination was observed in the piriform cortex, medial limbic allocortex, and neocortex and was also persistent in hippocampal subregions. However, only a minor population of cells in ventral pallial structures underwent recombination as shown by Beta-Gal staining. Emx-1-Cre was expressed in white matter structures including the corpus callosum, fimbria of the hippocampus, and layer I of the neocortex, indicating its potential expression in non-neuronal cells, including possible expression in oligodendrocytes. Cultures of astrocytes isolated from the P1 neocortex also showed evidence of successful recombination (Gorski et al., 2002). These results showed that Emx-1 mediated Cre recombination is not limited to neuronal lineages and can also occur in the brain cells of non-neuronal fate.
Another study performed by Kummer et al. (2012) highlighted another feature of the Emx-1-Cre models. Even though the vast majority of neurons in the cortical region are glutamatergic, 10–20% of cortical neurons are GABAergic. Therefore, this group focused on studying the efficacy of Emx-1-Cre mediated gene recombination in GABAergic cortical neurons. The inducible expression of a red fluorescent protein (tdTomato) under the control of Emx1-Cre-recombinase was examined in NeuN-positive neurons of the upper layers of the visual neocortex. While only 12.4% of NeuN-positive cells did not express tdTomato, more than 92% of the tdTomato-negative cells were found to be GAD67-positive, indicating that most of these neurons were GABAergic (Kummer et al., 2012). These data indicate the differential efficacy of Emx-1 mediated regulation of Cre-recombinase activity in GABAergic and glutamatergic neurons. This highlights the importance of selecting appropriate promoters in specific neuronal subtypes for biomedical research. A split-Cre system would be very appropriate to ensure the proper labeling of GABA-ergic neurons.
Thymocyte differentiation antigen 1-Cre
Thymocyte differentiation antigen 1 (Thy1 or CD90) protein was originally discovered as a thymus-specific protein and is the first identified T-cell marker (Reif and Allen, 1964). This protein is also found in several other cell types, e.g., myoblasts, epidermal cells, and keratinocytes. Importantly, Thy1 is also expressed in the brain tissue at high levels, although it is not limited to neuronal cell linages (Kemshead et al., 1982) and some glial cells are shown to express it, especially at the late stages of their differentiation (Kemshead et al., 1982). In terms of brain subregion specificity, Thy1 protein is expressed at the highest concentrations in the hippocampus and striatum, followed by the neocortex, spinal cord, cerebellum, retina, and optic nerve. The wide distribution of Thy1 protein in the brain makes it a good candidate for widespread expression of a protein of interest, as shown for the brain-specific amyloid precursor protein in a transgenic animal model of Alzheimer’s disease (Moechars et al., 1999). Expression of Thy-1 mediated Cre recombination in mice does not interfere with normal Thy-1 expression, therefore it can be used for examination of cortical and dorsal root ganglion neurons, and in neural cells of the retinal ganglionic cell layer in the retina as well as in cerebellar Purkinje cells (Campsall et al., 2002).
The effectiveness of Thy1-Cre recombinase for significant downregulation of target protein has been assessed by RT-PCR and western blotting (Price et al., 2013). The immunohistochemical examination also confirmed the successful downregulation of its target protein in NeuN positive primary motor cortex neurons, while oligodendrocytes in the corpus callosum, identified with adenomatous polyposis coli did not show any distinguishable difference when compared with control animals. Thy1-Cre expression was ~40% effective in the optic nerve and retina, indicating that despite predicted neuronal specificity, Thy1-Cre recombination might not reach the appropriate level of efficacy for neurons in the retina and optic nerve (Price et al., 2013). The safety and feasibility of using the Thy1-Cre system can be overshadowed by the gene of interest, as the non-neuronal expression of this system has been shown in the myocardium, vascular endothelium, lung alveoli/bronchiole cells, renal tubules, skeletal muscle, skin, hair follicles, testis, and bladder (Heffner et al., 2012). Therefore, depending on the importance of target proteins in the non-neuronal tissue, the Thy1-Cre system might cause lifethreatening abnormalities in mutant animals and may not directly reflect the importance of the targeted protein in the brain.
Calbindin 2-Cre
Calbindin 2 (Calb2) also known as calretinin is a member of the calciumbinding proteins family (1987) and is involved in various cellular functions. Cab2 is abundantly expressed in CNS where it plays important role in the modulation of neuronal excitability, including induction of long-term potentiation. Cortical interneurons and retinal neuroepithelium are major sources of Calb2 protein (Barinka and Druga, 2010). Calb2-Cre mediated gene knockout has been used for manipulating GABAergic neuronal populations in the cortex, allowing examination of its target protein in the inhibitory neurons (Taniguchi et al., 2011). Overall, 6.2% of neurons were positive for Calb2-Cre, of which 90.8% were located in the cortex. Using green fluorescent protein expression as a marker of Cre-recombinase in these mice showed that 71.3% of Calb2-positive neurons were also green fluorescent protein-positive (Taniguchi et al., 2011). Calb2-Cre recombinase efficiency was correlated with the endogenous Calb2 mRNA level, an indication of the suitability of this model to be used as a physiologically relevant approach in neuron-specific deletion studies (Kӧnig et al., 2020).
Glutamate decarboxylase 2
Glutamate decarboxylase 2 (GAD2) is a neuron-specific isoform of glutamate decarboxylase that catalyzes the production of γ-aminobutyric acid (GABA) and COfrom glutamate decarboxylation (Langendorf et al., 2013). GAD2 activity is the main regulator of GABA synthesis in the CNS (Langendorf et al., 2013). Employing GAD2 to drive Cre-recombinase activity resulted in 15% targeting efficiency in neurons, ~92.2% specificity in GAD67, and ~91% efficiency in cortical inhibitory neurons (Taniguchi et al., 2011).
Examination of micro-dissected brain regions from GAD2-Cre mutants and their control littermates by western blotting indicated a significant decrease of the targeted protein across various brain subregions (Kang and Shen, 2020) including olfactory bulb neocortex, hippocampus, striatum, thalamus, and midbrain, and spinal cord. These data suggested a considerable variation in GAD-Cre mediated knockout efficacy across the brain that correlated with the varying regional abundance of GABAergic neurons. The excitatory neurons and glial cells are not affected by the GAD2-Cre system, and therefore western blotting is not a reliable tool for efficacy assessment (Kang and Shen, 2020). Another group examined GAD2-Cre expression using immunofluorescence microscopy (Quina et al., 2020). They have identified that GAD2-expressing neurons are mostly present in rostral LHb (Lateral habenula), and central LHb nuclei. Additionally, they were often observed in clusters in the ventromedial part of the nucleus (Quina et al., 2020).
Cre-Containing Strains That Originate from Different Founder Lines Display Differences in Cre/LoxP Recombination Patterns
The importance of the founder line strain on the efficacy of Cre-recombinase promoter adds another variable factor that can affect the outcome. Numerous independent observations demonstrate either minor or major differences in efficacy and specificity of Cre/LoxP recombination in different Cre strains driven by the same promoter (Madisen et al., 2010; Taniguchi et al., 2011). Even minor technical variables in the primary genetic constructs such as the locus of Cre sequence incorporation into the promoter gene or sequence length can impact the pattern of Cre expression in future Cre founder lines (Yuan et al., 2011). We have listed some of the reported differences amongst the commercially available strains for some of the above-mentioned Cre promoters inAdditional Table 1
.Cre Promoters That Selectively Target Small Neuronal Populations
The use of specific promoters to examine the involvement of any given gene in neurodegenerative diseases has been a major tool in understanding the pathophysiology of these diseases including Parkinson’s disease which is caused by loss of substantial nigral dopaminergic neurons, or Purkinje cells in cerebellar ataxia.Additional Table 2
describes features of selected Cre promoters that target these specific populations of neurons.Brain Region-Specific Gene Downregulation
Although the Cre-recombination method enables gene knockout in specific groups of neurons, in some studies gene knockdown must be directed to specific brain regions. The animals must express the loxP-flanked gene of interest, and the Cre-recombination will be induced using stereotaxic microinjection of replication-deficient adeno-associated and lentiviral vectors in the cortex, hippocampus, or specific basal ganglia (Ahmed et al., 2004). There are some advantages of this approach over the conventional neuronspecific gene knockout including: 1) precise targeting of the gene of interest in a specific brain region, 2) avoiding the potential compensatory changes by other genes after downregulation of the target proteins during embryonic development, and 3) to study the potential differential effect of gene knockout at a different age, e.g., in youngvs.
old animals. This will specifically be useful in neurodegenerative diseases where aging-associated changes in some proteins have been linked to the induction of neurodegeneration.Table 1
lists some of the viral constructs that have been used for these studies.
Table 1 |Common viral constructs that have been used for gene delivery into central nervous system
Cre-Recombinase Delivery Methods
The currently available Cre/LoxP system requires the presence of LoxP-flanked DNA inserts in the cells or animals followed by induction of Cre-recombinase activity. As discussed above, the Cre-recombinase can be induced through breeding schemes and can take a few months for obtaining the optimal genotype. Using exogenous Cre-recombinase protein in a LoxP-flanked animal can significantly save time, however, a reliable delivery route and vehicle must be adopted.
Adeno-associated viral (AAV) constructs have been vastly proven to be effective tools for delivering genes of interest and are shown to have low immunogenicity; however, the presence of blood-brain barrier still poses an important obstacle limiting the efficacy of this system for CNS delivery. As discussed above intrathecal injection of a viral construct is an efficient way to bypass the blood-brain barrier and target the diseases known to affect specific regions in the brain. For neurodegenerative diseases that affect the CNS globally, AAV constructs may also be delivered by direct infusion into the cisterna. This method has been shown to cause widespread neuronal and glial transduction when using AAV9 vectors, although the age of receiving animals may affect the gene transduction in neurons and glial cells differentially (Bucher et al., 2014). Another issue with virus-mediated gene delivery of Crerecombinase recombinase is the continuous expression of Cre-recombinase in the cell/tissue after gene delivery which may cause its toxic accumulation and may be an important factor when designing clinical application of these approaches. New developments in the field of biomaterials have enabled direct protein delivery for the transduction of Cre-recombinase activity. Using reducible lipid nanoparticles, successful delivery of Cre-recombinase has been shown forin vitro
andin vivo
delivery in mice brains (Wang et al., 2016). In this method, lipid nanoparticles were directly injected into the mouse brain containing a LoxP-flanked STOP cassette. These particles are taken into the cells using pinocytotic vesicles. Once inside the cell, a specific surface electric charge facilitates their release from the endosomes and into the cytoplasm where they gain their recombinase activity after reduction by a cellular reducing environment. Using this approach, strong Cre-mediated gene recombination was detectable after 6 days post-injection (Wang et al., 2016). More recently a novel class of carrier particles has been derived from the brain’s neurotransmitters (Ma et al., 2020). These neurotransmitterderived lipidoids can be injected intravenously and cross the blood-brain barrier and have been shown to induce Cre-recombinase activity in neurons. This innovation negates the need for direct injections into the brain. Despite the popularity and clinical relevance of intravenous delivery of gene vectors, this method requires a large dose of the vector and also will cause systemic distribution of the vector. Intraarterial delivery of vectors might be a suitable alternative approach that can increase localized gene delivery into the brain. In this approach, the integrity of blood-brain barrier must be compromised transiently by a local injection of a hyperosmolar mannitol solution before the application of vectors. This method has been performed successfully in mice (Foley et al., 2014). This method requires a significant degree of expertise in rodent microsurgery. Other current innovations in the field of Crerecombination technology include using synthetic Cre-recombinase mRNA for intranuclear induction of recombinase activity (Leontovyc et al., 2017) and using TAT-mediated protein delivery of Cre-recombinase that can be activated using near-infrared light therapy (Morales et al., 2018). These applications have been successfully performed only in cell culture.Conclusions
Selecting a suitable animal model is the first step in completing a successful research project. Although Cre-recombinase is currently the gold standard method for generation of cell-specific knockout or knock-in mouse models, selection of the specific factors that drive gene targeting in the intended neuronal subtypes is of utmost importance, as not all the transcription factors deriving the Cre-recombination have similar efficacy and efficiency in different neurons. This is further complicated by the apparent effect of mice strain on the outcome even when using the same gene promoter. The use of alternative approaches for induction of Cre-recombinase activity can be considered to decrease the time for establishment of the required phenotypes through breeding of transgenic animals. The need for a detailed examination of experimental outcome is an important step and must be confirmed independently of the suppliers’ report. The screening approaches to assess the knockdown efficacy are another issue and must include both immunohistochemistry and western blotting. It is recommended that detailed microdissection of the brain regions is used for western blotting as mosaic efficacy may lead to differential regional efficacy as summarized for each transcription factor. The advent of new RNA sequencing and single-cell/nuclei sequencing are other approaches that might be used for confirmation of the intended outcome. While using fluorescent protein probes for confirmation of Cre-recombinase might be a great control experiment, it is not clear whether one transcription factor may have similar transduction efficacy for different genes/proteins. Considering these aspects might help to specifically improve the utility of knockout technology in neurons.
Author contributions:
TS performed the literature search, drafted the manuscript, drew the illustrations, and provided the tables. EE wrote additional materials, edited, and reviewed the final version. Both authors approved the final version of the manuscript.
Conflicts of interest:
The authors declare no conflicts of interest.
Availability of data and materials:
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Open access statement:
This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:
Danilo B Medinas, Biomedical Neuroscience Institute, Chile.
Additional files:
Open peer review report 1.
Commercially available promoters and their reported differential expression patterns.
Cre promoters that selectively target small population of neurons.
杂志排行
中国神经再生研究(英文版)的其它文章
- c-Abl kinase at the crossroads of healthy synaptic remodeling and synaptic dysfunction in neurodegenerative diseases
- The mechanism and relevant mediators associated with neuronal apoptosis and potential therapeutic targets in subarachnoid hemorrhage
- Microglia depletion as a therapeutic strategy: friend or foe in multiple sclerosis models?
- Brain and spinal cord trauma: what we know about the therapeutic potential of insulin growth factor 1 gene therapy
- Functions and mechanisms of cytosolic phospholipase A2 in central nervous system trauma
- Prenatal programing of motivated behaviors: can innate immunity prime behavior?
