Attribution of Antioxidation of Quercetin in Vitro and Arbor Acre Broilers
2022-06-25WuHaoLiuJiayanZhouShuaishuaiDingManyiFuYuxinYangQinlinMariaTabassumChaudhrySunDantongandLiYao
Wu Hao, Liu Jia-yan, Zhou Shuai-shuai, Ding Man-yi, Fu Yu-xin, Yang Qin-lin, Maria Tabassum Chaudhry,2,Sun Dan-tong, and Li Yao*
1 Institute of Animal Nutrition, Northeast Agricultural University, Harbin 150030, China
2 Faculty of Veterinary Sciences, Bahauddin Zakariya University, Multan 60800, Pakistan
Abstract: The antioxidant effects of quercetin were studied in vitro and in vivo. In vitro, vitamin C was used as a positive control to evaluate the antioxidant capacity of quercetin in three aspects: scavenging free radicals, protecting biological macromolecules and the total reducing power. In vivo, a total of 240 AA broilers (1-day age) with similar body weight were randomly divided into four groups with six replicates in each group, and 10 broilers in each replicate. The four groups were fed with corn-soybean basal diet supplemented with 0.00%, 0.02%, 0.04% and 0.06% quercetin to study its effects on antioxidant indexes of AA broilers, and to explore the optimal dose of quercetin as a dietary additive. The results showed that quercetin scavenged superoxide anion,hydroxyl radical and 1, 1-diphenyl-2-picrylhydrazyl (DPPH) in vitro, the scavenging effects of quercetin on O2- and •OH first increased and then decreased with the increase of the concentrations (P<0.01), and its maximum scavenging effect was observed at concentrations of 40 and 300 mg • L-1. The scavenging effects of quercetin on DPPH was increased constantly with increasing concentrations. The scavenging effect of quercetin on three free radicals was DPPH>•OH>O2-. The inhibition of vitelline lipoprotein peroxidation by quercetin was increased with increasing concentrations (P<0.01) and the inhibitory effect was higher than that of vitamin C. The inhibition of red blood cell hemolysis by quercetin was increased with increasing concentrations at 0.05-1.25 mg • L-1(P<0.01); however, the inhibition tended to decrease when the concentration was too high (31.25 mg • L-1), and the inhibitory effect was higher than that of vitamin C. The inhibition of mitochondrial expansion by quercetin was increased with increasing concentrations, according to the degree of mitochondrial expansion at 60 min, the integrity of mitochondria in the experimental groups was significantly higher than that in the model group (P<0.01). The total reducing power of quercetin was increased with increasing concentrations (P<0.01); however, the total reducing power was less than that of vitamin C. In vivo, malondialdehyde (MDA) and nitric oxide (NO) were significantly decreased with increasing quercetin (P<0.01). Quercetin supplementation had no effect on the content of lipid peroxidation (LPO) in livers (P>0.05); however, superoxide dismutase (SOD) activity was significantly increased,whereas glutathione peroxidase (GSH-Px) and catalase (CAT) activities were significantly decreased in livers with increasing quercetin (P<0.05). These results suggested that quercetin exhibited strong antioxidant effects in vitro and in vivo.
Key words: quercetin, antioxidant, in vitro, AA broilers
Introduction
Intensive livestock production often puts broilers under oxidative stress, which will inevitably produce a large number of free radicals in the body. Thus,the redox balance is broken, resulting in the decline of animal performance and immunity, severely restricting production (Linet al., 2017). Meanwhile,the endogenous antioxidant defense system will be weakened by oxidized sunflower oil in diet, and then contribute to liver damage in broilers (Wanget al.,2016). Moreover, antiradical drugs or antioxidant feed additives have been developed and utilized in animal production; however, synthetic antioxidants have toxicity, teratogenicity and potential carcinogenicity,and are prone to drug residues, affecting the quality of livestock products. Therefore, the search for natural and pollution-free antioxidants has become a research hotspot in the field of animal nutrition.
The special chemical structure of flavonoids determines their strong antioxidant effects. A lot of studies have shown that seabuckthorn flavone, citrus flavonoids, taxifolin, daidzein, buckwheat flavones,etc. exhibit antioxidant activities (Panduranganet al.,2011; Kanget al., 2016; Leeet al., 2016; Goliomytiset al., 2019; Xiaoet al., 2019). Quercetin is a typical representative of natural flavonoids and widely exists in fruits and vegetables. Quercetin has been proved to have strong antioxidant capacity (Lesjaket al.,2018); however, few researches have been done on the effects of quercetin in chickens. In this study,three free radical reaction systems were selected to study the scavenging effects of quercetin on free radicalsin vitro, and the total reducing power of quercetin was determined, based on the free radical scavenging test, the protective effects of quercetin on biomacromolecules under high concentration of free radicals was further explored. Meanwhile, broilers were used for determining antioxidation of quercetinin vivo. These results would provide scientific basis for the application of quercetin as a natural antioxidant in chicken production.
Materials and Methods
Materials and animals
Test substance: quercetin (purity 97%) and vitamin C were purchased from SIGMA-ALDRICH Company(St. Louis, MO). Pyrogallol, 1, 1-diphenyl-2-picrylhydrazyl (DPPH), salicylic acid, ferrous sulfate(FeSO4), trichloroacetic acid (TCA), thiobarbituric acid (TBA), potassium ferricyanide (K3[Fe(CN)6]),ferric chloride (FeCl3), absolute ethanol, hydrogen peroxide (H2O2), normal saline, Tris-HCl buffer,sucrose. All the chemicals were analytical grade of the highest purity and purchased from Tianjin Zhiyuan Chemical Reagent Co., Ltd (Tianjin, China).
Animals: male SD rats with (300±30) g body weight were purchased from Changchun City, the Experimental Animal Technology Co., Ltd.; 240 oneday-old AA broilers (initial weight, 105.92±1.61g)were purchased from Yinong Poultry Industry in Harbin City.
Care and use of animals: all the procedures in this study were approved by the Animal Care and Use Committee of the University. Housing, management and care of the SD rats and the birds confirmed to the guidelines of Agricultural Animal in Agriculture Research and Teaching of Heilongjiang Province (HEI Animal Management Certificate No. 11928. Date:8-Dec-2011).
Antioxidation of quercetin in vitro
The antioxidant capacity of quercetin was preliminarily determined by measuring the free radical scavenging rate, lipid peroxides (LPO), erythrocyte hemolysis,mitochondrial expansion and reducing power.
Scavenging effects of quercetin on free radicals
The scavenging effects of quercetin on superoxide anion radicals (O2-), hydroxyl radicals (• OH) and DPPH were determined using pyrogallol autoxidation method, salicylic acid method and DPPH method.Inhibition of quercetin on vitellin peroxidationPreparation of yolk suspension: 1:1 egg yolk suspension was prepared with egg yolk and equal volume of PBS (0.1 mol • L-1, pH=7.45), magnetic stirred for 10 min and then diluted into 1:25 suspension with PBS for refrigeration.
In a 10 mL centrifuge tube, 0.2 mL of 1:25 egg yolk suspension, 0.2 mL of 25.0 mmol • L-1FeSO4solution, 0.1 mL of quercetin (vitamin C) with different concentrations and 1.5 mL of PBS were added, respectively, and reacted for 1 h at 37℃. After removal, 20% trichloroacetic acid (TCA) with 0.5 mL was added, and mixed well. Then, stood for 10 min,centrifuged at 3 500 r • min-1for 10 min. Took 2.0 mL supernatant, added 0.8% thiobarbituric acid (TBA)1.0 mL, sealed, boiled in water bath for 15 min, and cooled rapidly. The absorbance value A of the solution was determined at 532 nm. The absorbance value was A0without quercetin (vitamin C) in the control tube.Each concentration was repeated three times. The blank tube was zeroed. The supernatant was replaced by PBS in the blank tube. The inhibition rate of the sample solution on lipid peroxidation of vitellin lipoprotein was: (A0–A)/A0.
Inhibition of quercetin on erythrocyte hemolysis
Preparation of red blood cells: SD rats were fasted overnight, carotid artery blood (heparin anticoagulant)was collected the next day, centrifuged at 3 000 r • min-1for 10 min at 4℃ to obtain red blood cells, washed three times with cold PBS buffer (10 mmol • L-1,pH=7.4), prepared into 0.5% red blood cell suspension,stored at 4℃ for 6 h.
Erythrocyte hemolysis test: added 0.3 mL H2O2with 30 mmol • L-1and 0.2 mL quercetin (vitamin C)solution with different concentrations into 1 mL red blood cell suspension. After 1 h in water bath at 37℃,added 5 mL of normal saline for dilution, centrifuged at 3 000 r • min-1for 10 min, and the absorbance value of the supernatant was measured at 415 nm. PBS buffer was used to replace H2O2and sample solution in the normal group. In the model group, PBS buffer was used to replace the sample, and other conditions remained unchanged. Each concentration was repeated three times. The hemolysis rate was calculated according to the following formula (Duan and He,2019):
Hemolysis rate=(Asample–Anormal)/(Amodel–Anormal)×100%Inhibition of quercetin on mitochondrial expansionMitochondrial preparation: SD rats were fasted overnight, cervical dislocation was lethal, the livers were quickly separated, washed with cold saline,fascia was removed and weighed by wiping with filter paper. The 10% liver homogenate was prepared with 0.25 mol • L-1sucrose solution in ice bath, centrifuged at 1 000 g for 20 min at 4℃, and the supernatant was dumped. The precipitate was washed twice with 0.25 mol • L-1sucrose solution, and the supernatant was combined, centrifuged at 10 000 g for 20 min at 4℃.The precipitation was washed twice with buffer solution (Tris-HCl 10 mmol • L-1, pH=7.4). After 10 min centrifugation at 10 000 g each time, the purified mitochondria were prepared into a suspension with protein content of 0.5 mg • mL-1using buffer solution and stored at 4℃ for further analyses.
Added 4 mL mitochondria and 0.4 mL quercetin of different concentrations into the reaction system. After mixing, added 0.4 mL FeSO4with 0.1 and 1 mmol • L-1vitamin C into the reaction system, respectively. The absorbance value was measured at 520 nm. Samples,FeSO4and vitamin C were replaced by buffer solution in the normal group; samples were replaced by buffer solution in the model group, and other conditions remained unchanged (Wanget al., 2016). Each concentration was repeated three times.
Determination of the total reducing power of quercetin
Aliquot 2 mL of quercetin (vitamin C) ethanol solution of different concentrations was added 2 mL of 2.5 mol • L-1PBS (pH=6.6) and 2 mL of 1% potassium ferricyanide solution. After incubating in a water bath at 50℃ for 20 min, quickly cooled down, added 2 mL of 10% TCA, centrifuged at 3 000 r • min-1for 10 min,added 2.5 mL supernatant with the same volume of distilled water and 0.5 mL of 0.1% ferric chloride into the tube, mixed well, the absorbance value was measured at 700 nm for three replicates (Maiet al., 2018).
Antioxidation of quercetin in vivo
Experimental birds, diets and managementA total of 240 one-day-old AA broilers (initial weight, 105.92±1.61 g) were randomly divided into four groups with six replicates. All the broilers were reared under the same environmental conditions and management. Breeding temperature was maintained at 32℃-34℃ in the starting 3 days and decreased by 2℃-3℃ per week to a final temperature of 24℃. Humidity of the experimental room varied from 60% to 65%.Continuous fluorescent light was provided during the experiment. Water and experimental diets were availablead libitum.
Corn and soybean basal diets (NRC, 1994) were formulated with four different levels of quercetin:0, 0.2, 0.4 and 0.6 g quercetin per kg diet. This experimental diet was offered to the birds from day 1 to day 42. Composition of basal diets and nutrient content is presented in Table 1. Quercetin dehydrate powder was mixed with basal diet and offered in mash form (5 mm) after grinding.
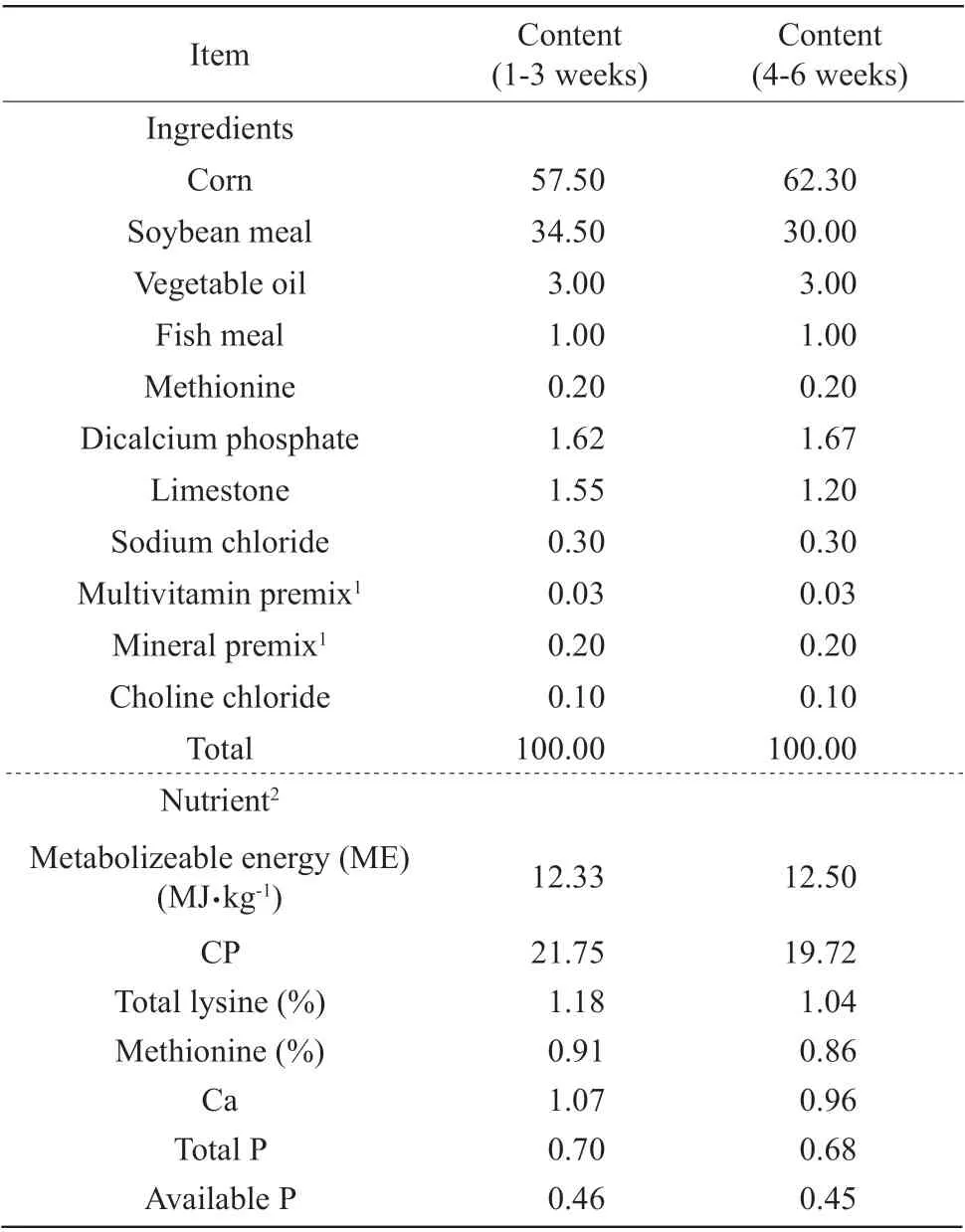
Table 1 Calculated composition of basal diets and nutrient content (air-dry basis, %)
Antioxidant index assay
The activities of antioxidant enzymes, including catalase(CAT), superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px), were assessed in livers of AA broilers. The livers were excised out, washed in ice-cold normal saline, patted dry and weighed.Approximately 0.1 g of livers were homogenized with 9 mL of ice-cold physiological saline (1:9, wt/vol)using an Ultra-Turrax homogenizer (Tekmar Co.,Cincinnati, OH), and then centrifuged at 3 000×g for 15 min at 4℃. The supernatant was used to determine the activities of CAT, SOD, GSH-Px and the total proteins using a colormetric kit and a 1 200 UV spectrophotometer (Mapada Instruments Co.,Ltd., Shanghai, China) according to the instructions of the commercial kits (Nanjing Jiancheng Institute of Bioengineering, Nanjing, Jiangsu, China). All the results were normalized against the total protein concentration in each sample for inter-sample comparison. The total protein concentration was determined according to the method by Bradford (1976) using bovine serum albumin (BSA) as the standard protein.The lipid peroxidation was assessed by the levels of malondialdehyd (MDA) measured using the thiobarbituric acid reactive substances (TBARS) assay at 535 nm (Ohkawaet al., 1979).
Statistical analysis
The results were expressed as "mean value ± standard deviation". One way ANOVA of SPSS 20.0 software was used for variance analysis. Duncan's method was used for multiple comparison.P<0.05 was used as the criterion of significant difference, andP<0.01 was used as the extremely significant difference. In addition, effects of dietary quercetin levels were tested using linear and quadratic regression analysis.
Results
Antioxidation of quercetin in vitro
Scavenging effects of quercetin on O2-
The scavenging effects of quercetin on O2-were increased first and then decreased with the increasing quercetin (P<0.01), and clearance rate was maximized at 40 mg • L-1(31.93%); while the scavenging effects of vitamin C on O2-were increased with the increasing concentrations (P<0.01), and IC50was 28.63 mg • L-1(Table 2). According to the overall trend and IC50,quercetin had a certain scavenging effect on O2-;however, the scavenging effect was less than that of vitamin C.

Table 2 Effects of quercetin and vitamin C on superoxide anion radicals scavenging
Scavenging effects of quercetin on ·OH
The scavenging effects of quercetin on ·OH were increased first, then decreased with the increasing quercetin (P<0.01), and the clearance rate was maximized at 300 mg • L-1(61.79%), IC50was 218.40 mg • L-1;while the scavenging effects of vitamin C on ·OH were increased significantly with the increasing concentrations (P<0.01); however, the scavenging effects of vitamin C on free radicals were not significant at 400 and 500 mg • L-1(P>0.05), IC50was 226.20 mg • L-1(Table 3). According to IC50, the scavenging effects of quercetin on ·OH were stronger than those of vitamin C at low concentrations; however, the scavenging effects of quercetin at high concentrations were less than those of vitamin C.
Scavenging effects of quercetin on DPPH
The scavenging effects of quercetin on DPPH were enhanced with the increasing quercetin, and the scavenging effects of quercetin on DPPH were significant at 2-8 and 16-32 mg • L-1(P<0.01), IC50was 1.47 mg • L-1; the scavenging effects of vitamin C on DPPH were significantly enhanced with the increasing concentrations (P<0.01); however, the scavenging effects of vitamin C on DPPH were not significant at 16 and 32 mg • L-1(P>0.05), IC50was 3.10 mg • L-1,the maximum clearance rate was 95.53% (Table 4).According to IC50, the scavenging effects of quercetin on DPPH were greater than those of vitamin C at low concentration; however, the scavenging effects of quercetin at high concentration were less than those of vitamin C.
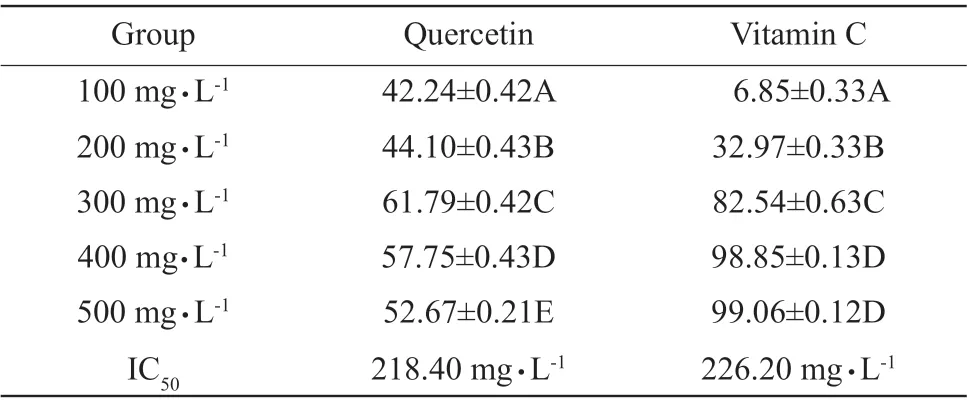
Table 3 Effects of quercetin and vitamin C on hydroxyl free radical scavenging
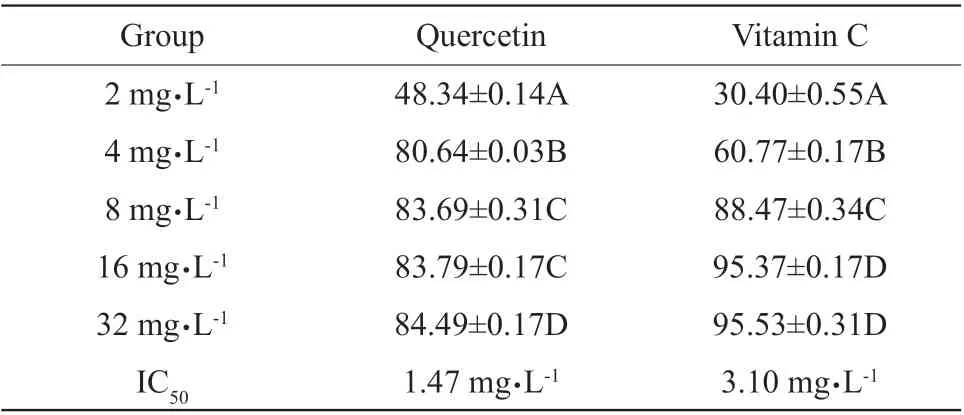
Table 4 Effects of quercetin and vitamin C on DPPH scavenging
Inhibition of quercetin on vitellin peroxidation
The inhibitory effects of quercetin on egg yolk lipoprotein peroxidation were significantly increased with the increasing quercetin (P<0.01), the maximum inhibitory effect of quercetin on vitellin peroxidation was maintained about 90.85% when the concentration of quercetin was 500 mg • L-1, and IC50was 11.89 mg • L-1;the inhibitory effects of vitamin C on egg yolk lipoprotein peroxidation were also increased with the increasing vitamin C, and the clearance rate was maximized at 5 000 mg • L-1(85.38%) (Table 5 a, b).However, according to the quercetin concentration and IC50, quercetin was stronger in inhibiting egg yolk lipoprotein peroxidation than vitamin C.
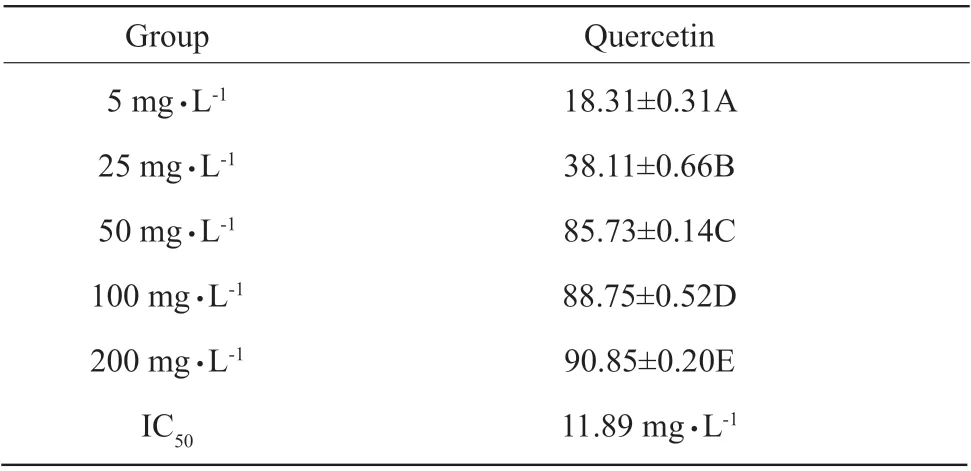
Table 5 a Inhibition of quercetin on peroxidation of lipoprotein in egg yolk

Table 5 b Inhibitory rate of vitamin C on peroxidation of lipoprotein in egg yolk
Inhibitory effects of quercetin on erythrocyte hemolysis
The inhibitory effects of quercetin on erythrocyte hemolysis induced by H2O2at 0.05-1.25 mg • L-1were significantly increased with the increasing quercetin(P<0.01). The inhibitory effects of quercetin on erythrocyte hemolysis remained unchanged (P>0.05)when the quercetin concentration was increased from 1.25 mg • L-1to 31.25 mg • L-1; however, the inhibitory effects tended to decrease (P=0.07) when the quercetin was too high, and IC50was 0.16 mg • L-1; the inhibitory effects of vitamin C on erythrocyte hemolysis were first increased, then decreased with the increasing concentration, in the process of increase and decrease,IC50was 9.21 and 127.60 mg • L-1, respectively.According to quercetin concentration and IC50, the inhibition effects of quercetin on erythrocyte hemolysis were greater than those of vitamin C (Table 6 a, b).

Table 6 a Inhibition of quercetin on erythrocyte hemolysis
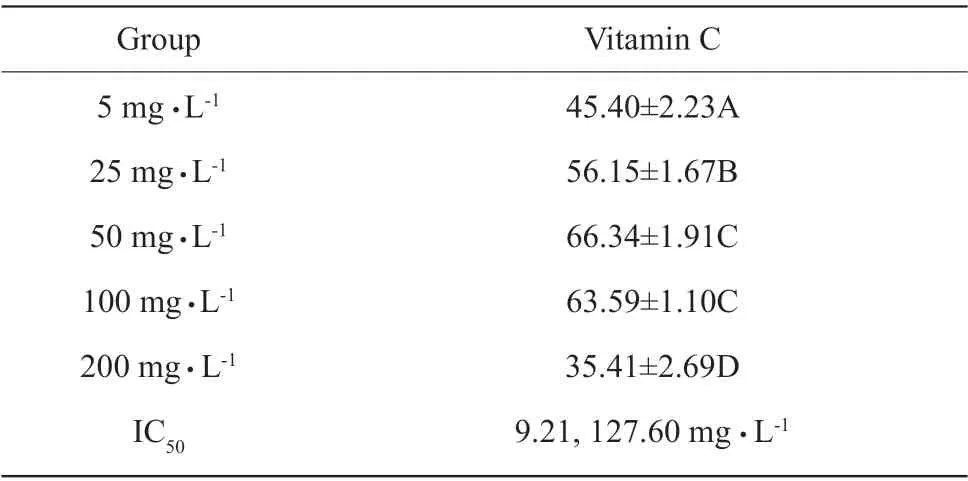
Table 6 b Inhibition of vitamin C on erythrocyte hemolysis
Inhibitory effects of quercetin on mitochondrial expansion
The inhibitory effects of quercetin on mitochondrial expansion induced by VC-Fe2+were gradually increased with the increasing quercetin (Fig. 1); the absorbance value of the normal group was significantly higher than that of the experimental groups and the model group at 60 min (P<0.01), and absorbance values of the experimental groups were significantly higher than those of the model group (P<0.01); the inhibitory effects of quercetin on mitochondrial expansion were gradually enhanced with increasing quercetin in the experimental groups, and the absorbance value of 10.0 mg • L-1quercetin group was significantly higher than that of 1.0 and 2.5 mg • L-1quercetin groups (P<0.05)(Table 7). Therefore, quercetin inhibited VC-Fe2+-induced ·OH damage in mitochondria.
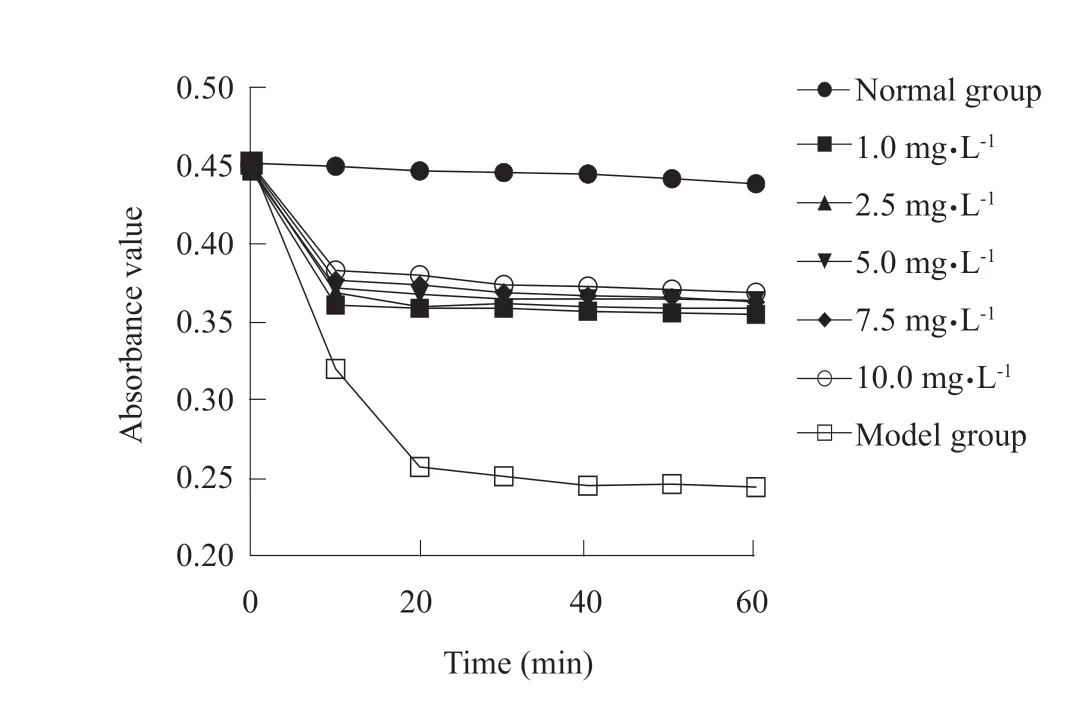
Fig. 1 Inhibition of quercetin on mitochondrial expansion
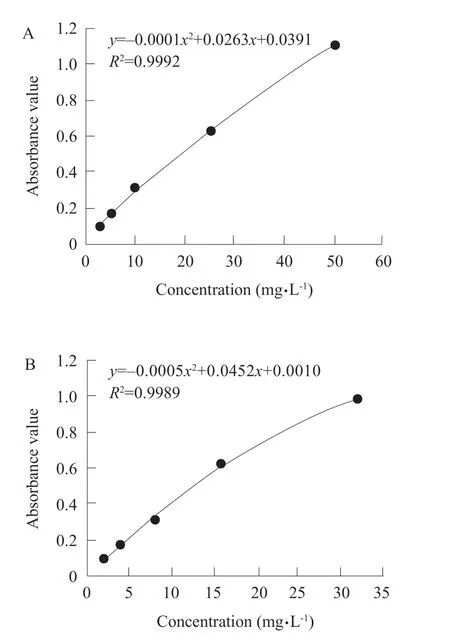
Fig. 2 The total reducing power of quercetin (A) and vitamin C (B)
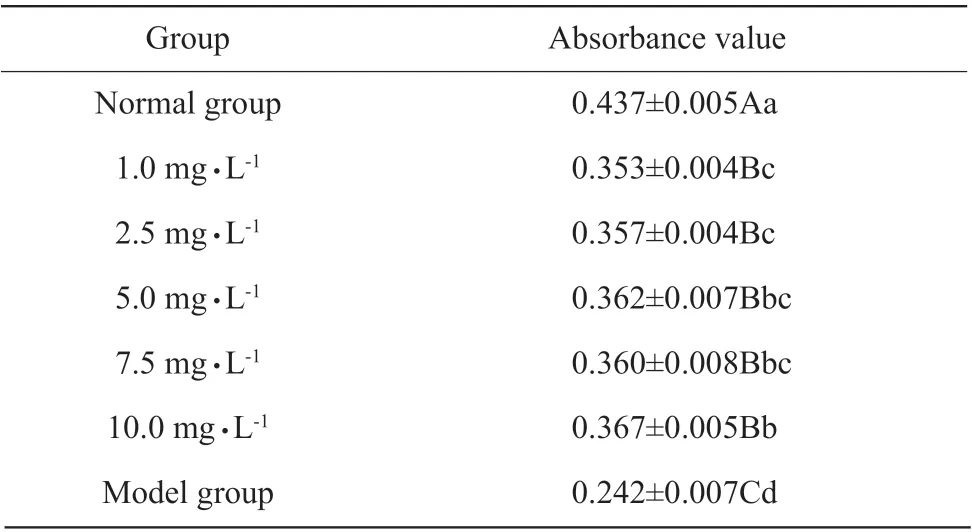
Table 7 Inhibition of quercetin on mitochondrial expansion at 60 min
The total reducing power of quercetin
The total reducing power of quercetin (the greater the absorbance value, the greater the reducing power) was increased with the increasing quercetin (P<0.01). The relationship between calculated concentration and reducing power wasy=–0.0001x2+0.0263x+0.0391,R2=0.9992. The total reducing power of vitamin C was also increased with the increasing vitamin C (P<0.01).The relationship between calculated concentration and reducing power wasy=–0.0005x2+0.0452x+0.0010,R2=0.9989, according to the regression equation, the total reducing power of quercetin was smaller than that of vitamin C (Fig. 2A, B).
Effects of quercetin on antioxidant activity in broilers
Quercetin supplementation for 42 days improved the broilers' antioxidant capacity (Table 8). In livers of broilers, compared with the control, quercetin supplementation significantly decreased content of MDA (P<0.01), the best results were obtained at 0.6 g quercetin per kg diet. SOD activity in 0.4 g quercetin per kg diet group was higher than that of the broilers without quercetin (P<0.05). GSH-Px and CAT activities in 0.6 g quercetin per kg diet group were significantly decreased comparing to those of the control group (P<0.01). Moreover, 0.4 and 0.6 g quercetin per kg diet were more effective than 0.2 g quercetin per kg diet. Furthermore, linear and quadratic effects were observed on CAT activity and NO content of livers as the increasing quercetin. In addition, quadratic effects were observed on SOD contents of livers.
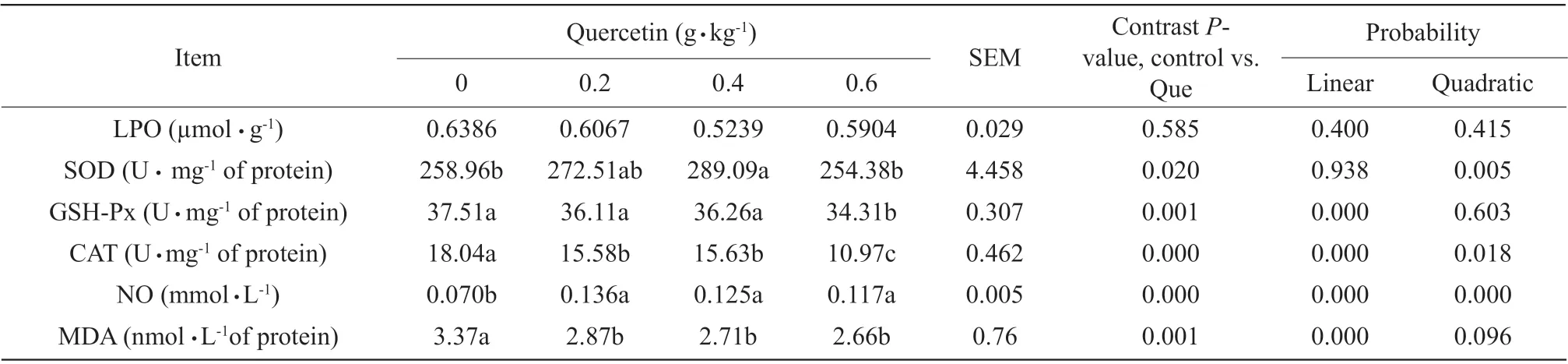
Table 8 Lipid peroxidation contents and antioxidant enzyme activities in livers of AA broilers supplemented with and without quercetin
Discussion
Oxidative stress is mainly caused by excessive free radicals, thereby it is of great significance to study the scavenging effects of antioxidants on free radicals.Many studies have shown that flavonoids have a strong scavenging effect on free radicals due to their special chemical structures. The cranberry flavonoids efficiently scavenged NO, • OH and O2-, as well as reduced DPPH radicalsin vitro(Lapshinaet al., 2015).The flavonoids inAstragalus mongolicushad a certain scavenging effect on O2-and were the main effective components ofAstragalus membranaceusantioxidant effects (Bian and Li, 2008). Seabuckthorn flavonoids scavenged • OH and O2-, and the scavenging effects were increased with the increasing flavonoids, and the scavenging effects of flavonoids on ·OH were stronger than those on O2-(Liuet al., 2006; Zhanget al., 2011).In this experiment, vitamin C was used as a controlin vitro, quercetin significantly scavenged O2-, • OH and DPPH. At the same concentration, the scavenging rate of quercetin was significantly less than that of vitamin C,and the scavenging effects on O2-were first increased and then decreased. In addition, the present study showed that quercetin also significantly scavenged• OH, and the scavenging effects were first increased and then decreased with the increasing quercetin with the maximum scavenging rate of 61.79%. It suggested that the antioxidation of quercetin was related to the concentrations. Quercetin strongly scavenged • OH at low concentrations (<300 mg • L-1) comparing to vitamin C; however, the scavenging effects of quercetin on • OH were less than those of vitamin C when the quercetin was more than 300 mg • L-1, thus quercetin had stronger scavenging effects on • OH at lower concentrations. In addition, the present study showed that quercetin significantly scavenged DPPH, and the scavenging effects on DPPH at low concentrations were significantly higher than those of vitamin C; however,the scavenging effects on DPPH were lower than those of vitamin C with the increasing quercetin, compared to the same concentration of vitamin C. According to IC50, the scavenging effects of quercetin on these three free radicals were DPPH >• Oh >O2-, which might be related to the chemical structures and the external conditions of the reaction system.
Red blood cells mainly have the function of transporting oxygen, and often are put in an oxygen rich environment, where the reactive oxygen species(ROS) contents are high, which easily induce lipid peroxidation and damages the lipid structures of red blood cell membranes. And red blood cells are rich in ferritin, which promotes ROS to induce lipid peroxidation, therefore, red blood cells are highly susceptible to oxidative damage, thus hemolyzed. Red blood cell hemolysis had been used for evaluating the antioxidant properties of compounds (Yanget al., 2017; Chenet al., 2018; Durantiet al., 2018).Mitochondria consumed 95%-98% oxygen during oxidation, were also the main production sites of oxygen free radicals. If the oxygen free radicals generated could not be metabolized in time, they would damage proteins, membrane lipids, DNA and other substances in mitochondria, thus disrupted the mitochondrial structure and function (Kowalskaet al.,2020). Therefore, it is of great significance to study the protective effects of antioxidants on erythrocytes and mitochondria. Some flavones significantly inhibited lipid peroxidation of cell membranes and then reduced erythrocyte hemolysis using absorption and fluorescence spectroscopy studies (Chaudhuriet al.,2007). Both persimmon leaf flavone and forsythia leaf flavone reduced erythrocyte hemolysis and hepatic mitochondrial expansion using spectrophotometry(Yanget al., 2002; Yanget al., 2007). The present studyin vitroshowed that quercetin inhibited H2O2-induced erythrocyte hemolysis, and the degree of inhibition was increased with the increasing quercetin,the inhibitory effects showed a downward trend when quercetin reached 31.25 mg • L-1(P=0.07), it suggested that appropriate quercetin exhibited antioxidation;however, the excess quercetin increased the release of free radicals. Meanwhile, VC-Fe2+was used to accelerate the lipid peroxidation of unsaturated fatty acids in mitochondrial membrane, which increased the membrane permeability, accelerated material exchange inside and outside the membrane, and finally led to the mitochondrial expansion. When mitochondria underwent expansion at 520 nm, their turbidity decreased. Clearly, the absorbance value was basically unchanged in the normal group, while was the largest decrease in the model group, and showed a corresponding attenuation in the extent of the decrease in the quercetin treatment, it indicated that quercetin inhibited lipid peroxidation while also reduced the mitochondrial expansion.
MDA is the main by-product of reactive oxygen species-induced oxidative stress and also the main marker of oxidative stress. LPO may be judged by measuring MDA content, thereby reflect the degree of oxidative damage of the body. Flavonoids may block LPO and inhibit the synthesis of MDA.Forsythiaflavonoids inhibited LPO in liver, kidney,heart, spleen, liver mitochondria and microsomes of mice, and protected the integrity of cell membrane(Yanget al., 2007). Low contents of flavonoids such as baicalein, oroxylin A and wogonin significantly inhibited lipid peroxidation, among which baicalein inhibited LPO more actively than other extracts(Liauet al., 2019). The total flavonoids ofTrifolium rubraexhibited strong antioxidation and significantly inhibited LPOin vitroat 4-32 mg • L-1(Liuet al.,2009). The present results showed that quercetin significantly inhibited vitellin peroxidation, and the inhibition rate was increased with the increasing quercetin, and the effects were better than vitamin C, which was consistent with the results of previous studies on other flavonoids. Considering the mechanism of antioxidant action, reducing power was also a reliable index for antioxidant capacity (Yildirimet al., 2001; Gulcin, 2003). Reducing power reflected the ability of antioxidants to give electrons. Free radicals trap electrons were released by antioxidants to form stable structures. Therefore, the stronger the ability of antioxidants to give electrons (reducing power), the stronger their scavenging free radicals.The extract from Acacia seed contained a large number of flavonoids, and had strong reducibility (Palet al., 2009). Both flavones from mulberry bark and buckwheat bark had certain reducibility (Wang and Ren, 2008; Zhou and Zhou, 2008). The present results showed that reducing effects of quercetin were similar to vitamin C, according to regression equation. This further confirmed reducing effects of quercetin, just like other flavonoids.
MDA and NO may be used as indexes to evaluate antioxidant capacity. MDA was the product of LPO,which might damage cells at enzyme, protein and DNA levels, consequently caused cell death (Ayalaet al., 2014). Supplementation of 0.5 and 1.0 g quercetin per kg diet linearly decreased contents of MDA during storage of meat (Goliomytiset al., 2014).Similarly, MDA levels were decreased in dosedependent manner when soybean isoflavone and alfalfa flavonoids were supplemented in broiler's diets (Jianget al., 2007; Ouyanget al., 2016). Oxidative stress was also evaluated by detecting contents of NO in serum and organs. NO was considered as pro-inflammatory mediator due to over-production (Kristeen-Teoet al.,2017). Reactive nitrogen species (RNS) and ROS resulted from excess production of NO and LPO played crucial role in infection and disease (Subbaiahet al., 2013). Increased production of ROS and NO was linked to increased oxidative carbonylation of mitochondrial proteins (Schildet al., 2003). Under conditions of oxidative stress, flavonoids protected NO from superoxide-driven inactivation (Duarteet al.,2014). Quercetin treatment significantly decreased the elevated NO and MDA in STZ-induced diabetic rats(Adewoleet al., 2006). Likewise, white mini broilers supplemented with 3 to 5 g • kg-1of onion (about 19.93 mg of quercetin per 100 g) significantly decreased contents of serum MDA and NO levels (Anet al.,2015). The results of this experiment were consistent with those previous studies.
Oxidative stress was regarded as an imbalance between the generation of oxidants and antioxidants,oxidants were in a dominant position (Luoet al.,2020). This imbalance or loss of cellular redox homeostasis damaged essential biomolecules, such as carbohydrates, proteins, amino acids, lipids and nucleic acids (Ardestani and Yazdanparast, 2006; Sharififaret al., 2006). As a result, generation of free radicals was increased, which increased lipid peroxidation,thus increased MDA levels in blood and tissues (Sevenet al., 2009). Lipid peroxidation might maintain and enhance the cell membrane integrity and fluidity,which adversely influenced the immune responses(Woodet al., 2003). Antioxidant molecules from plant origin resisted oxidative stress by scavenging free radicals and inhibiting lipid peroxidation (Karouet al.,2005). Flavonoids were ubiquitously present in plant kingdoms, moreover, the valuable effects of flavonoids had been indorsed to their antioxidant and antiinflammatory properties (Kimet al., 2004; Zhuanget al., 2019; Yinget al., 2020). Their planar structure,number and position of hydroxyl groups were essential for free radical scavenging activities (Procházkováet al., 2011). Antioxidant enzymes including SOD,GSH-Px and CAT were important components of the antioxidant defense mechanism in animals. The SOD catalyzed the dismutation of superoxide anion to hydrogen peroxide and inhibited the production of free radicals. Catalase converted H2O2into H2O. In the present experiment, administration of quercetin decreased contents of LPO in livers of broilers, which was in line with the study of Goliomytiset al. (2014),which supplementation of quercetin from 0.5 to 1.0 g • kg-1diet linearly decreased MDA levels during storage of meat. MDA levels were also decreased in dose-dependent manner when soybean isoflavone and alfalfa flavonoids were added in broilers' diet (Jianget al., 2007; Ouyanget al., 2016). Grape pomace provided a rich source of polyphenols and strong antioxidation, the broiler chicks diet supplemented with grape pomace (15 to 60 g • kg-1feed) linearly decreased MDA levels in refrigerated breast meat(Breneset al., 2008). Correspondingly, fruit pomaces significantly decreased MDA concentrations in raw,frozen and cooked turkey meat (Juskiewiczet al.,2017). In this study, SOD activity was significantly increased in livers of broilers with increasing quercetin. Similar results were obtained by Liuet al.(2014), who found that SOD was increased in livers of laying hens supplemented with 0.2 to 0.6 g quercetin per kg diet. Furthermore, SOD was increased in serum and breast fillets of broilers fed by diet with soybean isoflavone and bioflavonoids (Jianget al., 2007;Kamboh and Zhu, 2013). Serum SOD was increased in broilers fed by diets with alfalfa flavonoids supplementation (Kristeen-Teoet al., 2017). GSH-Px and CAT were significantly decreased in the present study. On the other hand, supplementation of quercetin had no effect on livers GSH-Px and CAT activity (Liet al., 2015). Walnut polyphenol extract administration significantly inhibited oxidative damage according to ·OH, SOD, GSH-Px and MDA levels in murine splenic lymphocytes (Yanget al., 2016). Secondary metabolites were found in above-mentioned flavonoids and polyphenols prevented oxidative injury by modulating gene expression of antioxidant enzyme systems (Puiggròset al., 2005). The results were not in agreement with the findings of Jianget al.(2007), and Ouyanget al. (2016), who reported GSHPx and CAT activity was increased with flavonoids supplementation, whereas MDA was not affected. It probably resulted from species of animals, dose of flavonoids, feeding management, etc. In addition,in previous studies, it showed that a certain dose of quercetin had no effect on SOD activity in the livers of AA broilers; however, 0.02% quercetin decreased the levels of CAT (Yinget al., 2020). Whereas in another experiment, SOD activity in the livers of laying hens was enhanced (Sunet al., 2014). These experimental results had certain agreement with our results.
Conclusions
Quercetin exhibited antioxidation through scavenging free radical and high total reducing powerin vitroand decreasing contents of MDA and NO in livers of broilers, thus protected biomacromolecules from free radical damage and health. The results of this study provided a theoretical basis for application of quercetin as natural antioxidation in livestock production.
Data AvailabilityThe data used to support the findings of this study are available from the corresponding author upon request.
Disclosure
The manuscript is based on the thesis of Dr. Maria Tabassum Chaudhry and Master Dantong Sun.
Conflicts of InterestThe authors of this paper declared that they have no conflicts of interest.
杂志排行
Journal of Northeast Agricultural University(English Edition)的其它文章
- Study on Antibacterial Effects of Purple, Yellow and White-skinned Onions
- Effects of Different Ventilation Modes and Outlet Height on Nursery Piggery Environment
- Measurement of Grain Production Efficiency in Main Grain-producing Areas and Analysis of Inter-provincial Differences
—— A Study Based on Super-SBM Model and Malmquist Index - Effects of Planting Density and Cutting Time on Hay Yield and Nutritional Value of Forage Soybean HN389
- Effects of Encapsulated Enzyme and Yeast Products on in Vitro Rumen Fermentation
- Study on Exogenous Ethylene Induced Rice Resistance to Rhizoctonia solani
