Liposome-based delivery of biological drugs
2022-06-18KosheliThpMgrGeorgeFrimpongBofoXiotongLiZhongjinChenWeiHeb
Kosheli Thp Mgr, George Frimpong Bofo, Xiotong Li, Zhongjin Chen,Wei Heb,,*
a Department of Pharmaceutics, School of Pharmacy, China Pharmaceutical University, Nanjing 211198, China
b Shanghai Skin Disease Hospital, Tongji University School of Medicine, Shanghai 200443, China
ABSTRACT Biological drugs are attracting tremendous attention in disease treatment.However, their application is significantly limited by their inherent properties, such as high hydrophilicity, poor membranepermeability, low stability, and larger size.Liposome-based drug delivery systems are emerging as promising tools to improve their delivery, owing to their ability to reduce toxicity, improve bioavailability,and enhance the therapeutic efficacy of the drug by optimizing delivery to the specific target site.Here,we reviewed the types of liposomes and their applications as carriers for biological drugs to treat various diseases, emphasized the commercial products, and ultimately provided perspectives in this field.
Keywords:Liposomes Drug delivery Biological drugs Endocytosis Toxicity
1.Introduction
Biological drugs are peptide and protein-based medicines usually developed from animals, bacterium, or yeast cells utilizing the recombinant technique.They are particularly promising in medical advances due to their high efficacy and specificity [1].Antibody and associated analogs, especially human monoclonal antibodies, are dynamically developing classes of tailored therapeutic drugs.Small interfering RNA (siRNA), cytokines, enzymes, and a range of peptide medicines are among the biologicals that have been investigated the most [2,3].However, these biological agents have a comparatively large molecular size and structural flexibility compared to chemically manufactured small molecules, which prevents biologics like oligonucleotides (ODNs/ON), immunomodulators (anti-tumor necrosis factor (anti-TNF) agents (etanercept,adalimumab, infliximab) from entering the cellular membrane [4–6].In addition, many biologically active compounds, when used as drugs, exhibit adverse actions on normal organs or tissues.Adverse actions occur due to the unwanted distribution of the drug in the whole body, where it is partly inactivated upon reaching the target site [5,7].
The use of drug carriers such as lipid and lipidoid nanoparticles (NPs) [8,9], liposomes, polymer-based particles [10], drug particles, erythrocytes, and immunoglobulins [11], inorganic particles[12], amongst others, are now ideal solutions that allow for desired drugs with selective action on targeted organs or tissues[13–15].Liposomes, among these carriers, display unlimited capacities of efficient drug delivery to target site.Liposomes have been known to possess several advantages such as increasing stability through encapsulation, increasing the efficacy and therapeutic index of drugs, improving pharmacokinetic effects (i.e., decrease elimination and increase circulation lifetime), and reduce the toxicity of encapsulated agents [16,17].Also, using liposomes as drug carriers have enabled transport across membranes, provided selective passive- targeting to tumor tissues, and a flexible ability to join site-specific ligands in order to attain active targeting [18].Furthermore, the simple nature and easy production, biodegradability and repeatability, a wide range of clinical applications, and safety make liposomes likable to other carriers [19–23].So far, liposomes have been extensively studied as drug carriers for enhancing the targeted delivery of biological drugs to specific areas.In this review, we mainly summarized the use of liposomes as carriers for biological drugs, and highlighted their clinical use, and offered perspectives in this field.
2.Liposomes
Liposomes were first discovered in the 1960s by Alec.D.Bangham [24].Liposomes, meaning "Fat Bodies", are small-sized sphere-shaped vesicles in which an aqueous mass is wholly enclosed by a phospholipid bilayer [24,25] (Fig.1).Liposomes are synthesized or generated from cholesterols, glycolipids, sphingolipids, non-toxic surfactants, long-chain fatty acids, and membranous proteins [25].There are many methods used in prepar-ing liposomes for drug delivery.However, all the methods used in preparing liposomes involve four primary stages.These are (1)drying down lipids from organic solvents, (2) dispersing the lipid in the aqueous medium, (3) purifying the resultant liposomes, and(4) analyzing the final product.In the case of loading a drug into a liposome, passive and active loading techniques are used.The passive loading technique is known to comprise three different methods: solvent dispersion method, mechanical dispersion method,and detergent removal method (i.e., to remove free drug) [25,26].
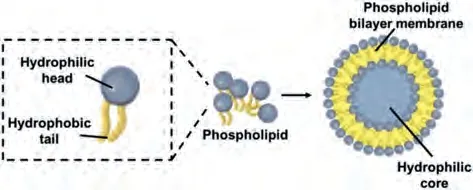
Fig.1.The basic structure of a liposome.
The accomplishment of liposomes as drug carriers have been mirrored in several liposome-mediated formulations that are now commercially available and approved for use in clinical trials [27].After the first liposome formulation (Doxil®) was designed [28], a considerable number of other anti-cancer agents have been efficaciously developed, like Depocyt®, DaunoXome®, OnivydeTM, and Myocet [27,29].Additionally, the application of a liposome is not restricted only to the use as therapies for anti-cancer but also for anti-fungal delivery (e.g., Ambisome®, Abelcet®, Amphotec®), antibacterial (Ampicillin), anti-viral (e.g., Epaxal®, Inflexal®), nucleic acids (NAs), and pain relief (DepoDurTM, Exparel®) agents respectively as seen in Table 1 [30–37].

Table 1Typical liposomal products.
2.1.Types of liposomes
Liposomes can be expressed and designed to vary in their composition and applications (i.e., conventional liposomes, longcirculating liposomes, pH-sensitive liposomes, light-sensitive liposomes, temperature-sensitive liposomes [38], magnetic-response liposomes, enzyme-sensitive liposomes, and immunoliposomes(ILs)) (Fig.2), based on their structural parameters in terms of size,charge, lamellarity (i.e., multilamellar, oligolamellar, and unilamellar vesicles) (Fig.3), and on the basis of liposomal preparations(i.e., extrusion techniques, reverse phase evaporation method, sonication, and dehydration method) [39].The size of a liposome may differ between 0.025 μm and 2.5 μm, from very small to large vesicles, respectively.Furthermore, liposomes may possess one or bilayer membranes.A liposome’s vesicle size is a critical variable in determining the circulation half-life.Both the number and the size of bilayers affect a liposome’s ability to encapsulate drugs.In terms of the number and size of bilayers, liposomes are classified into unilamellar vesicles (ULV), multilamellar vesicles (MLV), and multivesicular vesicles (MVV) [39–41].Unilamellar vesicles can also be categorized into: (1) giant unilamellar vesicles (GULV), size range>1 μm; (2) large unilamellar vesicles (LUV), size range 100-1000 nm; (3) small unilamellar vesicles (SUV), size range<100 nm.
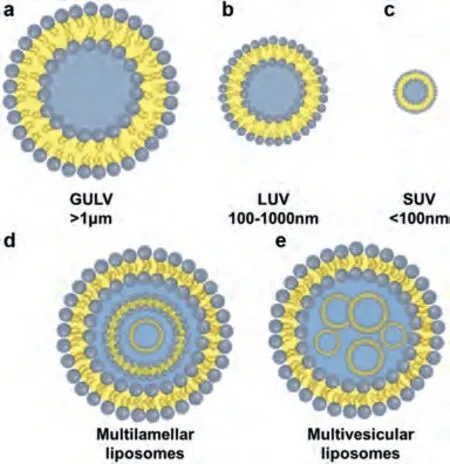
Fig.2.Categorization of liposomes according to their size and lipid-bilayer structure: (a) GULV, (b) LUV, (c) SUV, (d) multilamellar liposome and (e) multivesicular liposome.
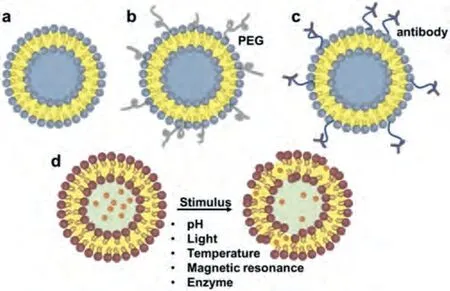
Fig.3.Liposome classification according to composition: (a) conventional liposomes, (b) long circulating liposomes, (c) pH-sensitive liposomes, (d) light-sensitive.
In a unilamellar liposome, the aqueous solution is entrapped by a single spherical phospholipid bilayer, whereas the vesicles in multilamellar liposomes have an onion structure.Classically, several unilamellar vesicles with smaller sizes will come together and form on the inside of one another, making a multilamellar structure of concentric phospholipid spheres separated by layers of water [41,42].LUV, SUV and MLV are suitable for a variety of routes,such as oral delivery, while MVV is typically utilized for parenteral delivery [43].
2.2.Interaction between cells and liposomes
Liposomes act within the human body through various interactions with the cells.The liposome interactions with cells can be classified into two main categories; that is, cationic and pHsensitive interactions [44].In a cationic interaction, a liposome comprising of positively charged lipid (lipofectin) and co-lipids(dioleoyl phosphatidylethanolamine, DOPE or dipalmitoyl phosphatidylcholine, DOPC) react with negatively charged deoxyribonucleic acid (DNA) molecule to form a stable [26,45].This interaction is typically applied in gene therapy.In a pH-sensitive interaction,negatively charged liposomes entrap DNA within its aqueous compartment rather than form stable complexes (lipoplexes).A pHsensitive liposome possesses potential duringin vivoDNA delivery[44].In other words, the interaction of cells and liposomes is associated with the following (Fig.4) [42]: (1) Attachment of liposomes to the cellular membrane and appearing to fuse with them in order to release their contents into the cell; (2) Engulfment of liposome by the cell incorporates its phospholipid in the cell membrane, releasing the encapsulated drug; (3) In phagocytic cells, liposomes are taken up; the lysosomes digest their phospholipid walls and then release the biological ingredient.
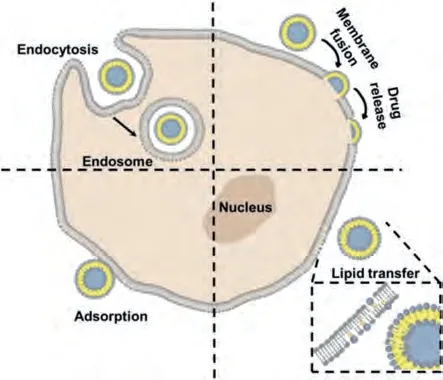
Fig.4.Basic diagram showing the interaction of liposomes and cells.
Liposomes are taken up intracellularly by endocytosis.Liposomes interact with endosomes and lysosomes, and they require membrane fusion or lipid mixing with endosomal or lysosomal membranes to deliver entrapped molecules intracellularly.Clathrin-mediated endocytosis (CME), caveolae-mediated endocytosis (CavME), and macropinocytosis are the most popular endocytic pathways in the intracellular delivery of liposomes in normal cells [46–48].According to Inohet al., CavME prefers to internalize relatively small lipoplexes, whereas CME and macropinocytosis prefer to take up the bigger ones by bone marrow-derived dendritic cells (DCs) [49].Nevertheless, Baeet al.found that cholesterol ester liposomes and 1,2-dioleoyl-3-trimethylammonium propane (DOTAP) liposomes with various sizes were internalized mainly through the CME pathway in COS-7 cells [50].
Endosomal escape, which is based on membrane fusion, is a crucial step for lipoplexes to be entrapped in endosomes in order to achieve the desired efficacy of NA therapy.Numerous research studies have reported that liposomes can transfer entrapped drugs directly into the cytosolviamembrane fusion instead of endocytic pathway [47,51,52].A correlation has been found between liposomal compositions’intracellular pharmacokinetics and their uptake mechanisms.There are unique combos of liposomes with the same lipid compositions but different uptake methods.For example, cationic liposomes with lysine head and ditetradecyl tail chains (K3C14) showed significant cellular uptake and lysosome disruption keeping the entrapped chemicals’activity [53].On the other hand, cationic liposomes made of K3C16 showed cellular internalizationviamembrane fusion pathway [54].
3.Liposomal delivery of nucleic acids (NAs)
NAs therapeutics are hydrophilic and negatively charged natural biopolymers, which are recognized as a difficult therapeutic payload that necessitates intelligent drug delivery systems (DDS)to assist its transport [55].While gene delivery systems are introduced into the physiological community, a sequence of eradicating processors are thought to work together to clear the foreign species, such asviaa reticuloendothelial (RES) system and enzymatic degradation by nucleases [56,57], thereby significantly hampering clinical application of NAs.Accordingly, there is a significant concern in using liposomes as carriers for nucleic drug delivery, either as plasmid vectors for the application of gene therapy or to deliver small NA species like ribozymes, antisense ODNs/ON,and currently, siRNA for the purpose of downregulating specific genes [57,58].Recent research has demonstrated that introducing single-stranded, double-stranded RNA (dsRNA) into the cell can affect photothermal and Ribonucleic acid interference (RNAi), resulting in gene silencing [59].
Liposomal delivery of nucleic drugs is determined by the biochemical and physical characteristics of liposomes, including size,stability, hydrophobicity, surface charge, interaction with serum proteins, and interaction with non-target cell surfaces.Liposomal carriers for NAs delivery are known to possess the following characteristics: (i) well-tolerated and safe; (ii) suitable pharmacokinetic properties to guarantee delivery to specific disease sites; (iii) effective intracellular delivery of intact NAs; (iv) non-immunogenic,enabling the use of multi dosing treatment regimens; and (v) high stability upon manufacture so that large batches can be prepared with uniform, reproducible specifications [57,60].
The drawbacks of NA-based products,i.e., accelerated enzymatic degradation and systemic clearance of, as well as their low selectivity for the target tissue and poor cellular absorption, severely restrict their medical use [61].Cationic lipids, including DOTAP, 1,2-dioleoyl-3-dimethyl ammoniumpropane (DODAP), 1,2-dioleyloxy-3-dimethylamino propane(DODMA), 2-di-O-octadecenyl-3-trimethylammonium propane(DOTMA), 3β-[N-(N′,N′-dimethylaminoethane)-carbamoyl] cholesterol (DC-Chol), 1,2-dilinoleoyl-3-dimethylaminopropane (DLin-DMA), (6Z,9Z,28Z,31Z)-heptatriacont-6,9,28,31-tetraene-19-yl 4-(dimethylamine) butanoate (DLin-MC3-DMA), DLin-K-DMA,DLin-KC2-DMA,etc., were integrated into the membrane of liposomes for this reason [61–63].The negatively charged NA-based materials combine physically with the positively charged (cationic)liposomes to create a complex lipoplex system.These lipoplexes are presumed to infiltrate the cell by fusing with the cell membrane, and after internalization, they facilitate the release of nucleotides from endosome [63].However, their rapid systemic removal, toxicity, instability, and induction of immunostimulatory response limit the possible clinical use of cationic liposomes.
Conversely, to improve the possible use of liposome-based structures in gene therapy, various strategies have been dedicated to developing cationic lipid-containing liposomes that effectively encapsulate nucleotides inside their lamellae but possess anionic surface charge or a net neutral charge.Coated cationic liposomes(CCL), lipidic nanoparticles (LNP), stable nucleotide lipid particles(SNALP), and liposome-polycation-hyaluronic acid particles (LPD)are several forms of these cationic lipid-containing liposomes.Moreover, vesicle targeting modern technologies can improve the surface-neutral liposome NA complex’s cell precision [64].Longcirculating cationic liposomes encapsulating c-myb asODNs was designed by Pastorinoet al.[65] to significantly reduce tumor growth and metastasis in the murine model of melanoma and neuroblastoma by targeting ganglioside GD2.SNALP is a unique lipid bilayer consisting of a mixture of cationic and fusogenic PEGcoated lipids.It has been formulated to protect siRNAs against serum nucleases, allowing endosomal cytotoxicity and further cytoplasmic siRNAs to be released.A procedure for using an antigen to provide SNALP encapsulation of siRNAin-vitrohas recently been described by Wilner and Levy [66].A specific targeted delivery could be attained by using SNALP together with an effective siRNA-mediated gene knockdown.LNPs were studied clinically to deliver RNA RNAi molecules to specific sites.In a clinical study,the PCSK9 gene, which regulates low-density cholesterol lipoproteins (LDL-C) levels, was suppressed by the LNP siRNA system.The LNP siRNA system has been substantially confirmed to lower LDLC without adverse effects.In another study, the LNP siRNA system was found to significantly decrease transthyretin (TTR) concentration in the body as a result of treating TTR-induced amyloidosis[67].
In order to achieve increased efficacy of gene therapy, genetic modification can also be merged with low molecular treatment.For instance, Saadet al.[68] designed cationic liposomes for the co-delivery of doxorubicin (DXR) and siRNA targeting multi-drug resistance (MDR) protein to enhance the anti-cancer efficacy of DXR in lung cancer cells.Again, the effectiveness of a new thermosensitive magnetic liposome for the co-delivery of both SATB1 and DXR short hairpin RNA (shRNA) to gastric cancer cells was later assessed by Penget al.[69].It was shown that DXR and SATB1 shRNA was delivered into MKN-28 cells, a human gastric adenocarcinoma, with increased drug delivery efficacy and high gene transfections, which led to growth inhibition in gastric cancer cells both in cells and animal models, as compared to individual delivery.
Despite the high efficacy of cationic liposomes, they have several inconveniences and setbacks; hence, the second wave of LNPs was designed based on pH-sensitive cationic lipids [70].These lipids overcome common lipid drawbacks with permanent cationic charges.Zimmermannet al.[71] reported the first effective LNPs incorporating siRNA made with ionizable cationic lipid DL in-DMA to silence genes in hepatocytes.These LNPs could suppress the apolipoprotein B (ApoB) gene in hepatocytes of cynomolgus monkeys.Also, a much more effective lipid, KC2, introduced by Sempleet al., was efficiently used to silence the coagulation factor VII(FVII) gene in hepatocytes (the ED50 value was 0.01 mg/kg) [62].A significant reduction in liver delivery and improved delivery to macrophages and dendritic cells (DCs) were observed when the diameter of KC-based LNPs was increased from around 100 nm to about 300 nm in the bone marrow [72].
A study conducted by Kobilovaet al.[22] reported on the design of the new folate-containing lipoconjugate (F-LP) made of 1,2-di-O-ditetradecyl-rac-glycerol and folic acid linked to a PEG spacer.The F-LP was also evaluated as a targeting component for the delivery of NAs in tumor cells expressing folate receptors (FR).The FR-targeting liposomes were made of polycationic lipids and had diameters of 60 nm with no cytotoxicity.Liposome-NA complexes were prepared to optimize liposome/cell interactions at different nitrogen and phosphate ratios (N/P).They exhibitedin vitrothat at low N/P (1/1 and 2/1), the FR-mediated delivery of different NAs mediated by liposomes 2 × 3-DOPE/FC occurs.Also, FC-containing liposomes have 3–4 times higher transfection efficiencies than conventional formulations under such circumstances.Forin vivostudies, the targeted liposomes and cargo (Cy7-labeled-siRNA targeted MDR1 mRNA) efficiently accumulated in the tumors (15%–18% of the total amount) and the kidneys (71%) for over 24 h and resulted in downregulation ofp-glycoprotein.
4.Liposomal delivery of plasmid DNA (pDNA) and gene editing
A non-viral vector, pDNA, serves as a carrier for therapeutic genes to be delivered into target cells for treating certain diseases, including cancer, cardiovascular disease, infection, and inflammation.However, because of the high negative charge and high molecular weight of pDNA, its delivery shares some difficulties, including high loading capacity and preventing aggregation.More specifically, unlike mRNA, pDNA must reach the nucleus for protein translation instead of simply the cytoplasm; and it incorporates into the genome and causes mutation [73].Recently, liposomal delivery platforms have been designed to improve the efficient transport of pDNA to target specific sites.According to previous studies, co-lipid, especially DOPE, could increasein vivointracellular trafficking in target cells.As a result, DOPE was found in the majority of cationic liposomal mediated pDNA delivery platforms[74].
The process used to prepare DNA-complexed liposomes can significantly affect transfection efficiency and cytotoxicity.Cationic liposomes were synthesized using co-lipids, DC-Chol, DOTAP, DOPC and DOPE in a ratio of 1:1:1:1 by self-assembling and microfluidic mixing.Liposomes prepared by the self-assembling method have several layers, while the microfluidic mixing method produces fewer lipid bilayers, a higher number, but fewer DNA molecules per cell.Therefore, liposomes prepared by microfluidic mixing delivered the same amount of pDNA, resulting in more transfection incidents but a lower transfection rate per cell [75].A study group found that microfluidic liposomal preparation containing cationic and co-lipids showed similar effect when the transfection efficiency was examined in cells and animal models.The transfection efficacy sequence was found to be DLin-KC2-DMA>DLin-MC3-DMA>1,2-dilinoleyloxy-3-dimethylaminopropane(DLin-DMA) 1,2-dilinoleoyl-3-dimethylaminopropane (DLin-DMA)>1,2-dilinoleoyl-3-dimethylaminopropane (DLin-DMA)>1,2-dil(DLin-DAP).In addition, saturated phosphatidylcholine (PC) and phosphatidylethanolamine (PE) could be replaced with unsaturated PC and PE in the liposomal pDNA preparations to improve transfection efficacy.The fact that hardly any transfection was recorded for formulations without helper lipids confirmed the necessity of helper lipids [76].It has also been looked at how versatile membrane-tethered DNA works.Cholesterol-anchored DNA was conjugated using a versatile tetra(ethylene glycol) (TEG) linker at the 5′ terminus of the strands.The DNA was anchored in the bilayers of DOPE and DOPC liposomes by the cholesterol moieties.According to quantitative analysis, cholesterol DNA could stack neatly on the liposome membrane.Despite doubling the negative charge,double-strand DNA had a stronger affinity for the liposomal membrane compared to similar lengths of single-strand DNA [77].
Recently a research group designed liposomal formulations that could be applied to the skin and enhanced delivery of pDNA to the target cells.They used various cationic lipids (DOTAP,DC-Chol, DOTMA) and co-lipids (DOPE, 1,2-distearoyl-sn-glycero-3-phosphorylethanolamine (DSPE)) to prepare different liposomal formulations.They synthesized lipoplexes by combining liposomes with pDNA at various pDNA/cationic liposome ratios.Their findings demonstrated that DOTMA lipoplexes could provide the higher transfection, DC-Chol lipoplexes offered double transfection rate as essential as DOTAP lipoplexes, and DSPE increased cell viability rate and showed similar transfection efficiency [78].Study by Hosseinpouret al.[79] addressed the issues related to liposomal gene delivery (such as constrained efficacy and toxicity at higher doses) by using photobiomodulation (PBM, a therapeutic technique that is highly controllable and effective) in order to change the behavior of the target cells.They investigated the effects of PBM on cationic liposome pDNA transfection efficiency and toxicity utilizing Lipofectamine 2000 to load GFP-encoding pDNA for targeting pre-osteoblast MC3T3-E1 cells.The findings showed that by using PBM with diode lasers at 810 nm and 970 nm improved the transfection performance of liposomal formulations in cells while also protecting them from Lipofectamine toxicity.The effects were wavelength dependent and irradiance dependent, with the best effect at 12 J.These findings backed up the idea that PBM with nearinfrared lasers could be utilized to enhance gene therapy as a safe,controllable, reliable, and cost-effective process.At the same time,more research is required to determine the biological mechanisms underlying these effects, as well as their transfection effects in different cells.
5.Liposome-mediated gene editing via CRISPR/Cas9 system
CRISPR technology has reinvented in molecular biology as a modern genetic editing technique.CRISPR/Cas9 therapeutics target and downregulate genes of interest for the treatment of various genetic diseases and cancers [80].CRISPR/Cas9 therapies have generally been provided as Cas9 protein with sgRNA, Ca9 mRNA with sgRNA, or CRISPR/Cas9 plasmid [81].Because of the unstable effect of these molecules, they are all susceptible to degrading in serum by enzymes.Due to the electrostatic repulsion of cell membranes and plasmid, there could be a low cell internalization effect.Recently, the liposome-based gene delivery platform has shown promising efficacy to solve these obstacles [82].
Cationic liposomes are the most common carrier for CRISPR/Cas9 delivery due to their significant efficacy on cell internalization and adequate endocytic egress.For instance,cationic lipid (DOTAP) containing liposomes have been designed for delivering CRISPR/Cas9 to downregulate the polo-like kinase 1 (PLK1) gene in pancreatic cancer [83].Recently a research group investigated the cumulative efficacy of cationic liposomes containing cholesterol domain and DOPE, a fusogenic lipid, and polyethylene glycol (PEG) on transfection efficiency and other particle properties by developing two-, three-, and four-component cationic liposomes.The prepared cationic liposomes (DOTAP/DOPE/cholesterol/Chol-PEG) recorded the maximum amount of transfection efficacy amongst those developed cationic liposomes.This technique successfully increased the transfection efficiency of Cas9/sgRNA by 39% gene-editing efficacy to downregulate the GFP reporter.The findings investigated that these liposome formulations did not show any cytotoxic effect, and they could completely protect plasmids in serum against enzymes [74].
Another study group designed a DOTAP-liposome-templated hydrogel nanoparticle (LHNP) for suppressing genes in tumors by safe and efficient delivery of Cas9 proteins and NAs.This study found that the LHNP delivered CRISPER/Cas9 more efficiently compared to the commercially available product into the cell.These findings suggested that this strategy could be used for the efficient delivery of functional CRISPR/Cas9 in cancer biology research [84].In a research study, liposomal lipoplexes were constructedviaa combination of DNA and blank liposomes at a charge ratio of +4/1.These cationic liposomes loaded CRISPR/Cas9 plasmid and donor vector for specific mucopolysaccharidosis type I care (MPS I) gene editing in cell and animal models.PEGylated DOTAP-liposomes loaded with CRISPR/Cas9 plasmid showed higher serum stability, endosomal escape, transfection efficiency, and potent efficacy on MPS I gene editing.This gene-editing efficacy was determined by analyzing the production of alpha-L-iduronidase (IDUA) activity, which is less produced in MPS I patients [85].They recorded an increased level of IDUA after the treatment with liposomal formulations.This study indicated that an increased level of IDUA in various tissues could have a practical impact on gene therapy for Hurler patients.
A multifunctional peptide, R8-dGR, is highly expressed on cancer cells, including pancreatic cancer.It binds with integrinαvβ3and neuropilin-1, which targets the tumor [86].The combinatorial strategy, that is, paclitaxel (PTX) containing R8-dGR cationic liposome carrying CRISPR/Cas9, has shown potential efficacy on suppression of hypoxia-inducible factor-1 alpha (HIF-1α) in a pancreatic tumor model [83].A recent study suggested that the PEGylated liposomes encapsulated with Cas9/16E7-expressing plasmids were able to affect and remove human papillomavirus type16(HPV16) E7-driven tumors in syngeneic mice.This study investigated CRISPR/Cas-mediated cell death as the immunogenic cell death (ICD) and preventing cancer reoccurrence [87].
The folate receptor-targeted liposome (F-LP) has been developed for delivering CRISPR pDNA /Cas9 and single guide RNA against ovarian cancer-related DNA methyltransferase 1 (DNMT1)gene (gDNMT1).Increased mutation of endogenous DNMT1in vitrooccurred with F-LP/gDNMT1 treatment and significantly expressed Cas9 endonuclease and downregulated DNMT1in vivo.Also, they showed a significant effect against tumors on PTX-sensitive and-resistant ovarian cancers, which has less adverse effects than PTX.Therefore, CRISPR-Cas9-targeted DNMT1 therapy could be a promising treatment therapy for ovarian cancer [47].
Despite the advantages of the liposomal platform, there are some factors that influence gene delivery efficiency in the liposomal CRISPR/Cas9 delivery system.For instance, fusogenicity of liposomes, their size, surface charge, PEGylation level, targeting ligand’s structure, and kinds of CRISPR/Cas9 are some of these factors [88].In addition, significant genome editing efficiencyin vitrodoes not always lead to an exact gene-editing abilityin vivo[89].The first issue is the lack of systematic research into the effects of surface ligandsin vivo.Sometimes these ligands could show immune responses and low tumor penetration effect.The relationship between surface ligand chemical structures and immune response/tumor penetration is unclear [89].Though cleavable PEGylated liposomes easily surpass uncleavable PEGylated liposomesin vitroandin vivo, the cleavage efficacy is inadequate.Developed cleavable PEGylated liposomes are less effective than unPEGylated liposomesin vitro.Therefore, research on highly cleavable PEG is necessary [90,91].The third issue is the lack of precise cellular absorption mechanism cationic preparations (lipoplexes and lipopolyplexes) [82].In addition, the efficient quantity of CRISPR/Cas9 successfully reached cytosol from endosomes after the uptake has not been determined.Therefore, advanced cell trafficking techniques are needed to research the comprehensive mechanism,which could help with more cationic formulation optimization.Also, developing a molecular imaging strategy could provide useful mechanisms of the CRISPR/Cas9 delivery process in the body.
6.Liposome-mediated delivery of protein therapeutics
Protein therapeutics, mainly cytokines, antibodies, enzymes, tumor antigens, pro-apoptotic proteins/peptides, have been widely used in pharmaceutical fields [92].These proteins have been used as therapeutic agents due to their unique properties like low toxicity, high target specificity, lower chances to have multi-drug resistance.They do not need a long time for their clinical trials and approval by Food and Drug Administration (FDA) [93,94].However,intracellular delivery of these proteins has shown challenges like instability in blood, degradation by enzymes, short half-life, and cell membrane impermeability due to electrostatic repulsions.
Recently, liposome-mediated protein delivery nanoformulations have been prepared for clinical use as a result of the rapid growth of nanomedicine.Liposome surfaces can be modified by protein-repellent polymers, including PEG, to avoid after challenges mentioned for protein delivery [95,96].Protein therapeutics’stability can be enhanced by entrapping them into liposomes, as the lipid bilayer protects them from degradation.In addition, PEGylated liposomes can extendin vivocirculation and be modified by active ligands to enhance active targeting [97,98].A study group prepared recombinant human insulin (rhINS)-loaded bile salt (BS) liposomes to observe if they could improve oral absorption and gastrointestinal stability while increasing transcellular permeation.BS-liposomes improved rhINS stability, efficient cellular absorption, and transport with very few side effects after 24 h of oral administration in cell and animal models.These findings depended on the BS liposome size [98,99].Protein corona liposomes (PcCLs) were prepared for efficient insulin delivery in which insulin was enveloped with CLs by bovine serum albumin(BSA).This study proposed that the hydrophilic and neutral charge protein corona of PcCLs can efficiently penetrate mucus and degrade gradually by enzymes.These PcCLs could decompose to improve insulin delivery through the transepithelial membrane.The evidence made clear about improved PcCLs cellular uptake and its transepithelial permeability.The decreased blood glucose levels and higher oral bioavailability up to 11.9% were observed in type I diabetic rats with PcCLs treatment.Thus, PcCLs is an emerging strategy to deliver peptide/protein drugs for reducing GI-related challenges [100].
PEGylated liposomal NPs loaded with the recombinant proteins cysteine-rich domains 2 and 3 (CRD2 and CRD3) were developed for cancer treatment [101].These liposomal NPs showed improved lymphocyte proliferation by 1.5-fold, which confirmed the liposome NPs’adjuvant effect as a vehicle for recombinant protein.They could be utilized as adjuvant antigens to produce a distinctive agonist antibody for stimulating TNFR1 in animal models.Thus,liposomal NPs containing recombinant protein might be a potential strategy to trigger immune responses against cancer [102].Cross-presentation is a critical response for removing intracellular pathogens and tumors, and also it plays a vital role in identifying effector cytotoxic T lymphocytes (CTL) from CD8+T-cells.However,efficient induction of CD8+CTL responses is quite challenging.As a way to overcome this challenge, liposome formulation containing pore-forming protein (PFP), stycholysin II (StII), and antigen ovalbumin (OVA) platform (Lp/OVA/StII) was developed.This platform was enabled to produce an antigen-specific strong CTL response against intracellular pathogens.Furthermore, they significantly reduced tumor growth in E.G7-OVA tumor carrier mice.However,the CTL response mechanism produced by liposomes was unknown[103].
In another study, the mechanism of SIINFEKL-specific B3Z CD8+T cells activation by liposome formulation, Lp/OVA/StII, in bone marrow-derived DCs (BM-DCs) and bone marrow-derived macrophages (BM-MΦs) with uptake inhibitors was evaluated.This study found that after the uptake of Lp/OVA/StII by BMMΦs, cells were enabled to induce targeted T cell responseviaa phagocytic process, thereby occurring OVA’s cross-presentation through the vacuolar pathway, while BM-DCs were unable to activate targeted T cells with this liposomal formulation due to a specific inhibitor that decreased cross-presentation of OVA.Macrophages’CTL response produced by this liposomal formulation in animal models was investigated by reducing macrophages with clodronate-based liposomes.Mice with reduced macrophages could not induce CTL response which suggests the importance of liposomal formulations for producing antigen-presenting cells(APC) [104].
Despite the several advantages of liposomes for protein therapeutics delivery, significant barriers have been demonstrated in liposomal protein delivery.They are highly costly to generate due to the complicated production, purification, and characterization processes.Numerous challenges are connected with inefficient liposome and protein production since many of the components must be manufactured individually, incorporated, and purified; therefore, significant discrepancies in the amount of targeting moieties present on the surface of each liposome, as well as irregular loading of proteins and small molecules within each liposome [105].It would be beneficial to develop an alternative approach for producing multifunctional lipid-NPs that eliminates multiple stages from the existing liposome production process.Producing proteoliposomes by exploiting bacterial vesicle production presents a unique opportunity to resolve many manufacturing issues and incorporate issues related to liposome manufacturing, allowing for the development of complex, low-cost NP therapies in the future [106].
7.Liposomal delivery of antibody and enzyme
Due to the biocompatibility, biodegradability, and controlled release properties of liposomes, they have been developed as an attractive delivery agent for antibodies and enzymes.Considerable efforts have been made on liposomes designed by various ligands/antibodies for specific targeting [21].The ILs concept was developed as a way to synergize the actions of antibodies and liposomes.Gregoriadiset al.[107] first investigated the utilization of IgGs produced against different cells and found that they could selectively ingest liposomes.Lesermanet al.[108] showed that antibodies coupled to the surface of liposomes resulted in particular contact with target cells.Cationic liposomes called PULSin (polyplus-transfection) were used to carry mouse IgG, antitransmembrane Golgi protein giantin, and anti-nuclear pore complex [109].Their surface charge and interactions regulated these liposome complexes’efficient cellular uptake.
Nevertheless, extremely positive charge biological agents having weak hydrophobic groups cannot be delivered efficiently.The choice of cationic and helper co-lipids is crucial for antibody delivery, and lipid compositions differed from one target to the next.For instance, cationic lipid bisguanidinium-tren-cholesterol (BGTC)and DOPE may effectively transportβ-gal and may show excellent cell function [110].A liposome-mediated anti-CD44 antibody NPs system (NPs-αIL6R Ab-CD44) was designed by using lipid mixtures of DOPC, DOPE, cholesterol, and DSPE-PEG3400-NHS decorated antibody.This liposome formulation was enabled to target CD44+efficiently, suppress IL6R-Stat3, and inhibit gene translations.In addition, this formulation had shown an effective antitumor metastasis activityin vivotriple-negative and luminal breast cancer [111].In another study, new membrane fusogenic liposomes (MFLp/DOX+S-mAb) were prepared for speedy co-deliveryof tumor-suppressing anti-S100A4 antibody (anti-S100A4 antibody inhibits apoptotic p53 protein and shows tumor metastasis inhibition activity) and doxorubicin (DOX).This fusogenic liposome could be transported directly to cytosol in order to avoid inefficient escape and endosomal degradation.The results showed a synergistic effect of the preparation.Liposomal delivery of anti-S100A4 antibody blocked intracellular S100A4, thereby recognizing the cytoskeleton of 4T1 cells, inhibiting cell motility.At the same time, DOX’s anti-tumor efficacy was markedly increased with S100A4 effect inhibition on sequestrating tumor-suppressor protein p53 [112].Incorporating cell-penetrating peptides into liposomes has been well studied,e.g., researchers used a liposome system to transport octaarginines (R8) and GALA for robust intracellular antibody transduction [91].However, this strategy is often linked to endosomal entrapment, which prevents effective cytosolic delivery.
An innovative approach based on nanoliposome-mediated co-delivery of drug and phage lytic proteins/enzymes against multidrug-resistant bacteria has certainly gained scientist’s interest.For example, nanoliposome co-encapsulated with vancomycin and anti-staphylococcal protein lysostaphin was directly applied on methicillin-resistant Staphylococcus skin aureus (MRSA) infected mice to suppress the bacterial infection.These nanoliposomes significantly suppressed bacterial infection in cells and animal models compared to control liposomes.This study suggested a new nano-carrier (co-loaded lysostaphin and vancomycin) could be applied on the topical antimicrobial platform to treat MRSA skin infections [113].A recent study was conducted for the treatment of MRSA with pH-sensitive liposomes loaded with Endolysin LysRODI.This study found that the prepared liposomes were significantly inhibitedS.aureuscells in mildly acidic environment (pH 5).Although the liposome formulation inhibits biofilm, the cell’s thick matrix encapsulated liposome action was remarkably blocked.So,they suggested that adjusting the nanovesicles’constitution or a combination therapy containing matrix-degrading enzymes could be used to avoid such problems [114].
Based on the enzymatic biocatalytic precipitation approach, a recent study developed a facile and practicable potentiometric immunosensing platform.This platform was applied for the sensitive detection of thyroid-stimulating hormone (TSH) utilizing a horseradish peroxidase (HRP) incorporated liposomes for signal amplification.For the determination of target TSH, two tagging techniques with or without the liposome had been tested; HRPentrapped liposome provided better analytical characteristics.This strategy has the potential to introduce new frontiers in protein diagnostics and biosecurity [115].
8.Liposomal delivery of vaccine
Vaccines are developed based on well-characterized antigens,including recombinant proteins and peptides.However, their immunological response is usually inadequate due to their synthetic nature, which is mainly owing to the antigens’incapacity to promote maturation of DCs, the major APCs that react to foreign infections and drive the immune reaction [116].Liposome has been used as a drug carrier, but its use as a vaccine transporter is raised recently.Liposome has been preferred over other carriers for vaccine delivery because of liposome’s physicochemical properties and tolerability by the human body, low cytotoxicity, as well as chemical and structural flexibility.Chemical flexibility is a liposome’s capability to incorporate a hydrophilic antigen or adjuvant or a lipophilic portion that may be translocated between bilayer lipids.Hydrophilic antigen’s surface conjugation is feasible that improves antigen accessibility and thus promotes phagocytic uptake.Structural flexibility is related to the ability to modifying liposomal properties through adjusting lipid concentration.For instance, different cationic lipids compositions have been widely used during optimized liposome formulations that enhance cytosolic antigen release [117].Gregoriadis and Allison [118] first reported liposomemediated vaccine adjuvants or associated antigens.Epaxal, Inflexal, and Mosquirix are clinically approved liposomal vaccines,which are classified as virosomes because virus-derived proteins are incorporated into PC membrane vesicles.PC membrane vesicles could protect the antigen from enzymatic degradation.Details about these approved vaccines are presented in Table 2.

Table 2 Liposomal vaccine products approved for infection [119].
Liposomal subunit vaccines could transport molecular adjuvants and antigens to APCviapattern recognition receptors (PRR).They can upregulate major histocompatibility complex (MHC)-II and costimulatory factors, thereby inducing an immune response against the antigen (Fig.5) [120].Antigens and adjuvants can incorporate into liposomes by electrostatic interactions with the lipid surface, covalent, and noncovalent lipid anchoring, and enclose into the lipid bilayer [121].Recently, scientists are more focused on the combination of DOTAP based cationic liposomes with immunostimulating ligands to develop potential vaccine delivery systems because of improved interaction with immune cells [122–126].For instance, combining the polymer-based delivery system and cationic liposomes loaded with conjugate 5 showed higher antitumor activity with an 80% survival rate in model mice after single immunization [127].
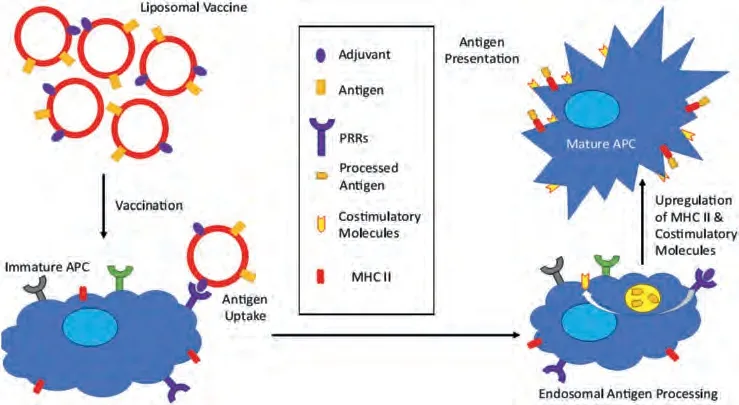
Fig.5.Subunit vaccine containing antigen and adjuvant to APCs.Interaction between adjuvant and PRR shows the increased MHC II and co-stimulatory molecules needed for significant T cell response.
Many plaque-specific antigens such as PS plaque-specific antigen, peptide plaque-specific antigens, cholesterol plaque-specific antigens have been incorporated into liposomes for treating inflammatory atherosclerotic disease [91].PS-loaded liposome was the first plaque-specific antigen liposome utilized to imitate immunogenically and inhibit inflammation on cancer cells.They were immunized in ApoE–/–mice to reduce atherosclerotic plaque and the necrotic core size.However, their specific mechanism in the treatment of atherosclerosis was unclear [128,129].LDL and apoB-100 are lipoprotein plaque-specific antigens that were also loaded into liposomes.These antigen-loaded liposomal formulations were used for reducing atherosclerotic plaque.In a study, liposomes have been used to transport CD4+T-cell-specific peptide (OVA323),proprotein convertase subtilisin/kexin type 9 (PCSK9), and peptide plaque-specific antigens.Subcutaneous immunization of these liposomes was able to deliver OVA323 and PCSK9 in BALB/c mice successfully.The results demonstrated that the liposomal formulations were able to decrease the plasma concentration of PCSK9 by approximately 50%, reduce PCSK9–LDLR interaction and inflammatory T cells [130].
Liposomal DNA vaccines produced efficient immune responses and memory responses for a long time to slow the tumor progression [131].For instance, for the first time, a study group developed MPLA-liposome loaded DNA vaccine (MPLA-pVAX1-cpc)againstLeishmania.This liposomal vaccine enormously boosted the T cell and antibody responses againstL.donovaniand reduced intracellular parasites in the liver and spleen ofL.donovaniinfected BALB/c [132].In a recent study,ex vivotransfection of autologous DCs with melanoma encoding DNA liposomes resulted in the substantial production of protective immunity against melanoma for a long-time [133].
Recently a study group produced 1,2-dimyristoyl-sn-glycero-3-ethylphosphocholine (EPC) containing adjuvant liposomal vaccine loaded with TLR7 agonist imiquimod and solubleLeishmaniaantigen (SLA) (Lip EPC+ Imiquimod + SLA) to treatLeishmaniasis.They immunized Lip EPC+ Imiquimod + SLA subcutaneously in BALB/c mice and examined parasite burden, footpad swelling, IgG isotype,IL-4, and IFN-γconcentration.Their results showed minor footpad swelling andparasiteburden comparing to the control group.Furthermore, upregulated IFN-γand reduced IL-4 levels were observedin vivo.Their study findings investigated that liposome vaccines containing the drug could produce a Th1 immune reaction inLeishmania[134] .
9.Conclusions and perspective
Biological agents have significant advantages over synthetic active drugs in human health, but their complete therapeutic effi-ciency cannot be achieved due to unexpected delivery problems.Therapeutic outcomes of NAs, such as pDNA, siRNA, miRNA, and antisense ONs, have been limited due to their pharmacokinetic properties.Interaction with cells and transfection into specific cells have significantly been constrained due to their strong negative charged backbones.Liposomes are developed as a most successful drug delivery platform for the biological drugs because of their various advantages such as excellent encapsulation efficacy and improvement in drug stability [135].Among the liposomes, cationic liposomes are most commonly used to deliver the biological drugs,whereas its systematic toxicity remains an obstacle to limit the translation.The incorporation of ionizable cationic lipid represents a promising approach to address this problem, because it allows the liposomes to have anionic surface charge or a net neutral charge in normal physiological conditions, effectively encapsulate the biopharmaceuticals inside the cores, possess positive charge under low pH environment and thus facilitate the endosomal escape for intracellular delivery.These pH-sensitive cationic liposomes are increasingly attracting attention across the world due to their translation potential.However, the liposomal formulations are overwhelmingly dosed by invasive route such as intravenous injection and intramuscular injection.The most popular administration pathways used in the clinical practices, oral administration and transdermal delivery, are almost not employed in the product, mainly owing to the poor stability in the gastrointestinal tract and modest transdermal ability,etc.The involvement of other techniques such as surface coating, microneedles, and weak electric current may help overcome the drawbacks [136].
PEGylated liposomes are used to improve stability and extend blood circulation time.A larger PEG ratio improves circulation lengths while hindering cellular uptake and endosomal escape.Optimization of PEGylated lipids ratio may compromise the limitation,e.g., Yagiet al.[137] designed wrapsomes, in which the core is siRNA/DOTAP, and the wrap is a neutral lipid bilayer made of egg phosphatidyl-choline and PEG lipid.Wrapsomes were discovered to have prolonged circulation time and improved stability.The PEG on the surface of liposomes acts as a steric shield against opsonin, thereby reducing liposome uptake by mononuclear phagocyte system (MPS) cells and allowing them to be taken up by other cells.However, a research study observed that the first dose of blank PEGylated liposomes could trigger an immune reaction in rats and Rhesus monkeys which affects the pharmacokinetics and bioavailability of the second dose.Due to the high uptake of these liposomes by liver and spleen cells, the plasma half-life of their second dosage was drastically reduced when given five days and up to four weeks after the first dosage, which is described as the Accelerated Blood Clearance (ABC) phenomenon.Ishida and coworkers [138] later provided a mechanism for the initiation of the ABC phenomenon, which is induced by the production of anti-PEG IgM.This phenomenon is a key problem for PEGylated formulations which require multiple dosing regimens in clinical practice.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (Nos.81872823 and 82073782), the Shanghai Science and Technology Committee (No.19430741500), the Key Laboratory of Modern Chinese Medicine Preparation of Ministry of Education of Jiangxi University of Traditional Chinese Medicine, China(No.TCM-201905).
杂志排行
Chinese Chemical Letters的其它文章
- Comment on “Acid-induced tunable white light emission based on triphenylamine derivatives”
- Strategies for efficient photothermal therapy at mild temperatures:Progresses and challenges
- Macrophage-targeted nanomedicine for chronic diseases immunotherapy
- Advances, opportunities, and challenge for full-color emissive carbon dots
- Fluorine-containing agrochemicals in the last decade and approaches for fluorine incorporation
- Critical review of perovskites-based advanced oxidation processes for wastewater treatment: Operational parameters, reaction mechanisms,and prospects
