Effects of Encapsulated Enzyme and Yeast Products on in Vitro Rumen Fermentation
2022-06-25ZhangMeimeiLiangGegeChenMingmingJinLongXieXiaolaiZhangYonggenandJiaoPeixin
Zhang Mei-mei, Liang Ge-ge, Chen Ming-ming, Jin Long, Xie Xiao-lai, Zhang Yong-gen, and Jiao Pei-xin,*
1 College of Animal Sciences and Technology, Northeast Agricultural University, Harbin 150030, China
2 Agriculture and Agri-Food Canada, Lethbridge Research and Development Centre, Lethbridge T1J 4B1, Canada
Abstract: Batch cultures of mixed rumen micro-organisms were conducted to evaluate the effects of encapsulated yeast (+EY)and encapsulated enzyme (+EE) using plant proteins (barley and oats grain) on rumen fermentation in vitro, investigate the abilities of encapsulated yeast and encapsulated enzyme to prevent rumen digestion in vitro. Treatments of the study were the control,+EY, +EE products (3.33 mg • mL-1 of the incubation medium), unencapsulated yeast (-EY) and enzyme (-EE) products (0.17 and 0.17 μL • mL-1 of the incubation medium, respectively). +EY group increased dry matter disappearance (DMD, P<0.01) and the total volatile fatty acids (TVFA, P<0.01) at 3 h of the incubation compared with the control, regardless of encapsulation of yeast.Gas production (GP) of +EY group was higher (P=0.05, 29.94 mL • mL-1 organic matter, OM) than that of the control (25.08 mL• g-1 OM) at 3 h of the incubation. Supplementation +EY increased DMD (P=0.04, 0.394 vs 0.352, respectively) and acetic proportion(P=0.04, 52.6 vs 49.8 mol • 100 mL-1, respectively) at 6 h of the incubation and increased A : P ratio (P<0.01, 3.11 and 2.86,respectively) at 24 h of the incubation, as compared to unencapsulation of yeast. Supplementation of enzyme had higher (P≤0.04)GP, DMD and TVFA at 3 and 6 h of the incubation compared with the control, regardless of encapsulation. Moreover, the addition of +EE produced greater GP at 6 (P<0.01, 92.35 vs 78.21 mL • g-1 OM, respectively), 12 (218.47 vs 159.18 mL • g-1 OM) and 24 h(380.97 vs 297.78 mL • g-1 OM, respectively) of the incubation, higher DMD (0.347 vs 0.313, respectively) at 3 h of the incubation as compared to -EE group. The study showed that the encapsulation might protect part of yeast and enzyme from releasing to the rumen throughout the digestion in vitro, resulting in higher or no difference of rumen fermentation parameters compared with unencapsulated groups at any incubation times. In comparison with -EY and -EE, the higher rumen fermentation parameters at the early incubation time were observed, which could be attributed to the higher concentration of yeast or enzyme. However, regardless of the encapsulation, the results indicated that both yeast and enzyme only improved the speed rather than the extent of rumen fermentation in vitro.
Key words: dry matter digestion, encapsulated enzyme, encapsulated yeast, gas production, in vitro, volatile fatty acid
Introduction
Various alternative solutions have been explored to the growing concern over the use of antibiotics and other growth promoters in the animal feed industry(Yoon and Stern, 1995). During the past decades, yeast and enzyme products have been increasingly studied as promising solutions to manipulate the microbial ecosystem to maximize the utilization of feed sources and enhance production efficiency of cattle without causing disturbance of intestinal microbial balance.The potential of exogenous enzyme to improve feed utilization for ruminants has been extensively studied,which depends on various factors, including diet composition, type of enzyme preparation, amount of enzyme, enzyme stability and method of application(Beaucheminet al., 1999; 2019). The positive effects of yeast cells have been mainly demonstrated on growth and activity of fibre-degrading bacteria and fungi, ruminal microbial colonization, stabilisation of rumen pH and prevention of lactate accumulation (Jiaoet al., 2019; Aminet al., 2020). However, the activity of supplemental yeast or enzyme may be limited to the early rumen digestion or low capacity to resist to rumen environmental stress because of the complex rumen ecosystem and rumen microbial competition for adhesion of substrate (Chaucheyras-Durandet al.,2008). Despite of the extensive researches in the effects of yeast and enzyme on rumen fermentation characteristics in the rumen ecosystem, few studies are directly conducted to investigate the effects of encapsulated yeast and enzyme on rumen fermentation parameters of cattlein vitro. Previous researches showed that microparticles and nanoparticles of various formulations of whey protein either alone or combined with other biopolymers are able to develop and display the desirable properties of protecting nutraceutical compounds in the stomach, and release them in the intestinal environment using simulated gastrointestinal techniques (Chen and Subirade, 2009;Wanget al., 2011). Although previous studies have showed that encapsulation of active dry yeast improves nutrient digestion and reduces the totalE. colicounts of feces in beef cattle, the survival and release activities of encapsulation yeast have not been directly detected in animals (Jiaoet al., 2017; Ranet al., 2018).Therefore, the objectives of the study were to evaluate the effects of encapsulated yeast and encapsulated enzyme using plant proteins (barley and orts grain) on rumen fermentation parametersin vitroand investigate the abilities of encapsulated yeast and encapsulated enzyme to prevent rumen digestionin vitro.
Materials and Methods
Materials
The additive samples were active dry yeast (Saccharomyces cerevisiae, 1.71×1010CFU • g-1) products provided by AB Vista (Marlborough Wiltshire, UK)and enzyme (alpha-amylase, 120 KNU-T • mL-1)products (Novozymes A/S, Bagsvaerd, Denmark). The encapsulation of yeast and enzyme was conducted at Department of Agricultural University of Alberta using the method described by Chen and Subirade (2009).The substrate containing high concentration diet was consisted of 10% barley silage, 87% barley grain and 3% vitamin and minerals (DM basis).
Batch culture incubations in vitro
The study was designed with four treatments including encapsulated yeast (+EY) products, unencapsulated yeast (-EY) products, encapsulated enzyme (+EE)products and unencapsulated enzyme (-EE). Glass bottles (volume capacity of 100 mL) fitted with rubber stoppers to prevent the escape of fermentation gases were used for incubations. Triplicate bottles were used for each treatment, each of which contained two acetone-washed and pre-weighed ANKOM bags (F57;ANKOM Technology, Macedon, NY, USA). One bag contained substrate (approximately 300 mg), the other bag contained the +EY or +EE products (approximately 200 mg). The bottles containing substrate and -EY or -EE products (approximately 10 μL or 10 mg,respectively) in ANKOM bags were conducted as the positive controls. Another three bottles, each of which contained substrate in an ANKOM bag and an empty ANKOM bag, were used as the controls. The freshly anaerobic medium was prepared according to the method described by Goering and Van Soest (1970)prior to the study and warmed at 39℃ in a water bath.
Rumen fluid was obtained from two ruminally fistulated beef cattle fed a diet consisting of 750 g • kg-1barley silage and 250 g • kg-1concentrate (DM basis).Ruminal contents were collected 2 h after morning feeding. The whole ruminal contents were obtained from various locations within the rumen and squeezed through PeCAP®polyester screen (pore size 355 µm;B & S H Thompson, Ville Mont-Royal, QC, Canada).The strained ruminal fluid was immediately transferred to the 39℃ water bath in an insulated, air tight container. Rumen fluid was re-strained through four layers of cheesecloth to remove any contaminating particles that might interfere with dispensation of rumen fluid into serum vials. Forty-five of pre-warmed media and 15 mL of strained rumen fluids were mixed into each bottle purged with CO2to remove air from the headspace. Each bottle was sealed with a 14 mm butyl rubber stopper plus an aluminum crimp cap immediately after loading, and incubated at 39℃ for 3, 6, 12 and 24 h, respectively. The three additional substrate-free bottles with two empty bags were served as blanks by adding 45 mL of medium and 15 mL of rumen fluid. The experiment was repeated in two runs on different days.
At 3, 6, 12 and 24 h of the incubation invitro,headspace gas production (GP) was measured and recorded. The GP was measured by inserting a 23-gauge(0.6 mm) needle attached to a pressure transducer(model PX4200-015GI, Omega Engineering, Inc.,Laval, Que., Canada) connected to a visual display(Data Track, Christchurch, UK). The transducer was then removed leaving the needle in place to permit venting. The gas volume was calculated using the equation described by Mauricioet al. (1999):
Gas volume=0.18+(3.697×gas pressure)+(0.0824×gas pressure2)
After incubation, the bottles were placed in cold water to stop fermentation. Substrate bags were washed and dried in oven at 55℃ for the measurement of dry matter disappearance (DMD) after removing from the vials, whereas the other bags with encapsulated products were dried at a pre-chilled (-40℃) freeze dryer (FTS Systems Dura-Top MP) for 72 h.
Activities of encapsulated yeast and encapsulated enzyme after incubation
After freeze drying, the residues of encapsulated yeast were removed form bags, digested in 1% pancreatin medium for 1 h to remove the encapsulation protection. The activity of encapsulated yeast was enumerated by spread plate method using PDA (Potato Dextrose Agar) as medium. Appropriately diluted encapsulated yeast solutions were inoculated in plated and all the plates were transferred to incubator for incubation at 30℃ for 3 days after inoculation.
Enzyme activity (EC 3.2.1.1) was assayed according to the procedures reported by Colombatto and Beauchemin (2003). The mixture of 1 mL substrate(1% soluble starch, wt • vol-1; Sigma Chemical Co.)and 0.9 mL buffer (0.1 mol • L-1citrate-phosphate,pH 6.0) were incubated at 39℃ for 10 min. The incubation was extended for another 10 min after adding the enzyme dilutions. The tubes with substrate only or enzyme only were incubated and served as blanks. The three millilitres of 3, 5-dinitrosalicylic acid were used as suspension agent to stop the reaction.All the tubes contents absorbance was red at 540 nm in a spectrophotometer (8500II, Thermo Electron Corporation, Rochester, NY, USA). The enzyme activity was expressed in μmol glucose equivalents/min/g enzyme product by a standard curve made with glucose (from 0 to 0.8 mg • mL-1).
Chemical analysis
Chemical analyses of DM and OM were performed according to the procedures of the Association of Official Analytical Chemists (AOAC 1999, method 930.15, method 985.01, respectively). The total volatile fatty acids (TVFA) and composition of volatile fatty acid (VFA) were measured using a gas chromatograph(model 5890; Hewlett-Packard Lab, Palo Alto, CA) with a capillary column (30 m by 0.32 mm i.d.; 1 μm phase thickness; Zebron ZB-AAP; Phenomenex, Torrance,CA) and flame ionization detection; crotonic acid(trans-2-utenoic acid) was used as an internal standard.
Data processing
Data were analyzed using the MIXED model procedure of SAS (SAS Inst. Inc. Cary, NC) including fixed effects of treatments and the random effects of day. For repeated measures, various covariance structures were tested with the final choice exhibiting the lowest value for Akaike information criteria. LSMEANS included the Tukey correction was used to adjust the PDIFF option statement to account for multiple comparisons among treatments. Differences among treatments were declared significant atP≤0.05.
Results
The +EY group had higher GP (P=0.05, 29.94 mL • g-1OM)) at 3 h of the incubationin vitrocompared to the control (25.08 mL • g-1OM). There was higher DMD(P<0.01 andP=0.04, respectively; 0.315 and 0.394,respectively) with +EY group in comparison to the control (0.261 and 0.330, respectively) at 3 and 6 h of the incubation. In addition, -EY group had higher DMD(P<0.01, 0.332), compared with the control (0.261) at 3 h of the incubation (Table 1).
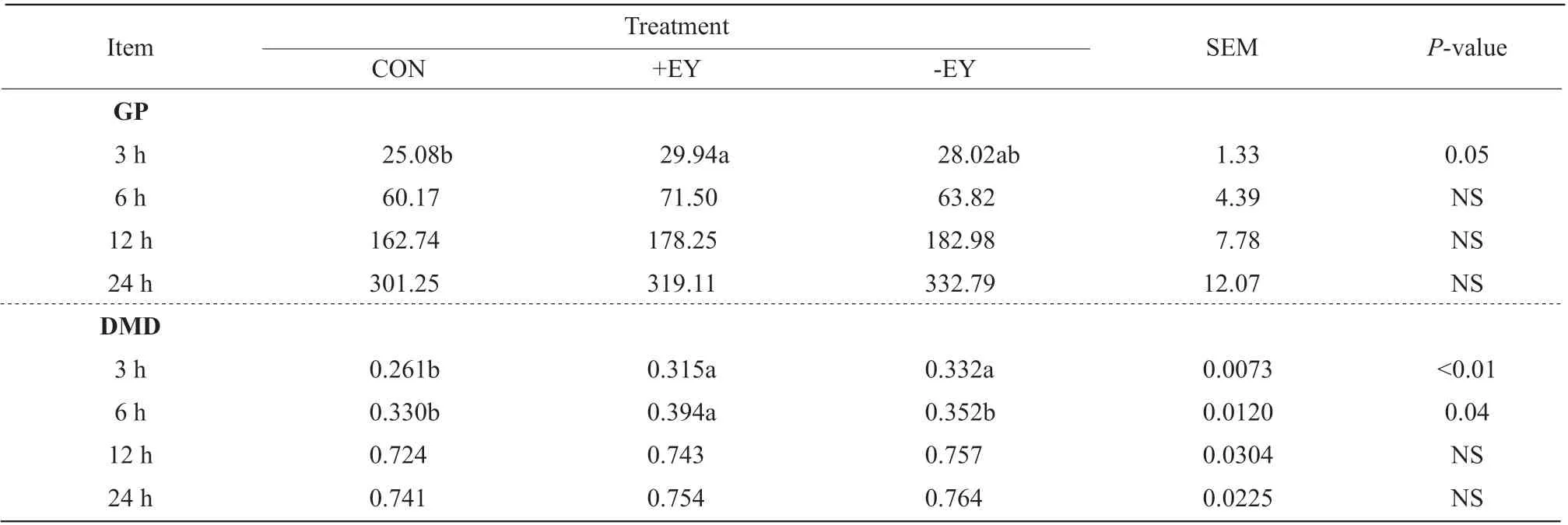
Table 1 Gas production (GP, mL·g-1 organic matter, OM) and dry matter disappearance (DMD) of high concentration diet containing encapsulated yeast (+EY) and unencapsulated yeast (-EY) at 3, 6, 12 and 24 h of in vitro incubation
The addition of +EY and -EY increased TVFA(P=0.02, 16.5 mmol • L-1and 18.0 mmol • L-1, respectively) compared with the control (11.6 mmol • L-1) at 3 h of the incubation. However, +EY and -EY groups had lower (P=0.01) propionate production (16.8 and 17.3 mol • 100 mol-1, respectively) than the control(18.6 mol • 100 mol-1) at 24 h of the incubation. At 6 h of the incubation, the production of acetate was higher(P=0.04) with +EY group (52.6 mol • 100 mol-1) than with -EY (49.8 mol • 100 mol-1). For the ratio of A: P,+EY group had the highest value (P<0.01, 3.11) than the control (2.57), and -EY (2.86) was the lowest value compared with the control (Table 2).
The GP of +EE was the highest (P<0.01; 41.72,92.35, 218.47 and 380.97 mL • g-1OM, respectively)throughout all the incubation time (3, 6, 12 and 24 h)(Table 3). At 3 and 6 h of the incubation, -EE group had higher GP (P<0.01; 38.38 and 78.21 mL • g-1OM,respectively) than the control (24.49 and 60.17 mL • g-1OM, respectively). In consistence with GP at 3 and 6 h of the incubation, the DMD of +EE (0.347 and 0.378, respectively) and -EE groups (0.313 and 0.401,respectively) were higher (P<0.01) than that of the control (0.261 and 0.330, respectively).

Table 2 Volatile fatty acid (VFA) profile of high concentration diet containing encapsulated yeast (+EY) and unencapsulated yeast(-EY) at 3, 6, 12 and 24 h of incubation in vitro

Table 3 Gas production (GP, mL • g-1 organic matter) and dry matter disappearance (DMD) of high concentration diet containing encapsulated enzyme (+EE) and unencapsulated enzyme (-EE) at 3, 6, 12 and 24 h of in vitro incubation
For TVFA at 3 and 6 h of the incubation, +EE group(P<0.01; 20.3 mmol • L-1;P=0.04, 34.5 mmol • L-1)and -EE group (P<0.01, 17.8 mmol • L-1;P=0.04,34.1 mmol • L-1) was higher than the control (11.6 and 29.2 mmol • L-1, respectively) (Table 4). In addition,+EE group produced the highest (P=0.01, 20.3 mol • 100 mol-1) propionate among the three treatments, whereas -EE group had the lowest (P=0.01)propionate production (17.1 mol • 100 mol-1) at 12 h of the incubation. At 3 h of the incubation, +EE group produced higher butyric (P=0.03, 16.7 mol • 100 mol-1)than the control (14.6 mol • 100 mL-1), however,+EE group produced lower butyric (P<0.01, 13.8 mol • 100 mol-1) than the control (15.0 mol • 100 mol-1),so did -EE group (12.9 mol • 100 mol-1) at 6 h of the incubation. However, -EE group produced the highest butyric (P<0.01, 22.9 mol • 100 mol-1) among the three treatments at 12 h of the incubation. +EE group had the lowest ratio of A : P (P=0.05, 2.41;P<0.01, 2.41)at 3 and 12 h of the incubation. However, at 24 h of the incubation, +EE had higher ratio (P=0.03, 2.94)than the control (2.57) (Table 4).
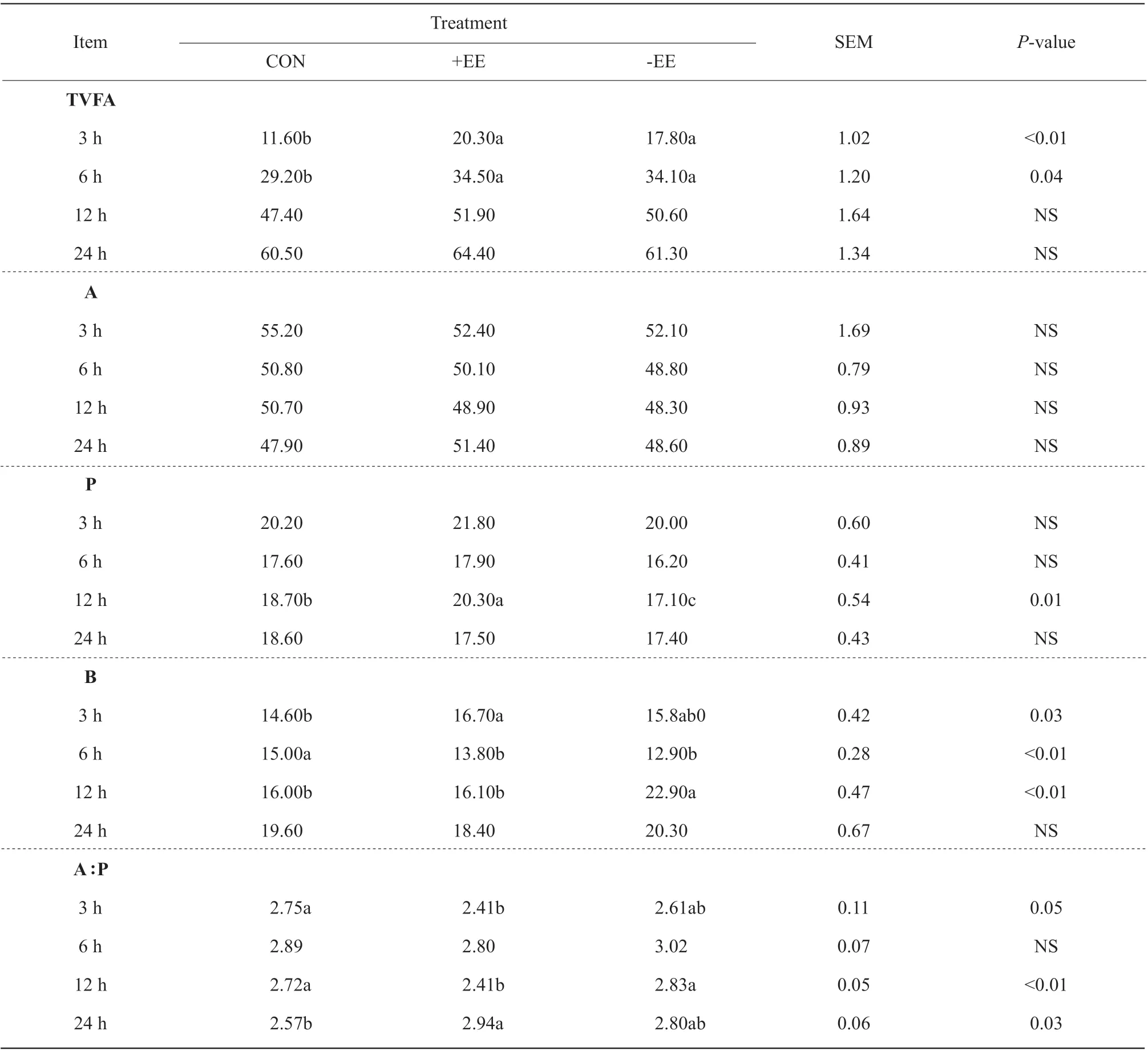
Table 4 Volatile fatty acid (VFA) profile of high concentration diet containing encapsulated enzyme (+EE) and unencapsulated enzyme (-EE) at 3, 6, 12 and 24 h of in vitro incubation
The dry matter of encapsulated yeast disappeared(EYDMD) was increased as the increasing incubation time (Table 5). The activity of +EY was increased from 9.47×108CFU • g-1at 0 h of the incubation to 2.1×109CFU • g-1at 3 h of the incubation, then decreased to 6.03×108CFU • g-1at 24 h of the incubation. The dry matter of encapsulated enzyme disappeared (EEDMD)was increased as the increasing incubation. The activity of +EE was decreased from 41.93 µmol • min-1• g-1at 0 h of the incubation to 10.23 µmol • min-1• g-1,followed by an increase to 19.74 µmol • min-1• g-1at 24 h of the incubation.
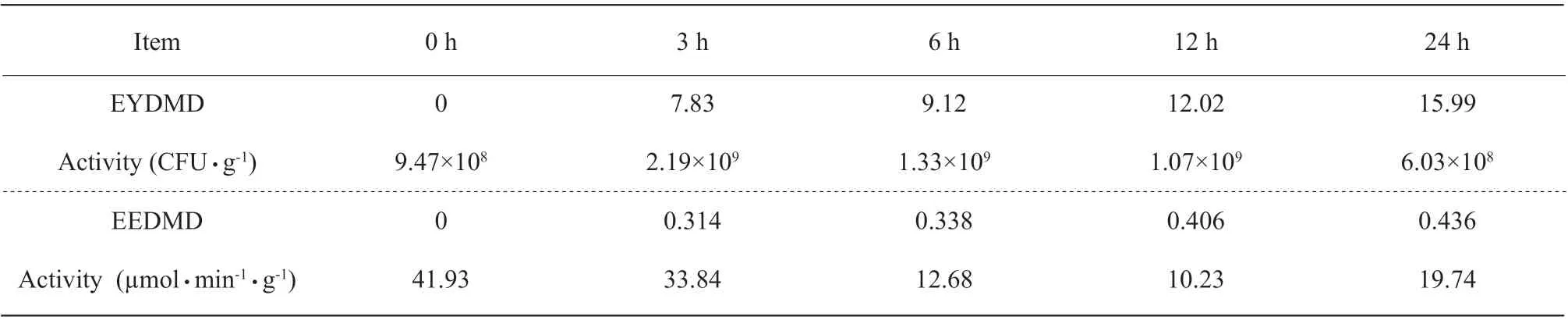
Table 5 Activities and dry matter disappearance of encapsulated yeast (EYDMD) and encapsulated enzyme (EEDMD) at 0, 3, 6, 12 and 24 h of incubation
Discussion
Gas production which was mainly composed of hydrogen, carbon dioxide and methane depended on nutrient availability for rumen microorganisms(Mahala and Fadel Elseed, 2007). Meanwhile, gap production was an important feed digestion characteristic which was positively correlated with ruminal DMD (Menke and Steingass, 1988). This was consistent with the current result that +EY group had higher GP and DMD compared with the control at 3 h of the incubation, indicating that the addition of +EY encouraged nutrient digestion in rumen fermentation.A better rumen fermentation environment might be created byS. cerevisiaefor rumen microorganisms which was sensitive to O2to improve microbial activities (Chaucheyras-Durandet al., 2008). Newboldet al. (1996) reported thatS. cerevisiaemight consume excess oxygen which was toxic to anaerobic bacterial and release vitamins or other growth factors to be closely correlated with bacterial cells (Jouany, 2006),which might contribute to the higher GP and DMD at 3 h of the incubation in the current study. This higher GP and DMD shown in +EY group at the early incubation time compared to the control indicated that the addition of +EY affected the early rumen fermentation rather than the extent of rumen fermentation. This resulted from the low fiber concentration in the substrate used in current study. Girard and Dawson (1995) suggested that yeast might enhance growth and/or activities of fibrolytic bacteria, which improved fiber digestion in the rumen. However, the low fibre substrate might limit the fibre source digested by fibrolytic bacterial and the stimulating effects of yeast, resulting in no difference of GP and DMD among treatments at longer incubation time.
A number of reports showed that effects of yeast on TVFA or VFA composition were variable, which was in consistence with the current study (Jiaoet al., 2018;2019). Supplementation of yeast obtained significantly higher TVFA concentrations after feeding a few hours in non-lactating dairy cows from Enjalbertet al(1999). The result was in consistent with the current experiment, which had higher TVFA concentrations at 3 h of the incubation with supplementation of +EY or -EY. In this study, +EY and -EY decreased the proportion of propionate and increased the ratio of A : P. However, supplementation of yeast might increase the proportion of propionate with a decrease of acetate resulting in lower A : P ratio of beef cattle(Penget al., 2020). Zeleňáket al. (1994) reported that the ratio of A : P was increased by yeast with the diet consisted of 800 g • kg-1meadow hay and 200 g • kg-1barley grain. There was no effect of the same yeast neither on the diet of 500 g • kg-1hay and 500 g • kg-1barley grain, nor on the diet of 650 g • kg-1hay, 200 g • kg-1barley and 150 g • kg-1treated beech sawdust.Various effects of yeast on proportion of propionate and ratio of A : P might be resulted from different compositions of diets in this study and previous studies.
The higher GP and DMD were observed in +EE and -EE groups at 3 and 6 h of the incubation, this was consistent with the positive relationship between GP and DMD from Menke and Steingass (1988). The higher DMD might indicate higher starch digestionin vitro. Previous researches showed that the addition of amylase increased DMD and starch digestion (Guti'-errezet al., 2005; Rojoet al., 2001). The higher DMD due to addition of amylase at 3 and 6 h of the incubation might be explained by activities of amylase in the early rumen digestion. Moraet al.(2002) reported that starch digestion by amylase started before rumen proteolysis, which might contribute to the higher DMD and starch digestion at the early incubation hours. The release of amylase in +EE group mainly occurred at the first 3 h of the incubation (0.314, vs. 0.436 at 24 h of the incubation).In addition, the activity of +EE was decreased from 41.39 µmol • min-1• g-1at 0 h of the incubation to 19.74µmol • min-1• g-1at 24 h of the incubation, indicating that the main enzymatic activity happened in the early incubation time. This might explain the no difference in the DMD among +EE, -EE and the control groups at 12 and 24 h of the incubation.
Amylase supplementation might increase the availability of starch hydrolysis products in the rumen and alter the ruminal fermentation process by its hydrolytic action (Tricaricoet al., 2008). Alternatively,other than enhancing starch digestion by amylolytic organisms, the addition of amylase might primarily alter rumen fermentation by encouraging release of starch hydrolysis products including maltodextrins and oligosaccharides (Tricaricoet al., 2008). Thus,these hydrolysis products provided substrates to non-amylolytic organisms, resulting in modification of microbial population and rumen fermentation products, such as VFA (Tricaricoet al., 2008). This probably explained the differences of VFA profile shown with the amylase supplementation compared with the control group at 3, 6, 12 and 24 h. The higher TVFA concentrations in +EE and -EE groups might be associated with the higher GP and DMD at 3 and 6 h of the incubation. The addition of amylase increased TVFA by increasing both starch and non-starch digestion through its own amylolytic activity and encouraging non-amylolytic organisms, resulting in increased DMD. This also contributed to increased the ATP production which encouraged ruminal microbial growth (Sauvant and Van Milgen, 1995). However,previous researches showed various results on the effects of amylase on composition of VFA in rumen fermentation. Andreazziet al.(2018) and Tosetiet al.(2020) reported that the composition of VFA was not affected by amylase supplementation in dairy and beef cattle, respectively. Tricaricoet al. (2005) found thatAspergillus oryzaealpha-amylase supplementation increased the molar proportion of butyrate, reduced the molar proportion of propionate, and increased the ratio of A : P compared to the control. In consistence with the previous studies, the A : P ratio was greater in +EE group, when compared to the control at 24 h of the incubationin vitro. Different results were reported by Nozièreet al. (2014) that the addition of amylase increased the proportion of propionate, at the expense of the proportion of butyrate, and decreased the ratio of A : P in first-lactation cows with high starch diet.Further investigation was still needed to evaluate the effects of amylase from different sources on ruminal fermentation characteristics.
Conclusions
Encapsulation protected yeast and enzyme from releasing to the rumen throughout the digestion,resulting in higher rumen fermentation parameters compared with unencapsulated groups at the early incubation time. However, regardless of the encapsulation, the results indicated that both yeast and enzyme might improve the speed rather than the extent of rumen fermentationin vitro.
杂志排行
Journal of Northeast Agricultural University(English Edition)的其它文章
- Study on Antibacterial Effects of Purple, Yellow and White-skinned Onions
- Effects of Different Ventilation Modes and Outlet Height on Nursery Piggery Environment
- Measurement of Grain Production Efficiency in Main Grain-producing Areas and Analysis of Inter-provincial Differences
—— A Study Based on Super-SBM Model and Malmquist Index - Effects of Planting Density and Cutting Time on Hay Yield and Nutritional Value of Forage Soybean HN389
- Attribution of Antioxidation of Quercetin in Vitro and Arbor Acre Broilers
- Study on Exogenous Ethylene Induced Rice Resistance to Rhizoctonia solani
