Difference Analysis of Liver Metabolic Response Between Diploid and Triploid Rainbow Trout Oncorhynchus mykiss Under Fishing Stress
2022-06-25HanYueLvXiaonanChenSimiaoZhouQunQinMeichuanandHanYing
Han Yue, Lv Xiao-nan, Chen Si-miao, Zhou Qun, Qin Mei-chuan, and Han Ying*
1 College of Animal Sciences and Technology, Northeast Agricultural University, Harbin 150030, China
2 Beijing Fisheries Technology Promotion Station, Beijing 100000, China
Abstract: To elucidate the effects of fishing stress on the liver glucose metabolism of both diploid and triploid female rainbow trouts as well as differences in their stress response, blood and liver tissues were collected from 0 h to 24 h after the fishing stress.Blood indexes, such as the levels of white blood cells and red blood cells, cortisol, glucose and lactic acid, as well as activities of key enzymes of the glucose metabolism in the liver, were measured. Furthermore, the mRNA expressions of glucocorticoid receptors and enzymes related to energy metabolism were assessed. The results showed that fishing stress exerted significant effects on blood physiological and biochemical indexes and liver glucose metabolism. Differences were found between diploid and triploid female rainbow trouts in the liver glucose metabolism pathway, as well as the level and performance during stress. The basic glucose metabolism intensity of the triploid liver was higher than that of the diploid liver; however, the hepatic glycogen reserve was lower in the triploid liver. After fishing stress, triploid trouts entered the immunosuppressive state earlier than diploid trouts. Triploid trouts also showed an earlier and stronger stress reaction than diploid trouts, while their energy allocation ability and immune recovery ability were weaker than those of diploid trouts. These results showed that the regulatory ability of diploid female rainbow trouts in response to fishing stress was better than that of triploid trouts.
Key words: rainbow trout, polyploid, handing, energy metabolism
Introduction
Exogenous stress factors or stressors impose important effects on the physiological balance of fish. In intensively farmed conditions, fishes are often subject to many "unnatural" stressors related to man-made operations, including overcrowding, fishing and transportation. It has been shown that operation and restraint stress can decrease the level of plasma growth hormone (GH) in rainbow trouts, which is concomitant with activation of the hypothalamic-pituitary-renal axis(HPI) (Pickeringet al., 1991). The continuous increase of the plasma cortisol content, the decrease of disease resistance, the decrease of reproductive performance,and the slow growth and development of fishes under stress are closely related (Petitjeanet al., 2020). In response to the increasing energy requirements of the stress response, fish metabolism changes in specific tissues, especially in the liver (Houet al., 2019;Meileret al., 2020). The first stage of the metabolic reorganization in the liver is associated with increased levels of catecholamine (CA), while the second stage is regulated by cortisol (López-Patiñoet al., 2014).Changes in blood cells under stress can enhance the defence function of the body, and it can directly reflect the changes in the physiological functions. With the enhancement of the energy metabolism in response to stress, it will result a hyperglycaemia symptom, which is characterized by a significant increase of glucose and lactic acid in the blood (Qianget al., 2015).Increased energy demand will lead to an increase in the oxygen consumption metabolism, while insufficient oxygen intake aggravates lactic acid deposition in the blood (Jiang, 2017). In extreme environments, changes of gene expression in the liver are more severe than in other tissues (Shiet al., 2019). Consequently, the changes of mRNA expressions of key enzymes in the energy metabolism in the liver reflect the ability of fishes to resist external stimuli.
The rainbow troutOncorhynchus mykissis widely recommended worldwide as a high quality cold-water farmed fish and a common model animal in fishes studies (Liet al., 2017). Triploid fishes are sterile,they can allocate resources which are used by diploid fish for reproduction into growth. Therefore, they have more growth advantages than diploid trouts, and have thus increasingly become the main cultured form (Maet al., 2019). The stress resistance of triploid rainbow trouts is less resilient, with more mortality in triploid than diploid trouts under poor feeding conditions(Oppedalet al., 2003; Shrimptonet al., 2012; Verhilleet al., 2013; Fraseret al., 2015). Stress researches on the rainbow trouts mainly focus on changes of blood physiology and biochemistry (Wisemanet al., 2007).It is found that under stressful conditions, diploid and triploid rainbow trouts have significantly higher haematocrit, cortisol and blood glucose concentrations,while lymphocyte numbers are reduced. Stress can cause a dynamic imbalance in the neuroendocrine immune system and energy balance of the rainbow trouts (Houet al., 2019; Benfey and Biron, 2000). Few studies investigate fishing stress on rainbow trouts.The dynamics of the glucose metabolism of diploid rainbow trouts after fishing stress have been studied,but no such studies have been published on triploid female rainbow trouts (López-Patiñoet al., 2014).
Understanding the impacts of stressors on farmed fishes in aquaculture are very important to increase aquaculture production and improve animal welfare.In this study, technical methods, such as the double antibody sandwich method, colorimetry and RT-PCR,were used to assess changes in both physiological and biochemical indicators. Examples were white blood cells(WBC), cortisol (COR), glucose (Glu) and glucose 6-phosphatase (G6Pase), glucokinase (GK), and other liver metabolic enzymes, as well as mRNA expression changes. Fishing stress was analyzed in diploid and triploid female rainbow trouts to explore the difference in tolerance of both to fishing stress, and to provide a theoretical basis for their healthy breeding.
Materials and Methods
Animals and ploidy detection
Diploid and triploid female rainbow trouts weighing(200±10) g had the same genetic background were hatched from Harbin Academy of Agricultural Sciences(China), and shipped to animal lab at Technology Promotion Station (Harbin, China). Healthy and uninjured diploid and triploid female rainbow trout individuals (N=144 each) adapted to the lab environment in 200 L tanks for 14 days (n=9 tank-1). Running water was fully circulated during the feeding period, the water temperature was kept at 15±0.5℃ under a 12:12 LD photo cycle (the light was switched on at 6:00 a.m.).Fish feeding at 8:00 a.m. and 4:00 p.m. daily, after which, the remaining bait was removed. Thirty samples were randomly selected from the triploid population,and the relative DNA content was determined by flow cytometry to detect the chromosome ploidy of samples(Thorgaardet al., 1982), using diploid as the control.
Experimental design
All the fishes were stopped feeding on the day of experiment. The experiment was implemented under filtered and continuously renovated fresh water,oxygen was provided by aerator. The female rainbow trouts were subjected to a control group (resting state) and seven treatments. To prevent the secondary disturbances caused by sampling, tanks were separated according to different sampling time (2, 10, 30,60, 120, 240 and 1 440 min), and treatments were placed in the corresponding tanks after the stress.The experimental fishes in the treatment groups were chased by the fishing nets for 2 min and removed from the water for 1 min, then returned to the corresponding tank. Added MS-222 (250 mg • L-1) to each tank when the sampling time reached. Removed experimental fishes after deeply anesthetized, and collected samples(N=9). The control group was not stimulated and remained in a resting state all the time.
Sampling and determination
After anesthesia, the length and weight of the female trouts were measured, and the blood was collected in the tail intravenous. Each blood sample was divided into two parts, one was placed in an ethylenediaminetetraacetic acid tubes (EDTA-K2,K2E, 3.6 mg) for routine blood determination, and the other was placed in the Eppendorf tube sized 2.0 mL for serum extraction. Blood samples in Eppendorf tubes sized 2.0 mL were centrifuged at 3500 r • min-1for 15 min at 4℃, then the serum was isolated and kept at -80℃. Liver tissues were collected in a sterile environment and divided into two parts. One in sterile RNA-free 2.0 mL cryotubes, kept frozen in the liquid nitrogen and stored at -80℃, and the other for liver glycogen detection.
The contents of white blood cells, red blood cells and hemoglobin in blood samples were determined by fully automated blood cell analyser (NIHON KOHDEN, Tokyo, Japan). The blood serum samples used for determining the concentration of glucose(Glu) and cortisol (COR) in blood by commercially available enzyme immunoassay kits (LMAI, Shanghai,China; NJJC, Nanjing, China). Lactic acid (LD),the activity of hexokinase (HK) and pyruvate kinase(PK) were determined with colorimetric method by commercial kits (NJJC, Nanjing, China). The content of glycogen in the liver was measured by fresh liver samples by commercial kit (NJJC, Nanjing, China).
RT-PCR (qPCR)
The total RNA was obtained from each liver with the BioFast®Simply P®total RNA analysis kit (BIOER,Hangzhou, China). A complete of 2 μg RNA was back-transcribed to cDNA in each sample using the BioRT®Master HiSensi®cDNA first strand synthesis kit (BIOER, Hangzhou, China). Both total RNA and cDNA were tested for nucleic acid concentration by a NanoPhotometer N60 Touch (Implen, Germany)ultraviolet-visible spectrophotometer to ensure that no other genomic contamination was present in total RNA and cDNA. The RT-PCR was conducted using a BioEasy®Master Mix (SYBR Green, High ROX) RT-PCR kit (BIOER, Hangzhou, China) by LightCycler®480 system RT-PCR apparatus (Roche,Basel, Switzerland). The primers were designed,according to López-Patiñoet al. (2014), including G6Pase, PEPCK, GK, GR1, GLUT2 andβ-actin(Table 1).

Table 1 Primers for RT-PCR determination of β-actin, GK, G6Pase, GR1, GLUT2 and PEPCK expression
The samples were treated in equal numbers and tested in triplicate on the same plate. The real-time PCR reaction program was set up by the two-step method: 95℃ for 1 min; 95℃ for 15 s, 60℃ for 1 min,for a total of 35 cycles. The melting curve program was set as the followings: 95℃ for 1 min; 65℃ for 1 min; 95℃ for 20 s; 30℃ for 1 min. Theβ-actin was used as an internal reference gene, and its expression was standardized. The relative transcription level of the target gene was calculated by the 2-△△Ctmethod.
Data statistics
All the data were statistically analyzed using SPSS software (22.0). The main factors were stress reaction time (2, 10, 30, 60, 120, 240 and 1 440 min) and chromosome ploidy (diploid and triploid). Multivariate analysis of variance was used to assess the differences.The statistical values were presented as mean±standard deviation (mean±SD).
Ethical statement
The care and use of laboratory animals were in accordance with the animal welfare laws, standards and guidelines of the Chinese Society for Laboratory Animal Science as approved by the Ethics Committee of the Northeast Agricultural University. All the fishes were kept in an environment with sufficient oxygen,fresh water and adequate food. All the fishes used for tissue collection were completely anaesthetised with MS-222 and strictly followed the rules for dosing.After experiment, all the fishes that were not experimentally released back into the culture water.
Results
Comparison of fishes of different ploidy statuses in resting state
At rest (when fishes were not subjected to fishing pressure), no significant difference was found in the number of the WBC and HGB between diploid and triploid trouts. The number of the RBC in diploid trouts was significantly higher than that of triploid trouts (P<0.01) (Fig. 1). The contents of the COR,Glu and LD in the serum of diploid trouts were higher than those of triploid trouts, and there was no significant difference in the COR, Glu and LD concentrations (Fig. 2). The liver glycogen content of triploid female rainbow trouts was only 68% of that of diploid at resting state (Fig. 3A). The content of hepatic glycogen and the HK activity in triploid trouts were significantly lower than those of diploid trouts (P<0.05) (Fig. 3B), while the PK activity was not significantly different between diploid and triploid trouts (Fig. 3C).
Comparison of different ploidies after fishing stress
After fishing stress, the number of the WBC in diploid and triploid trouts blood increased first and then decreased. The WBC number of diploid trouts peaked before triploid trouts after fishing stress, and the WBC number was significantly lower than that of triploid trouts (P<0.05) (Fig. 1).
The number of the RBCs in diploid trouts blood fluctuated after fishing stress, and was significantly higher than that of the control group at 10 and 120 min (P<0.05). The number of the RBC in triploid trouts increased first and then decreased after stress,and was significantly higher than that of the control group (P<0.05). There was significant difference in the number of the RBCs between diploid and triploid trouts at 2, 10, 120 and 1 440 min (P<0.05). The HGB contents in blood of diploid and triploid trouts both increased and then decreased after fishing stress, and were higher than those of the resting group (P<0.05).The HGB contents in blood of triploid trouts were higher than those of diploid trouts at 240 min (Fig. 1).

Fig. 1 Time-course of WBC (A), RBC (B) and HGB (C) in diploid and triploid rainbow trouts after fishing stress

Fig. 2 Time-course of COR (A), Glu (B) and LD (C) in diploid and triploid rainbow trouts after fishing stress
After fishing stress, the concentrations of the COR,Glu and LD in diploid and triploid trouts increased first and then decreased (Fig. 2). The concentrations of the COR in triploid trouts blood peaked before diploid, and the fluctuations of triploid trouts were stronger than those of diploid trouts (P<0.05) (Fig. 2A).The blood Glu concentration of both diploid and triploid trouts peaked 10 min after fishing stress, and were significantly higher than those at 2 and 30 min(P<0.05) (Fig. 2B). The LD concentrations of both diploid and triploid trouts peaked 60 min after fishing stress, and were significantly higher than those at 30 and 120 min (P<0.05) (Fig. 2C).After fishing stress, the liver glycogen contents of diploid and triploid trouts decreased, and were significantly lower than those of the control group(P<0.05). Within 24 h after stress, liver glycogen decreased by 85.4% in diploids and 89.7% in triploids,and the triploids started to decrease before the diploids. The liver glycogen content of triploid trouts was significantly lower than that of diploid trouts(P<0.05) (Fig. 3A). The HK activities of diploid and triploid trouts first increased and then decreased, and were higher than those of the control group (P<0.05),peaking at 60 min after stress (Fig. 3B). The PK activities of diploid and triploid trouts showed a double-peak trend of rising and falling. The two peaks of diploid trouts were at 10 and 240 min, while those of triploid trouts were at 2 and 60 min (Fig. 3C).
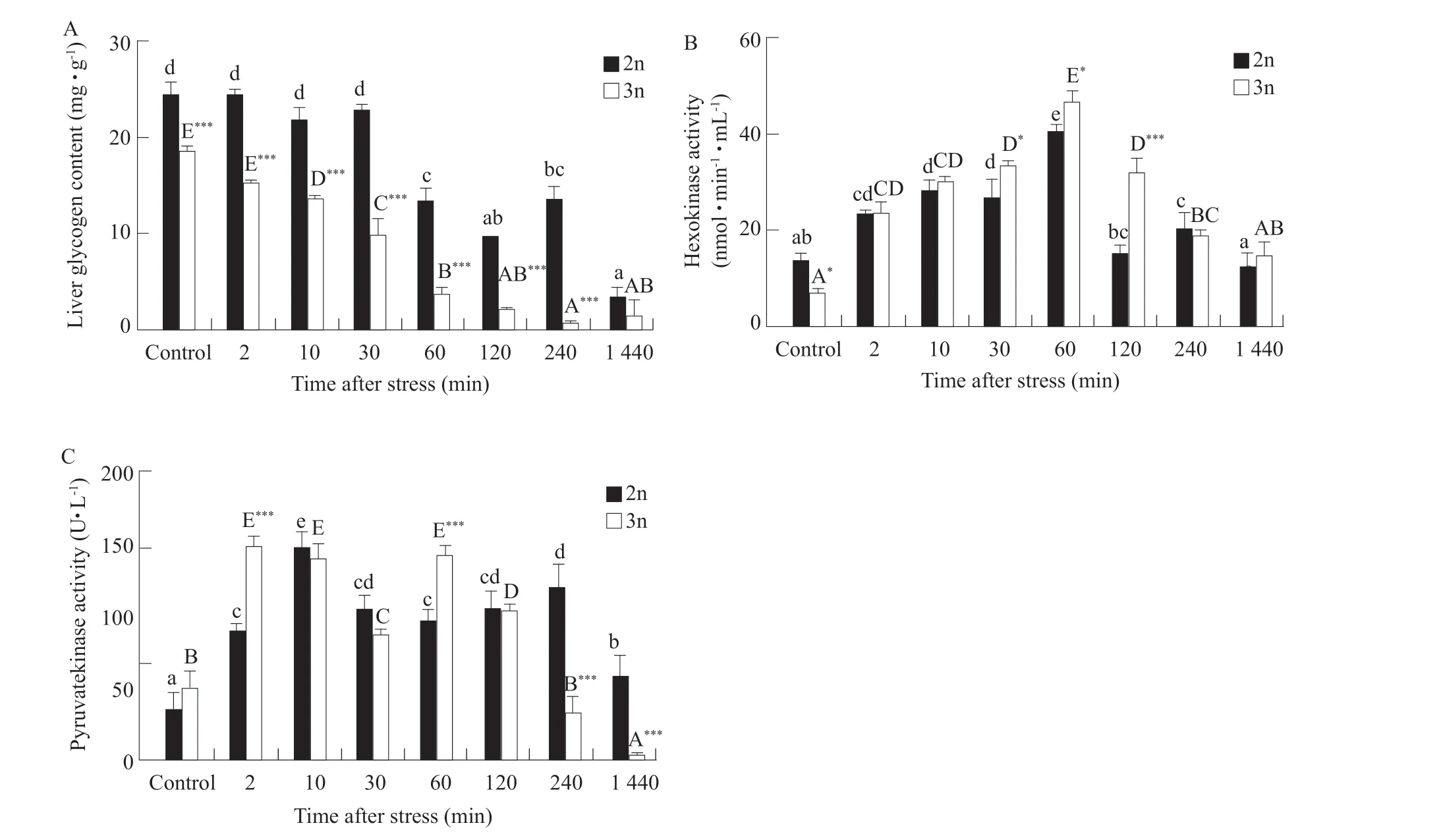
Fig. 3 Time-course of liver glycogen content (A), HK (B) and PK (C) activities in diploid and triploid rainbow trouts after fishing stress
Changes in mRNA expressions of glycolytic enzymes in diploid and triploid female rainbow trouts after fishing stress are shown in Fig. 4. After fishing stress, the PEPCK mRNA expression of triploid trouts at 1 440 min was significantly higher than that of the control group (P<0.05). The G6Pase mRNA expression of diploid and triploid trouts followed a fluctuating trend, triploid trouts peaked before diploid trouts, and there was a significant difference in the G6Pase mRNA expression between diploid and triploid trouts (P<0.05).The GK and GLUT2 mRNA expressions both increased first and then decreased, and triploid trouts peaked before diploid trouts. The GR1 mRNA abundance showed a decreasing trend. At 2 and 60 min, the GR1 mRNA abundance of triploid trouts was significantly higher than that of diploid trouts (P<0.01).

Fig. 4 Time-course of PEPCK (A), G6Pase (B), GK (C), GLUT2 (D) and GR1 (E) mRNA in diploid and triploid rainbow trouts after fishing stress
Discussion
Both diploid and triploid female rainbow trouts responded to fishing stress. The physiological, biochemical and glucose metabolism indexes presented time specificity and ploidy specificity, and the response amplitudes and glucose metabolism pathways of each index differed.
Red blood cells (RBC) were an important auxiliary component of the immune response of blood cells in fishes and participated in oxygen transport (Shenet al., 2018; Sadleret al., 2000). The results of the present study showed that the number of the RBC was significantly higher in diploid controls than that in triploids, and the HGB in the control group was not significantly different between diploid and triploid fishes. These results were consistent with previous reports in the same species (Benfey and Biron, 2000).Triploid trouts had fewer erythrocytes, and triploid and diploid trouts had a very similar oxygen delivery ability as well as the same blood response after stress(Sadleret al., 2000). The WBC participated in the innate and adaptive immunity of vertebrates, and stress could stimulate the proliferation of the WBC in fishes(Zhouet al., 2019). The number of the WBC was significantly higher in the diploid trouts stress group than that in the control group from 10 min to 30 min,while in triploid trouts, it was significantly higher from 2 min to 1 440 min. The number of the WBC in the stress group of triploids was significantly higher than that of diploid trouts from 2 min to 1 440 min. Similar findings had previously been reported in the same species (Chenet al., 2019), indicating that triploid female rainbow trouts were stronger in terms of immunity and disease resistance and less susceptible to disease than diploid trouts.
Cortisol regulated the energy metabolism of bony fishes, promoted glucose mobilization, increased protein degradation to overcome the stress state, acted within a few seconds to minutes, and was an important indicator of the primary stress response (Jorgeet al.,2019; Daset al., 2018; Balasch and Tort, 2019;Fanourakiet al., 2011). Cortisol levels of diploid trouts increased within 10 to 60 min after the stress and in triploid trouts, cortisol levels increased within 2 min to 60 min after stress. These changes were similar to other changes in the same species, with higher cortisol levels than the controls from 0.25 h to 5 h after fishing pressure (Vijayan and Moon, 1994; Jentoftet al., 2005;López-Patiñoet al., 2014). The time of high cortisol levels was longer in triploids than that in diploid trouts, indicating that the stress response in triploid trouts was more intense and lasted longer. The levels of glucose and lactate increased in both diploid and triploid female rainbow trouts after stress. The glucose level was the highest 10 min after stress and the lactate level was the highest 60 min after stress, both followed the same trend in diploid and triploid trouts. These results suggested that both diploid and triploid trouts used their glucose metabolism to resist stress.
Previous studies on diploid and triploid fishes had focused on their physiology and biochemistry, while largely ignoring metabolic differences between diploid and triploid fishes. The results of this study showed a significant reduction in the GR1 mRNA abundance in diploid and triploid female rainbow trouts following fishing stress, which was similar to a previous report on rainbow trouts (Teleset al., 2013). However, the GR1 mRNA abundance in triploid trouts was higher than that in the control 10 min after stress, which might be because cortisol levels in triploid trouts reached the maximum earlier than those in diploid trouts, and cortisol bound more rapidly to specific receptors, thereby activating the GR1 expression.
Glucose was the most effective and preferred energy substrate (Liuet al., 2017). The short-term supply of glucose in fishes after stress originated from glycogen decomposition (Dinget al., 2020). In the control group, the glycogen content was significantly higher in diploid livers than that in triploid livers, which was possibly because of differences between diploid and triploid trouts after stress. Diploid liver glycogen levels decreased 60 min after fishing stress and triploid liver glycogen levels decreased 10 min after stress(López-Patiñoet al., 2014). The rapid decline in the levels of hepatic glycogen might reflect a gradual increase in glycogenolysis, induced by catecholamines,which provided fuel for all the tissues, during the initial phase of stress (López-Patiñoet al., 2014). The liver glycogen content of triploid trouts decreased before that of diploid trouts, suggesting that triploid trouts responded more rapidly to fishing stress and preferentially prompted liver glycogen mobilization to provide energy, which might be a metabolic advantage of triploids compared to diploid trouts. However,this advantage also led to a rapid depletion of hepatic glycogen in triploid trouts.
Glycogen mobilization at 60 min after stress in diploid trouts and 10 min after stress in triploid trouts might result from an increase in the glycogen catabolic potential. The G6Pase mRNA abundance represented the glycogenolytic potential (López-Patiñoet al.,2014) and was found to be significantly elevated at 10 min post-stress for diploid trouts and 2 min poststress for triploid trouts. These results explained the difference in the timing of glycogen mobilization in diploid and triploid trouts. The G6Pase mRNA abundance of triploid trouts increased significantly for the second time at 60 min post-stress. Liver glycogen mobilization was eventually depleted when the gluconeogenic potential persisted, which explained why hepatic glycogen was almost depleted after 60 min in triploid trouts.
Gluconeogenesis played an important role in the maintenance of adequate blood glucose levels.Shortly after stress, 3% of the blood sugar of rainbow trouts originated from gluconeogenesis (Mommsenet al., 1988). A higher activity of the PEPCK mRNA abundance indicated a stronger gluconeogenesis ability. The PEPCK mRNA abundance of diploid and triploid trouts showed no difference between the control and stress groups from 2 min to 240 min after stress. The PEPCK mRNA expression of triploid trouts showed an up-regulation at 24 h after stress as reported by Sathiyaa and Vijayan (2003). These results were consistent with the claim by López-Patiñoet al. (2014) that the mRNA expression of PEPCK persisted for a long time after stress, even beyond 24 h. Hepatic gluconeogenesis was inhibited in response to stress, which contributed to the depletion of hepatic glycogen reserves in the liver. Triploid trouts had lower hepatic glycogen reserves than diploid trouts, therefore, first began to provide glucose by enhancing gluconeogenesis.
After the liver had been depleted of its reserve glycogen content, hepatic glycolysis became the main form of glucose supply (Wisemanet al., 2007). The HK activity in stress groups of diploids and triploids reached the maximum 60 min after stress, while the PK activity in stress groups of both diploid and triploid trouts was slightly decreased. These results implied that both diploid and triploid female rainbow trouts mobilized glucose rapidly and delivered it to the blood stream instead of using itin situafter fishing stress.
The GK mRNA expression showed a reduction from 10 min to 240 min in diploids and from 30 min to 240 min in triploid trouts. The GK mRNA abundance was significantly lower in triploid trouts than that in diploid trouts, suggesting that the liver of triploid trouts had a lower ability to utilize exogenous glucose. The GLUT2 mRNA abundance in diploid trouts reached the maximum 30 min after stress, while in triploid trouts,the maximum reached at 2 min after stress, which matched previous reported (Wisemanet al., 2007;López-Patiñoet al., 2014). Since GLUT2 was a twoway transporter protein, increasing mRNA expression might suggest an increase in the rate at which the liver transported glucose into the blood. These results showed that the glucose transport capacity of triploid trouts was better than that of diploid trouts.
For this study, samples were not collected from 4 h to 24 h after stress to avoid the effects of diurnal fluctuations on stress outcomes (Hernández-Pérezet al.,2019); however, this did not affect the description of the metabolic effects in diploid and triploid female rainbow trouts after fishing stress. The best fishing processes in aquaculture production were simulated,while in actual production, the fishing operation lasted longer and damaged more fishes. Triploid trouts had an advantage over diploid trouts in terms of their metabolic mobilization, because their stress responses preceded that of diploid trouts. However, since their hepatic glycogen reserves were lower than those of diploid trouts and their efficiency of glucose at the outer edge of the liver was also lower, excessive glycogen depletion complicated the adaptation to the high glucose consumption required for fish recovery during later stages of stress.
Conclusions
To understand the impact of fishing stress on fishes was essential to improve animal welfare and increase production in aquaculture production. In general,the present results supported two main metabolic differences between diploid and triploid female rainbow trouts under fishing stress. One point was that triploid trouts had a stronger stress response than diploid trouts and preferentially mobilized liver glycogen, which was an advantage of triploid trouts and one of the reasons for their poor tolerance. Another point was that the hepatic glycogen content and the ability to utilize exogenous hepatic glucose of triploid trouts were lower than those of diploid trouts and metabolized faster than diploid trouts, leading to the hepatic glycogen in triploid trouts was depleted before diploid trouts. Triploid trouts did not have any abilities to provide sufficient energies in time to resist disease and trauma during recovery from stress, resulting in a higher mortality rate than that of diploid trouts. In conclusion, diploid female rainbow trouts were more tolerant to fishing stress than triploids.
杂志排行
Journal of Northeast Agricultural University(English Edition)的其它文章
- Study on Antibacterial Effects of Purple, Yellow and White-skinned Onions
- Effects of Different Ventilation Modes and Outlet Height on Nursery Piggery Environment
- Measurement of Grain Production Efficiency in Main Grain-producing Areas and Analysis of Inter-provincial Differences
—— A Study Based on Super-SBM Model and Malmquist Index - Effects of Planting Density and Cutting Time on Hay Yield and Nutritional Value of Forage Soybean HN389
- Effects of Encapsulated Enzyme and Yeast Products on in Vitro Rumen Fermentation
- Attribution of Antioxidation of Quercetin in Vitro and Arbor Acre Broilers
