A comparative study on the reactivity of cationic niobium clusters with nitrogen and oxygen
2022-06-18BenenHuangMengzhouYangXinLeiWenGanZhixunLuo
Benen Huang, Mengzhou Yang, Xin Lei, Wen Gan, Zhixun Luo
a Beijing National Laboratory for Molecular Sciences (BNLMS), State Key Laboratory for Structural Chemistry of Unstable and Stable Species, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, China
b University of Chinese Academy of Sciences, Beijing 100049, China
ABSTRACT We have prepared well-resolved (n = 1–10) clusters and report here an in-depth study on the essentially different reactivity with N2 and O2, by utilizing a multiple-ion laminar flow tube reactor in tandem with a customized triple quadrupole mass spectrometer (MIFT-TQMS).As results, the clusters are found to readily react with N2 and form adsorption products Nbn; in contrast, the reactions with O2 give rise to Nbnproducts, and the odd-oxygen products indicate O-O bond dissociation, as well as increased mass abundance of NbO+ pertaining to oxygen-etching reactions.We illustrate how N2 prefers a physical adsorption on clusters with an end-on orientation for all the products, and allow for size-selective clusters to act as electron donor or acceptor in forming Nbn.In contrast to these nitrides, the dioxides Nbn display much larger binding energies, with O2 always as an electron acceptor,corresponding to superoxide or peroxide states in the initial reactions.Density-of-states and orbital analyses show that the interactions between and O2 are dominated by strong π-backdonation indicative of incidental electron transfer; whereas weak π-backdonation and simultaneous σ donation interactions exist in Nbn.Further, reaction dynamics analysis illustrates the different interactions for N2 and O2 in approaching the clusters, showing the energy diagrams for N2 adsorption and O-O bond dissociation in producing odd-oxygen products.Fragment analyses with orbital correlation and donor-acceptor charge transfer are also performed, giving rise to full insights into the reactivities and interactions of such transition metal clusters with typical diatomic molecules.
Keywords:Gas phase reaction Nb cluster Chemical adsorption Orbital analysis Energy decomposition analysis
Gas adsorption is one of the most basic interfacial phenomena and closely related to diverse applications in industrial and agricultural production and daily life.People may take it for granted that the adsorption of gas molecules on the metals is simply divided into physical adsorption and chemical adsorption; however,the underlying mechanism that determines the diverse interactions and surface reactivity at reduced sizes are illusive to be fully understood.The study of gas adsorption on solid surfaces is helpful to understand the micro-mechanism of various physicochemical processes; also, adsorption of gas molecules on nanometals is a key step in catalytic processes and important for various gas phase reactions.While physisorption is often dominated by molecular vander Waals force and generally emits rare energies, chemisorption can be largely exothermic and may involve covalent interactions and stepwise reactions [1].In this regard, molecular adsorption can be associated with three processes,i.e., inactivated adsorption, activated adsorption and precursor-mediated adsorptions [2].The adsorbate may reach a chemisorption state through a barrierless process directly from physisorption state; or it needs to overcome a potential barrier simply by heating or other reaction conditions; or undergoes a precursor-mediated adsorption, where the chemisorbed state could be attained through a precursor intermediate.
On this basis, the reactivities of transition metal clusters with small molecules have been extensively studied, illustrating the distinction of physical and chemical adsorptions on surfaces [2].Also,the interaction mechanisms and properties of metal cluster complexes have been widely analyzed by joint experimental and theoretical studies.Among them, niobium clusters have been an important research object unveiling size-dependent chemical and physical properties of transition metals at reduced sizes [3–10].Combining mass spectrometry and gas flow reactors [11,12], the reactivities of niobium clusters with many chemicals have been studied, such as H2[12–15], O2[16,17], N2[18,19], COx[20–22], NOx[23,24] and hydrocarbons [25–29].The reactions of Nb clusters with N2and H2(/D2) have similar adsorptive patterns; while the reaction with O2shows a difference due to the differences of atomic valence configuration and bonding nature of these diatomic molecules.In general, N2is inert in most reactions due to a very high dissociation energy (945 kcal/mol) of the N≡N triple bond and a high HOMO-LUMO gap (~10.82 eV); in comparison, groundstate O2is in triplet with two spin single electrons pertaining to its high activity [30].However, it is not fully unveiled how the stability of such metal clusters and atomic valence configuration and bonding nature cooperatively determine the experimental observation on their reactivity.
On the other hand, previously published studies have also estimated the reaction rate constants for niobium clusters with N2and O2showing a likely difference of three orders of magnitude[18,19].Further studies reported the similar adsorption of N2on niobium clusters [18,19,31], and there are adsorption products of polynitrogen molecules depending on the pressure and concentration of the reaction gas at room temperature [32].For example,Pillaiet al.[33] generated Nb+(N2)ncomplexes (n= 3–16) with nitrogen as buffer gas and investigated the vibration in forming such complexes by infrared photodissociation spectroscopy (IRPD),showing a preferred coordination of six ligands.Meanwhile, the reactions of small(n= 1–3) with O2have been studied by Lohet al.[16] who measured the cross section as a function of kinetic energy and proposed dissociation pathways and threshold values.It is anticipated that these experimental cluster physics studies of such small Nb clusters can be connected with theorical energy calculations in determining the thermodynamics and their reaction dynamics in gas phase [34].
On these bases, here we have performed a further in-depth study of the thermalized gas-phase(n= 1–10) clusters in reacting with sufficient N2and O2gas in a customized multiple ions laminar flow tube in tandem with triple quadrupole mass spectrometer (MIFT-TQMS).It is found that theclusters, produced by the home-made magnetron sputtering (MagS) source, readily react with both N2and O2, but form different series of products respectively, seen as(m= 1–3) and(x= 1–4).Theoretical calculations results reveal that theclusters all adopt end-on orientation adsorption for N2onwith much smaller binding energy than that of correlativeseries which correspond to strong hollow-site adsorption allowing for occasional dissociative adsorption.Further, we conducted a comprehensive study thermodynamic energetics, HOMO-LUMO gaps, charge transfer, Wiberg bond index (WBI), density of state (DOS), natural atomic orbital (NAO) and energy decomposition analyses based on natural orbitals for chemical valence (EDA-NOCV).In addition, we carried out potential scan for N2and O2in approaching ancluster until the formation ofand Nb3illustrating how cluster-molecule interactions initiate their reaction.Finally, we explore the reaction pathways of both N2and O2withas a representative, demonstrate the N2-adsorption behavior and the reaction dynamics in producing Nb3from O2-adsorption, to dissociative intermediate, and to ultimately exhaust of NbO (/NbO+).
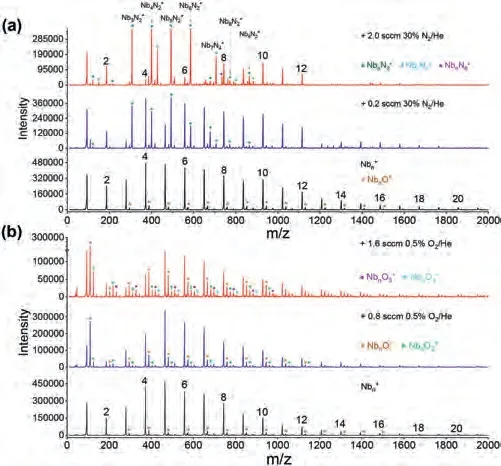
Fig.1.The mass spectra ofclusters in the absence and presence of (a) 30%N2/He and (b) 0.5% O2/He, respectively.The weak mass peaks marked with stars(*) in the nascent cluster distributions correspond to trace amount of oxygen contamination.
The experiments in this study are conducted on our customized multi-ions laminar flow tube reactor in tandem with a triple quadrupole mass spectrometer (MIFT-TQMS).A self-designed MagS source with a DC power supply of 5 kW was used to obtain the clean mass distributions of small niobium clusters [35].A niobium disk (99.95% purity, 50.8 mm diameter, 4 mm thickness) was used as the sputtering target.High-purity helium (>99.999%) and highpurity argon (>99.995%) were used as carrier gas and sputtering work gas, introduced from the rare of the MagS source and the magnetron head respectively, with the gas flow rates controlled by two mass flowmeters (Alicat, a range of 0–100 sccm and 0–100 slm, respectively).Typical parameters for the MagS source to produce the niobium clusters withn= 1–10 are: 170 V DC voltage,4 A current, a background pressure of source chamber at ~7 Torr,He carrier gas at ~12 slm helium, while Argon is 60–120 sccm.Different concentrations of N2and O2(ca., 0.5%–30% in He) are injected into the flow tube (60 mm diameter, 1 m long) which is maintained at 0.9 Torr pressure for stable laminar flow and suffi-cient collisional reaction.The detailed values for these parameters may differ with dependence on the target situation and vacuum status,etc.
All the DFT calculations were performed using Gaussian 09 software package [36].B3LYP exchange-correlation functional was used to optimize the geometric structures of all the niobium clusters and products, the basis sets of Lanl2dz was used for Nb with an extradpolarization function, while 6–311G(d) for N and O atoms [36,37].Vibrational frequency calculations were performed for each of the optimized structures and zero-point vibrations were corrected for all the energy calculations.Thermodynamic data,Wiberg bond index (WBI), partial density of states (PDOS), natural atomic orbital (NAO) and electron configuration are analyzed by using the Multiwfn software [38].The geometric structures,frontier orbitals, charge distributions by natural population analysis (NPA), and the PDOS patterns of the, Nbn(n= 1–10) are plotted by visual molecular dynamics (VMD) [39].Energy decomposition analysis was conducted based on natural orbitals for chemical valence (EDA-NOCV), calculated with Amsterdam Density Functional (ADF) program [40,41] at the B3LYP/TZP level of theory.

Table 1DFT-calculated energies (eV) for the likely diverse reaction pathways of (n = 1–10) with O2.All the data are calculated at the B3LYP/Lanl2dz level for and B3LYP/6-311G(d) level for N/O.
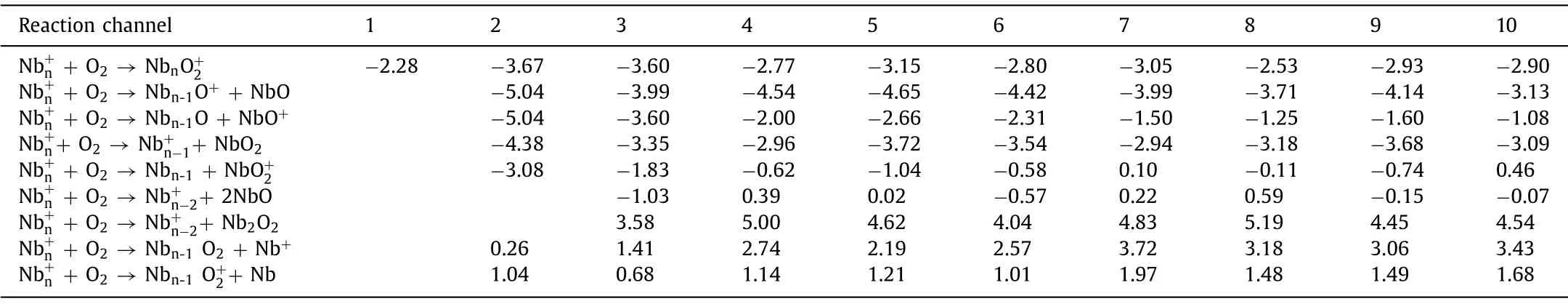
Table 1DFT-calculated energies (eV) for the likely diverse reaction pathways of (n = 1–10) with O2.All the data are calculated at the B3LYP/Lanl2dz level for and B3LYP/6-311G(d) level for N/O.
Reaction channel 1 2 3 4 5 6 7 8 9 10 Nb+n + O2 →NbnO+2-2.28 -3.67 -3.60 -2.77 -3.15 -2.80 -3.05 -2.53 -2.93 -2.90 Nb+n + O2 →Nbn-1O+ + NbO -5.04 -3.99 -4.54 -4.65 -4.42 -3.99 -3.71 -4.14 -3.13 Nb+n + O2 →Nbn-1O + NbO+ -5.04 -3.60 -2.00 -2.66 -2.31 -1.50 -1.25 -1.60 -1.08 Nb+n+ O2 →Nb+n-1+ NbO2 -4.38 -3.35 -2.96 -3.72 -3.54 -2.94 -3.18 -3.68 -3.09 Nb+n + O2 →Nbn-1 + NbO+2-3.08 -1.83 -0.62 -1.04 -0.58 0.10 -0.11 -0.74 0.46 Nb+n + O2 →Nb+n-2+ 2NbO -1.03 0.39 0.02 -0.57 0.22 0.59 -0.15 -0.07 Nb+n + O2 →Nb+n-2+ Nb2O2 3.58 5.00 4.62 4.04 4.83 5.19 4.45 4.54 Nb+n + O2 →Nbn-1 O2 + Nb+ 0.26 1.41 2.74 2.19 2.57 3.72 3.18 3.06 3.43 Nb+n + O2 →Nbn-1 O+2+ Nb 1.04 0.68 1.14 1.21 1.01 1.97 1.48 1.49 1.68
There is different case.As shown in Fig.1b, the(n= 1–10) clusters are highly reactive with O2even at a very low concentration (e.g., 0.8 sccm of 0.5% O2in He gas).Even in the presence of a small amount of oxygen, the dissociative products NbnO+are observed.This is reasonable considering the large Nb-O bond energy up to ~770 kJ/mol which is even larger than the O-O bond strength (~400 kJ/mol); and electron transfer readily occur between niobium and oxygen [43,44], with a variety of exothermic reaction channels as given in Table 1.Among the thermodynamically favorable channels, the O2-dissociaed equation to produce a NbO neutral suggests the maximal energy release for all the(n= 2–10) clusters.This could well explain the experimental observation of dissociative products NbnO+which was also found in previous experimental study [17].It is worth noting that,such a favorable reaction channel “+ O2→Nbn-1O++ NbO”largely differs from the previously determined reactions of[46] andDue to the large Nb-O bond energy, here the reaction channels with Nb0/+orremoval are much less favorable from the thermodynamics, which is well consistent with the experimental observation that Nb+anddo not display increased mass abundance in the varied reaction conditions.Also, this is consistent with the previous study by Radiet al.[17], where the dominant reaction pathway of massselected small niobium clusters with O2in a drift reactor was proposed to be the one in producing primary products of NbO and NbO+.
Utilizing DFT calculations we have provided further insights into the diverse reactivities of niobium clusters (for details of the lowest-energy structures, spin multiplicity, bond lengths, and point group see Fig.S6 in Supporting information).For the adsorption complexes, N2adopts an end-on adsorption orientation on theclusters of which the nascent structures and spin multiplicities almost do not change, except Nb2which shows aV-shape structure with a high spin multiplicity.In contract, Nbn(n >2) clusters all adopt hollow-site adsorption mode with elongated O-O bond length ranging from 1.2to 1.5, although the structures ofin Nbndo not show much changes.Moreover, the spin multiplicities of the odd-number small niobium clusters (i.e.,Nb2n+1) reduced in the formation of Nbn(except Nb9);whereas, for the even-number niobium clusters (i.e., Nb2n+), the spin of the cluster can be aligned opposite to that of the3O2molecule thus spin conservation in the Nb2nproducts [48,49].

Fig.2.(a) DFT-calculated HOMO-LUMO gaps of Nbn and Nb(n = 1–10).(b) Binding energy of Nbn and Nbn (c) NPA charge transfer of to N2 in Nbncompared with to O2 in Nbn All the data are calculated at the B3LYP/Lanl2dz level for and B3LYP/6-311G(d) level for N/O.
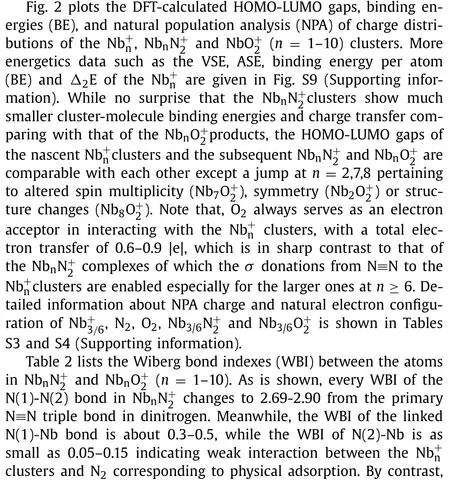
Table 2 The WBI analysis between the atoms in Nbnand Nbn(n = 1–10).
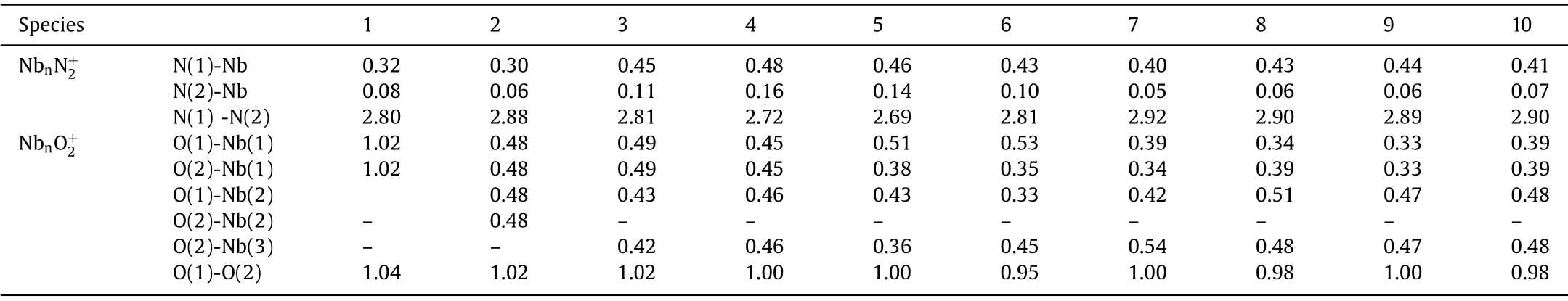
Table 2 The WBI analysis between the atoms in Nbnand Nbn(n = 1–10).
Species 1 2 3 4 5 6 7 8 9 10 NbnN+2N(1)-Nb 0.32 0.30 0.45 0.48 0.46 0.43 0.40 0.43 0.44 0.41 N(2)-Nb 0.08 0.06 0.11 0.16 0.14 0.10 0.05 0.06 0.06 0.07 N(1) -N(2) 2.80 2.88 2.81 2.72 2.69 2.81 2.92 2.90 2.89 2.90 NbnO+2 O(1)-Nb(1) 1.02 0.48 0.49 0.45 0.51 0.53 0.39 0.34 0.33 0.39 O(2)-Nb(1) 1.02 0.48 0.49 0.45 0.38 0.35 0.34 0.39 0.33 0.39 O(1)-Nb(2) 0.48 0.43 0.46 0.43 0.33 0.42 0.51 0.47 0.48 O(2)-Nb(2)–0.48––––––––O(2)-Nb(3)––0.420.460.360.450.540.480.470.48 O(1)-O(2) 1.04 1.02 1.02 1.00 1.00 0.95 1.00 0.98 1.00 0.98
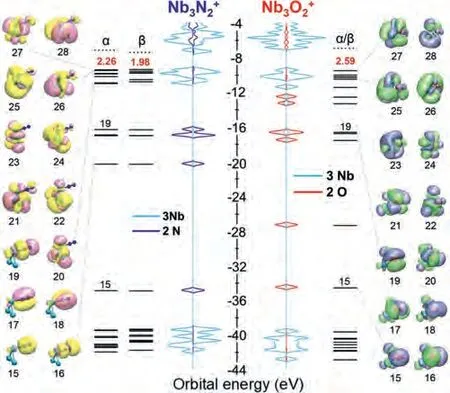
Fig.3.Energy level of and Nb3O+2 together with PDOS of 2 N atoms and 3 Nb atoms in Nb3 and PDOS of 2 O atoms and 3 Nb atoms in Nb3.Insets show the corresponding orbitals.
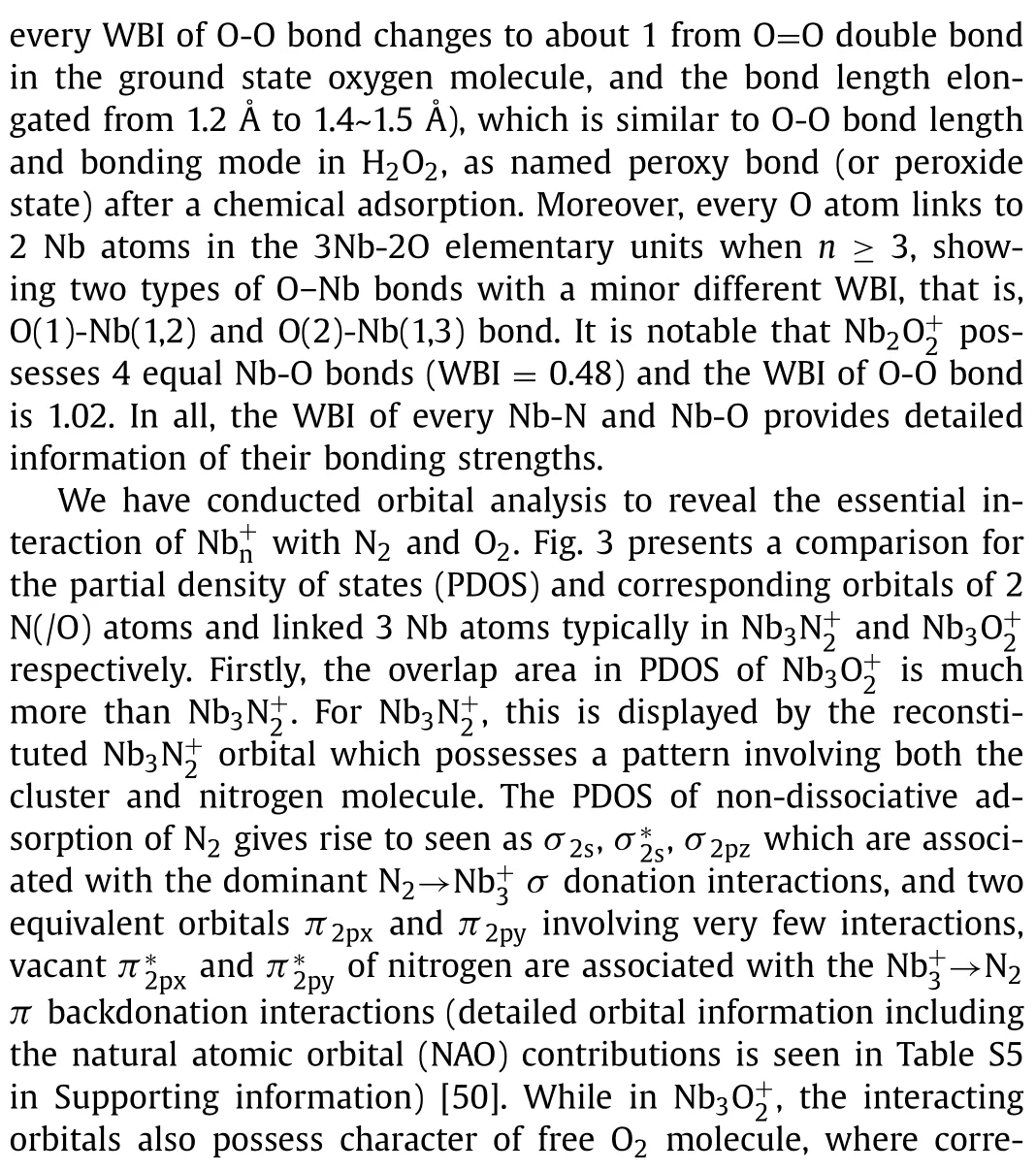

Table 3 EDA results for 3Nb3 and 1Nb3O+2 at the B3LYP/TZP level of theory using ADF, taking and N2 (/O2) as interacting fragments. ΔEint is the intrinsic interaction energies.ΔEpauli is the repulsion energy caused by the Pauli exclusion principle. ΔEelstst and ΔEorb are the attraction energies due to electrostatic and orbital interactions, respectively.The values in parentheses show the contribution to ΔEint and the total orbital interaction ΔEorb.

Table 3 EDA results for 3Nb3 and 1Nb3O+2 at the B3LYP/TZP level of theory using ADF, taking and N2 (/O2) as interacting fragments. ΔEint is the intrinsic interaction energies.ΔEpauli is the repulsion energy caused by the Pauli exclusion principle. ΔEelstst and ΔEorb are the attraction energies due to electrostatic and orbital interactions, respectively.The values in parentheses show the contribution to ΔEint and the total orbital interaction ΔEorb.
3Nb3N+2 1Nb3O+2 Energy term & assignment kcal/mol Energy term & assignment kcal/mol ΔEint -13.6 ΔEint -170.6 ΔEpauli 66.8 ΔEpauli 544.5 ΔEele -39.6 (49.2%) ΔEele -287.1 (40.2%)ΔEorb -40.9 (50.8%) ΔEorb -430.0 (59.8%)ΔEorb-α(1): σ donation + polarization -10.3 (25.1%) ΔEorb-α(1): π backdonation -177.8 (41.5%)ΔEorb-α(2): π backdonation -4.1 (10.1%) ΔEorb-α(2): π donation -20.0 (4.7%)ΔEorb-α(3): polarization + π backdonation -3.0 (7.3%) ΔEorb-α(3): π donation -8.3 (1.9%)ΔEorb-β(1): σ donation + polarization -9.5 (23.3%) ΔEorb-β(1): π backdonation -177.8 (41.5%)ΔEorb-β(2): π backdonation -5.8 (14.2%) ΔEorb-β(2): π donation -20.0 (4.7%)ΔEorb-β(3): π backdonation -3.2 (7.9%) ΔEorb-β(3): π donation -8.3 (1.9%)ΔEorb(4): rest -5.0 (12.2%) ΔEorb(4): rest -16.0 (3.7%)
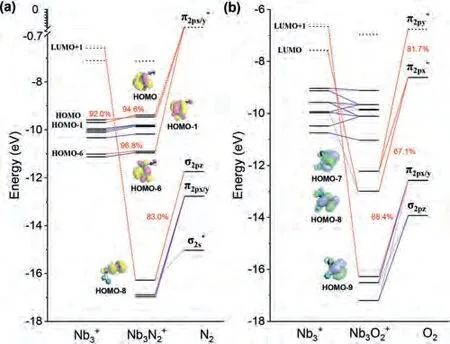
Fig.4.Kohn–Sham orbital correlation diagrams of (a) Nb3in the triplet state.(b)Nb3 in the stable triplet state.Blue lines are for orbitals composited by one part,and red lines are for orbitals interaction of two fragments with percentage which solid lines are for occupied orbitals and dotted line are for vacant orbitals.All the data are calculated at the B3LYP/TZP level of theory using ADF.
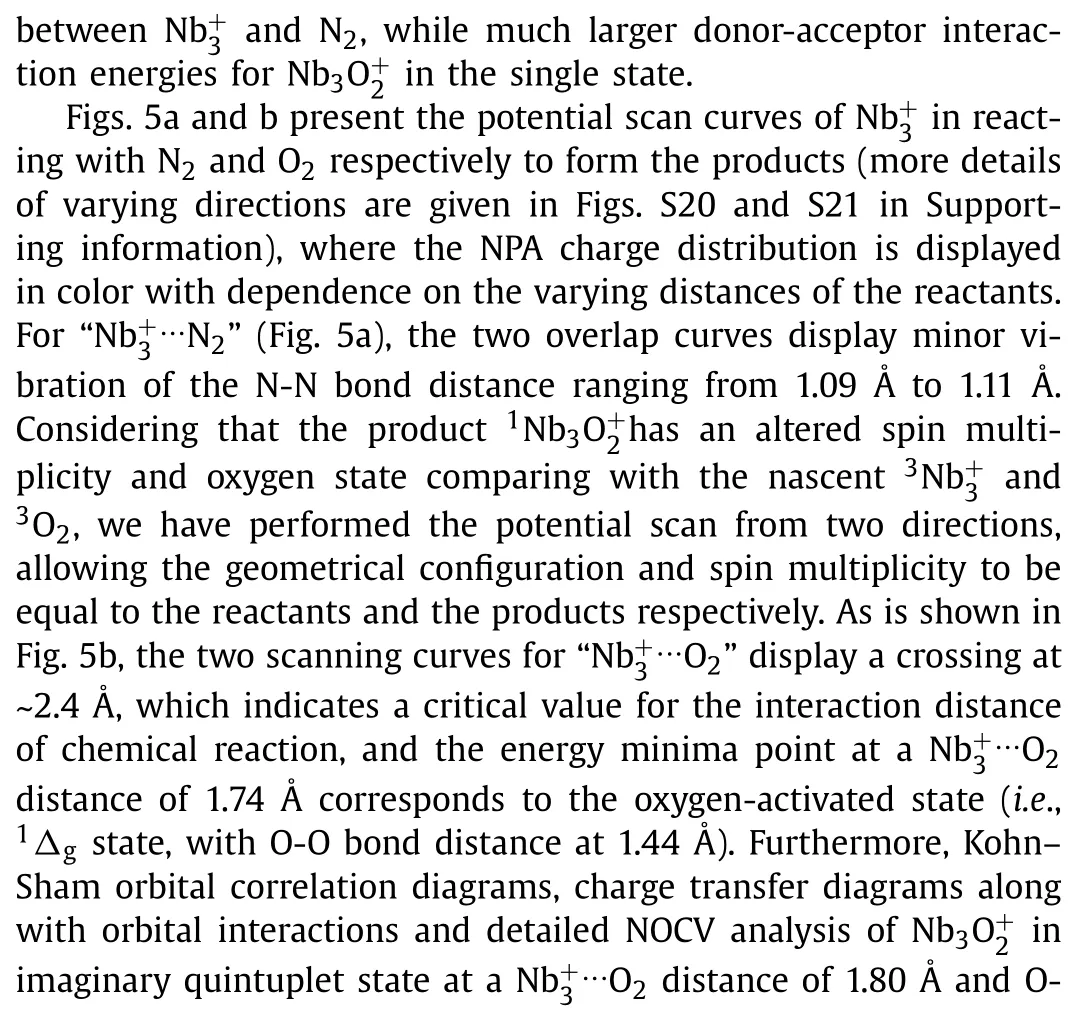
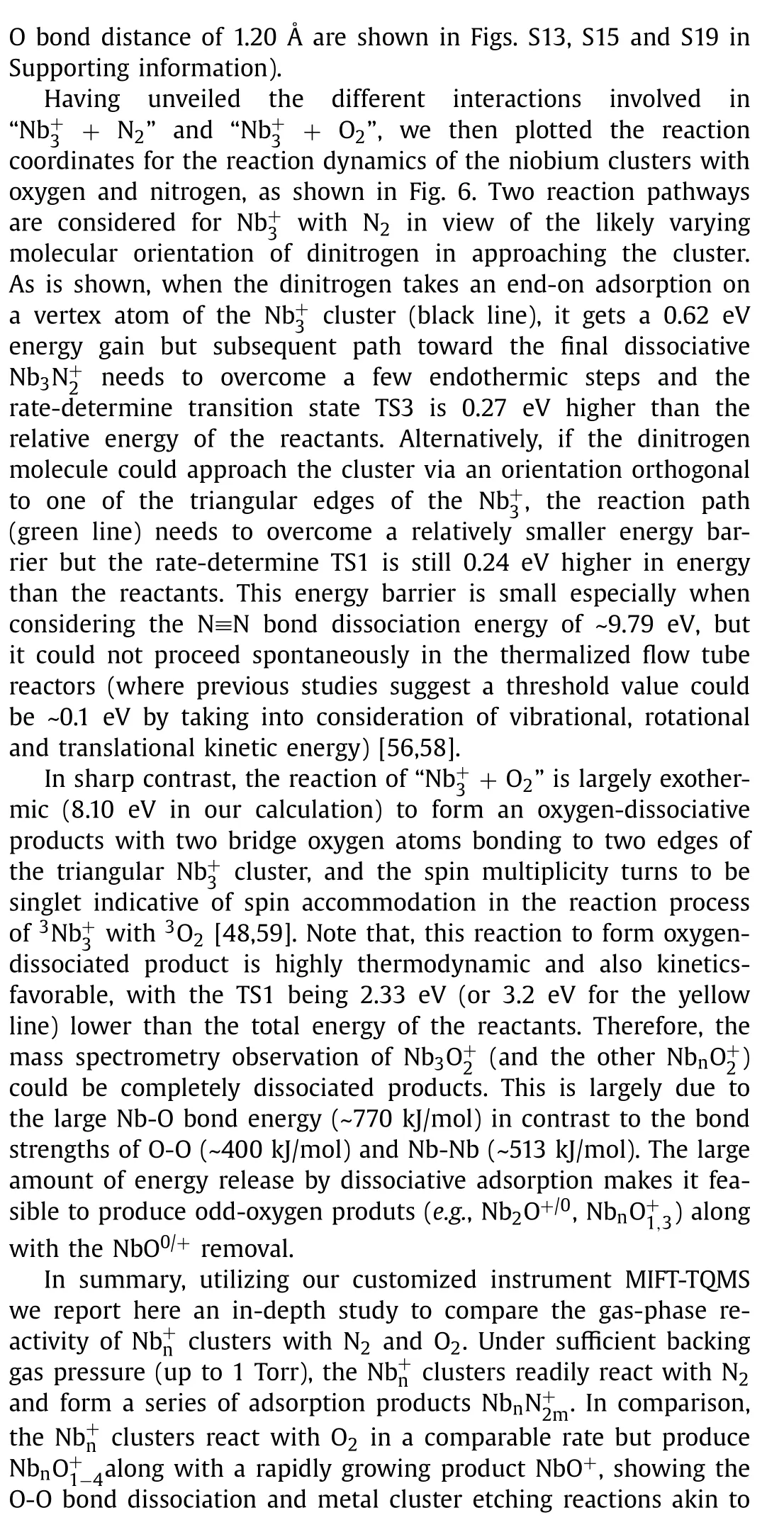

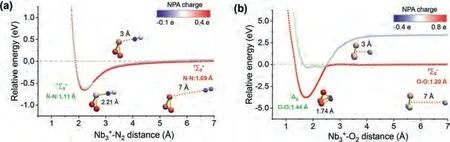
Fig.5.Potential scan curves of the cluster in approaching (a) N2 and (b) O2 from two directions with the geometrical configuration and spin multiplicity equal to the reactants and the products respectively.Red lines refer to real approaching process due to energy crossing point, while light blue/green lines refer to imaginary scanning processes.The insets show the NPA charge of every atom as a function of different interaction distance.
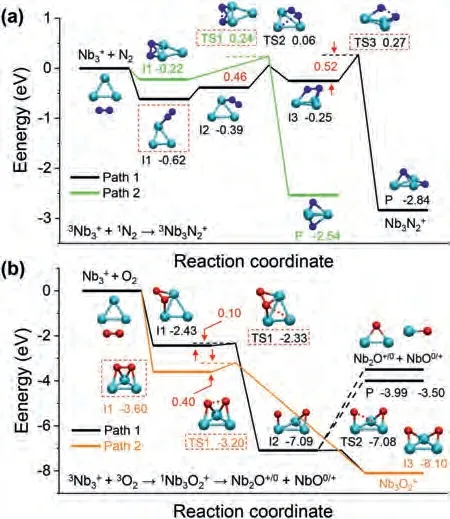
Fig.6.The calculated reaction pathways for (a) “+ N2” and (b) “+ O2”.All the thermodynamic data are calculated at the B3LYP/Lanl2dz for Nb and B3LYP/6-311G (d) for O and N.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was financially supported by the CAS Instrument Development Project (No.Y5294512C1), the National Natural Science Foundation of China (No.21722308) and Key Research Program of Frontier Sciences (CAS No.QYZDBSSWSLH024).
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.04.020.
杂志排行
Chinese Chemical Letters的其它文章
- Comment on “Acid-induced tunable white light emission based on triphenylamine derivatives”
- Strategies for efficient photothermal therapy at mild temperatures:Progresses and challenges
- Liposome-based delivery of biological drugs
- Macrophage-targeted nanomedicine for chronic diseases immunotherapy
- Advances, opportunities, and challenge for full-color emissive carbon dots
- Fluorine-containing agrochemicals in the last decade and approaches for fluorine incorporation
