Strategies for efficient photothermal therapy at mild temperatures:Progresses and challenges
2022-06-18PengGoHuiWngYiyunCheng
Peng Go, Hui Wng,*, Yiyun Cheng,b,*
a South China Advanced Institute for Soft Matter Science and Technology, School of Molecular Science and Engineering, South China University of Technology, Guangzhou 510640, China
b Shanghai Key Laboratory of Regulatory Biology, School of Life Sciences, East China Normal University, Shanghai 200241, China
ABSTRACT Photothermal therapy (PTT), typically ablates tumors via hyperthermia generated from photothermal agents (PTAs) under laser irradiation, has attracted great attentions in the past decades.Unfortunately,longstanding, frequent and high-power density laser irradiations are needed to maintain the hyperthermal status (>50 °C) for efficient therapy, which will damage the skin and nearby healthy tissues.Suppressing cancer cells with a mild temperature elevation is more attractive and feasible for PTT.Recently,low-temperature photothermal therapy (LTPTT), which could inhibit tumor under mild hyperthermia, has been widely investigated by researchers.Herein, we systematically summarized the strategies to achieve LTPTT.Diverse PTAs including organic and inorganic materials reported for LTPTT were introduced.The established strategies for LTPTT were intensively described.Finally, the challenges as well as future perspectives in this field were discussed.
Keywords:Photothermal therapy Mild hyperthermia Photothermal agents Nanomedicine Tumor targeted therapy
1.Introduction
Malignant tumors also called cancer, have been the most threatening diseases for human health for decades [1-4].Developing reliable methods for confronting cancer is the chief business for scientific and clinical investigations [5-11].But the main tactics in clinical cancer therapy still staying at chemotherapy, surgery and radiation therapy [12,13].The destruction of immune systems caused by high-dosage and repeated radiotherapy can induce deadly side effects to the patients [14,15].Serious severe effects to the healthy tissues and drug resistance are the critical problems for chemotherapy [13,16-20].While the surgery can cause irrecoverable tissue injury, and the disease lesions are hard to be completely removed during surgery, which may further cause cancer recurrence and metastasis [12,21,22].Therefore, tactics with higher controllability, better effectiveness and higher safety are highly desired for improved cancer treatment [23-31].
Phototherapy employs photo and photothermal agents (PTAs)or photosensitizers to kill cancer cells by either photo-thermal conversion (photothermal therapy, PTT) [32-35] or photo-induced reactive oxygen species generation (photodynamic therapy, PDT)[36-39].As a photo-induced chemical process, the therapeutic effect of PDT is largely associated with the oxygen concentration in the tumor tissue.However, hypoxia [40] and the reductive cellular microenvironments as the common features for most tumors,severely limited the effectiveness of PDT.In comparison, PTT does not require the participation of O2and the therapeutic effect is less restricted by the cellular microenvironments [41,42].Elevating the tumor temperature over the tolerance threshold value can effectively ‘burn’the cancer cells to death [43,44].Thus, the past decades have witnessed the rapid development of PTT for cancer therapy.
Numerous PTAs including small molecules and diverse organic/inorganic nanomaterials have been developed for PTT [45].Molecular PTAs have definitive pharmacokinetic characteristics;while the high surface area and abundant active sites of nanomedicines rendered them extraordinary maneuverability [46].It is easy to connect diverse targeting, recognition, responsive and therapeutic moieties with diverse PTAs, to develop versatile platforms for enhanced theranostics for cancer therapy [46].
Nevertheless, the well-developed defense systems in cancer cells can protect them from hyperthermia [47-49].To completely ablate the tumor tissues, longstanding, ultrahigh temperature(>50 °C) is usually required [50-52].As a result, PTT may simultaneously destroy tumor tissue and damage the nearby healthy tissues due to the inevitable thermal diffusion.Moreover, traditionalPTT can also induce intense inflammation response and other adverse effects [48,53,54].Killing cancer cells at mild temperatures are imperative for clinical practice [55,56].Aiming to the defense systems in cancer cells, researchers have developed a series of novel PTAs for reducing the tolerant capacity of cancer cells, in another word, sensitizing cancer cells to hyperthermia.For instance,PTAs for heat shock protein (HSP) inhibition [48,57,58], autophagy modulation [59-61], organelle targeting [62-66], gas sensitization[67-70], and the combination with many other treatments [52,71-76] have been enthusiastically developed for LTPTT.
In this review, we systematically summarized the emerging strategies for LTPTT.The intrinsic mechanisms and signatures of different strategies are discussed.A series of typical examples of LTPTT are highlighted.At last, we described the fundamental requirements for LTPTT, our opinions on the current challenges and future perspectives were provided.
2.Classification of PTAs
PTAs and laser are the two key elements for PTT.PTAs are materials with broad NIR absorption and good photothermal conversion effect [77-83].PTAs in the ground state can effectively absorb photo energy, and the adsorbed energy was released as heatvianonradiative decay [84].The joint participation of light and PTAs in PTT renders the therapy excellent spatiotemporal controllability since only the tissue with PTA accumulation and laser irradiation can be heated [85-87].An ideal PTA should possess strong longwavelength absorption and good photothermal conversion effect,good tumor targeting ability and biocompatibility.Compared with UV-vis light (400-700 nm), NIR laser (750-1350 nm) has better tissue permeability, since it lies in the "optical transparency window"and less temperature increment can be induced when irradiating normal tissues with NIR laser, which not only transfer enough energy to the deeper disease tissues but also avoid the excessive injury to the skin tissues [88,89].Therefore, combining PTAs with NIR laser enable selectively "burn" the disease lesions.To date, a considerable number of PTAs have been developed for PTT [90,91],and can be divided into organic PTAs and inorganic PTAs.
Inorganic PTAs are stable, easy to synthesis/modification and possess good photothermal conversion effects [92,93].Ever since the report of using gold nanoparticles (AuNPs) for PTT in 2003[94], a considerable number of inorganic PTAs including noble metal materials, carbon materials, transition metal oxides/sulfides and so on have been explored [95-97].Most of the inorganic PTAs show relatively high photothermal conversion effect.With the development of NIR-II region fluorescence imaging, some PTAs were also proved with certain photothermal effect in the NIR-II region [43,98,99].Importantly, the relatively high drug loading effect makes inorganic PTAs especially useful for combining with other treatments for enhanced PTT.However, the poor biodegradability of inorganic PTAs might cause long-term toxicity to living bodies,and the commercialized inorganic PTAs are still rare.
Organic PTAs including small-molecule dyes, semiconductor polymers, melanin nanoparticles and so on [50,100-102].Although some organic PTAs have been clinically approved for tumor theranostics, the easy clearance and weak photostability are the key limitations.Small molecules can assemble or be loaded with biocompatible vehicles such as liposomes and polymers and form relatively stable nanoPTAs [65].When being intravenously injected into the bodies, PTAs could effectively accumulate in tumor tissuesviaeither passive or active targeting.Under further laser irradiation, these organic molecules can elevate the localized temperature to ablate solid tumors.Subsequently, these small molecules can be excreted/degraded, which ensures the biosafety of PTAs [84].As for nanoscale organic PTAs, their easy modification and potential biodegradability also rendered them with good biocompatibility[103].Therefore, the past years have witnessed the extensive application of cyanine dyes, polydopamine, porphyrins and semiconductor polymers in PTT.Table 1 summarized representative PTAs employed for LTPTT [43,51,58,59,63,65,66,70,72,76,99,104-142].
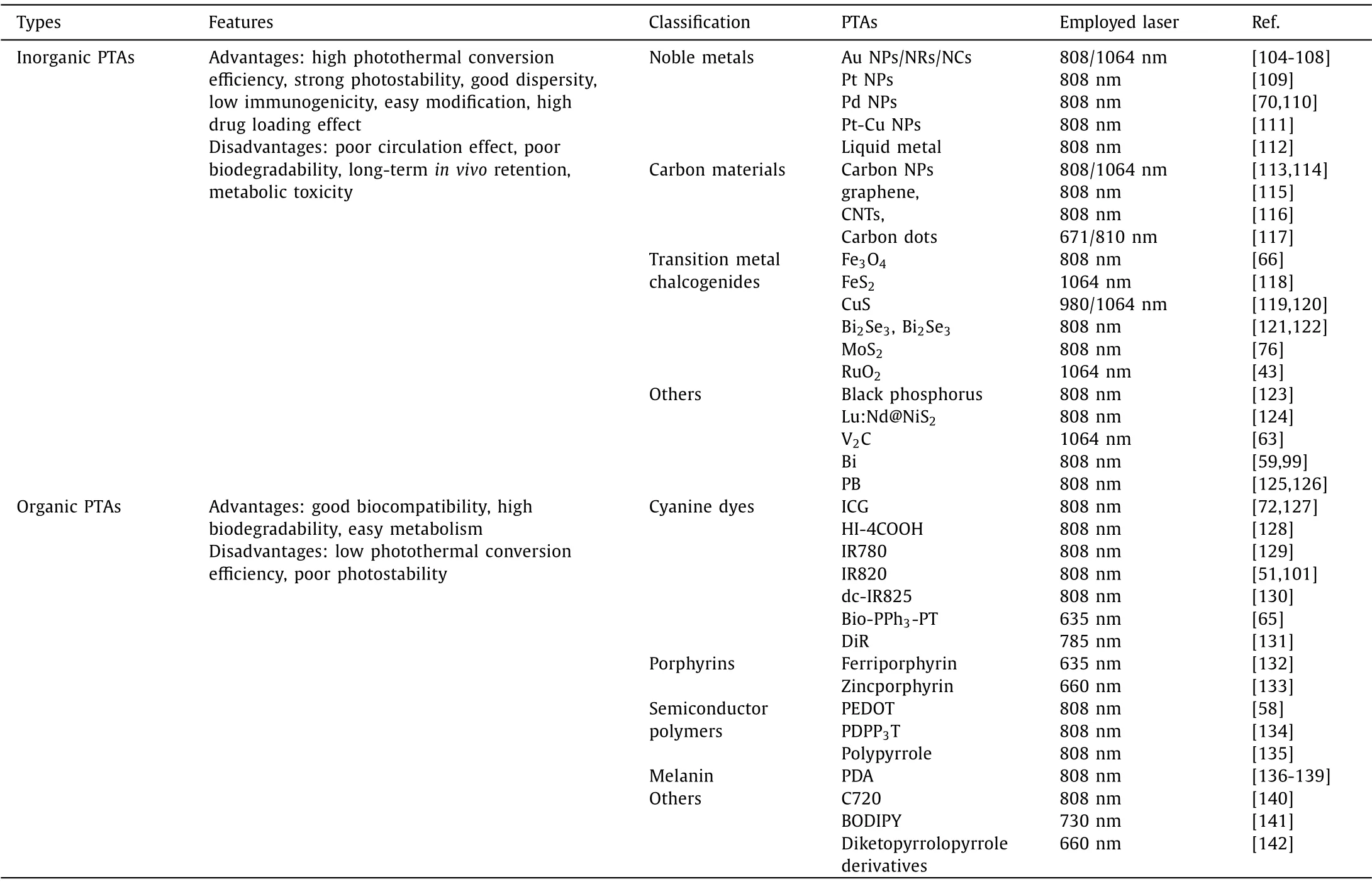
Table 1 Typical PTAs employed for LTPTT.
3.LTPTT by inhibition of HSPs
Heat shock proteins (HSPs) are a group of functional proteins that widely exist in almost all organisms from bacteria to humans[75].These ubiquitous molecular chaperones are the first defense system for cancer cells under stress.When the cells are exposed to dangerous environments such as hyperthermia, HSPs are rapidly expressed to facilitate intracellular protein refolding to further elevate the tolerance of cells [124,125].The intracellular expression levels of HSPs directly influences the therapeutic outcome of PTT.Inhibiting HSPs can reduce the thermal resistance of cancer cells to potentiate the cell-killing effect of hyperthermia.Therefore, nanoPTAs carried with HSPs inhibitors are widely developed to realize LTPTT.Currently, HSP inhibitors employed for the combination with different PTAs for LTPTT mainly include chemical inhibitors and small interfering RNAs (siRNAs) [72,143].
Benefiting from their low toxicity, high stability and good selectivity, chemical inhibitors are promising candidates for HSPs inhibition to attenuate the thermal resistance of cancer cells.However, most chemical HSP inhibitors suffer from poor tumortargeting effect, and they may cause toxic effects when being internalized by normal cells.Employing nanoPTAs as the carriers for these agents is therefore essential for enhanced drug delivery and stimuli-responsive release.In recent years, diverse HSP inhibitors such as epigallocatechin 3-gallate [124], VER155008 [57], gambogic acid [114,130,134], phenylethynesulfonamide [58], quercetin (Qu)[105,129], NVP-AUY922 [115], and triptolide [107] have been employed for combining with different nanoscale PTAs for LTPTT.
Metal-organic frameworks and coordination organic polymers with high surface area, excellent designability, good degradability, are suitable vehicles for chemical/biological drugs [144,145].Yanget al.reported the design and preparation of nanoscale coordination polymers (NCPs) by coordination between ICG, metal ions (Mn2+) and histidine (His)-tagged poly(ethylene glycol) (PEG)(pHis-PEG) [48].The developed Mn-ICG@pHis-PEG showed excellent tumor acidic pH-responsive tissue retention effect (Figs.1A and B).HSP90 inhibitor gambogic acid (GA) loaded Mn-ICG@pHis-PEG (Mn-ICG@ pHis-PEG/GA) effectively inhibited the expression of HSP90, enabling completely tumor growth inhibition under a mild temperature (43 °C) by the guidance of fluorescence, photoacoustic, and magnetic resonance imaging (MRI).In addition to directly loading HSP inhibitors onto PTAs, some natural HSP inhibitors with polyphenol structures could coordinate with metal ions to form nanoscale coordinative materials.Mao’s group reported the preparation of Qu-based NPs by the coordination between Qu, metal ions and poly(vinylpyrrolidone) (PVP)viaa onepot method [146].Qu-FeIIP consisting of Qu, Fe2+and PVP with the highest photothermal conversion efficiency could effectively ablate solid tumors under mild 808 nm laser irradiation benefiting from the HSP inhibition effect of Qu.Importantly, the obtained nanoscale PTA could avoid PTT-induced inflammation owing to the ROS scavenging and anti-inflammation effects of Qu(Fig.1C).siRNA featured by excellent specificity and high biosafety,are also explored for the down-regulation of HSPs in cancer cells[143].Nanoscale PTAs carrying siRNA targeting HSPs have been reported by several groups.Zhou’s group prepared HSP70 siRNA loaded cypate-conjugated porous upconversion nanocomposites(UCNPs) for LTPTT.The porous UCNPs simultaneously served as the MRI/upconversion luminescence dual-modal probe as well as the vehicles of cypate and siRNA.After intravenous injection, the nanoprobe with good dispersibility could accumulate in tumorviathe enhanced permeability and retention (EPR) effect.siRNA released from the pores could silence the HSP70 to enhance the therapeutic effect of PTT.Gu’s group developed HSP70 siRNA and PEG co-functionalized hollow gold nanoshell (HGN), which enables photothermal-controlled siRNA release and endosomal escape.More recently, Zhang’s group reported polydopamine (PDA)coated HSP70 siRNA loaded nucleic acid nanogel for programmed LTPTT (Fig.2).The PEGylated PDA shell effectively prolonged thein vivocirculation effect and prevented premature degradation of siRNA before reaching the tumor.
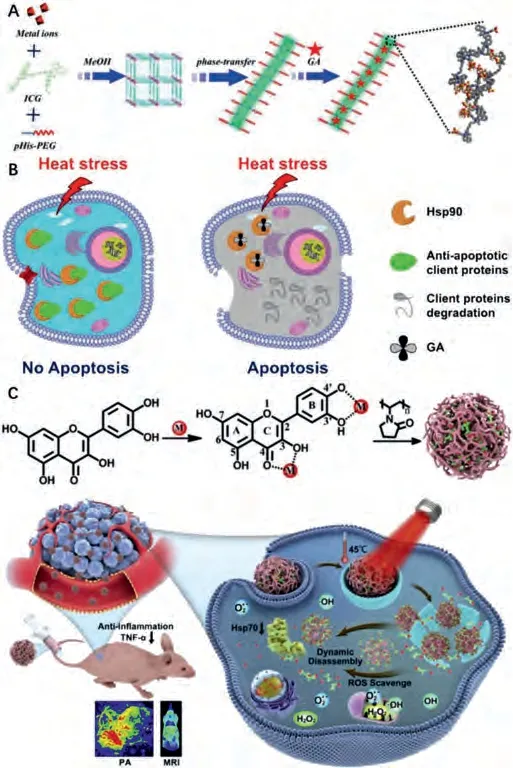
Fig.1.Schematic illustration of (A) the preparation and (B) the therapeutic mechanism of Mn-ICG@pHis-PEG/GA.Reproduced with permission [48].Copyright 2017,Wiley-VCH Verlag GmbH & Co.KGaA, Weinheim.(C) Schematic illustration of the preparation of Qu-based NPs for enhanced LTPTT.Reproduced with permission[146].Copyright 2019, Elsevier Ltd.
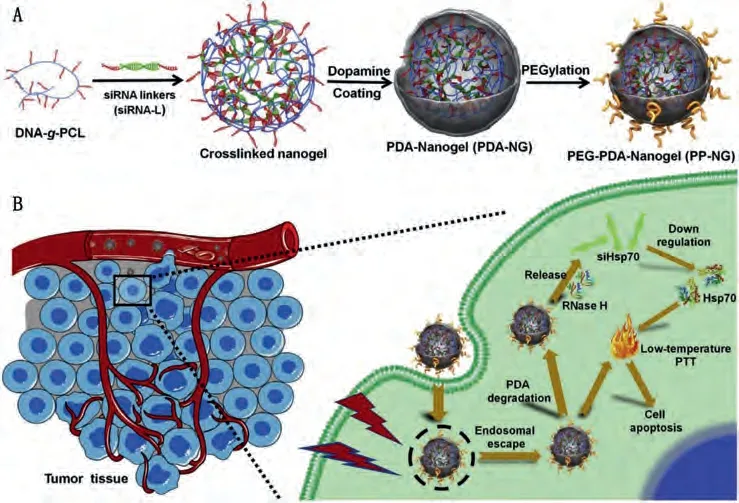
Fig.2.Schematic illustration of (A) the preparation and (B) the in vivo therapeutic effect of the PEGylated PDA coated siRNA delivery system.Copied with permission [139].Copyright 2020, Elsevier Ltd.
4.LTPTT by targeting organelles
Precise regulating the intracellular distribution of PTAs in cancer cells is another important strategy for LTPTT.For instance, the nucleus is the vital component for maintaining cellular functions and regulating homeostasis, which controls all the physiological processes during the whole life span of cells.Many clinically approved antitumor drugs especially chemotherapeutic drugs take the nucleus as their target.Any disturbance to the nuclear function can influence the gene expression of cells and affect the cell cycle.Minor temperature alternation in the nucleus may influence the activity of intranuclear enzymes that are vital to DNA replication and gene expression, which could further induce dysfunctions in cancer cells and cause cell death.Delivery of adequate PTAs into the nucleus is more easily to induce cell apoptosis under the same light irradiation dosage.Therefore, nucleus-targeted PTAs have received considerable research interest in recent years.To date, nucleus targeted Au-based nanomaterials [104], MXenes[63], Pd nanosheets [110], ruthenium(IV) oxide [43] and coordination polymers [128] have been reported for LTPTT.
Panet al.demonstrated the preparation of PEG/TAT dualfunctionalized gold nanorods (GNRs-NLS) for LTPTT (Figs.3A and B) [104].The PEG chain neutralizes the positive charge of TAT to ensure the long-term blood circulation and subsequent nucleus accumulation of GNRs-NLS in cancer cells.The therapeutic outcome of GNRs-NLS under 0.2 W/cm2808 nm laser irradiation was comparable with GNRs plus 2 W/cm2irradiation, although the tumor temperature of the former group is nearly 20 °C lower than the latter one.The nucleus-targeting strategy significantly reduced the required laser power density, opening a promising avenue for solid tumor treatment.In addition to directly entering the nucleus,nanomaterials accumulation in the perinuclear sites was reported to directly influence the function of cancer cells.Wu’s group reported that TAT-functionalized Pd nanosheets [110].accumulated in the perinuclear of MCF-7 cancer cells stimulated the overexpression of lamin A/C proteins to enhance the nuclear stiffness and inhibit cell migration.Further irradiation by 0.3 W/cm2mild 808 nm laser effectively accelerated the nuclear entry of PTAs, inhibiting cancer metastasis and inducing cancer cell apoptosis.In addition to modifying nanomaterials with nucleus-targeting ligands, PTA with intrinsic nucleus-targeting capability has been reported recently by Li and Wu’s groups [128].The coordination polymer consisting of heptamethine cyanine dye and hafnium ions effectively accumulated in the nucleus of tumor cells, and eliminated the 4T1 tumor by LTPTT.
Fig.3.(A, B) Schematic illustration of GNRs-NLS for nucleus-targeted PTT.Copied with permission [104].Copyright 2017, American Chemical Society.(C) Schematic illustration of the preparation of V2C-PEG-TAT@Ex-RGD and (D) the related therapeutic mechanism.Reproduced with permission [63].Copyright 2019, American Chemical Society.
Laser in the NIR-II (1000-1350 nm) window is more permeable in tissues, and the maximum permissible exposure (MPE) for skin exposure at NIR-II region is much higher than that at the NIR-I region (750-1000 nm).Employing NIR-II laser for nucleus targeted LTPTT was recently reported by Zhang and Dong’s group [63].TAT modified small fluorescent V2C quantum dots (QDs) were encapsulated within tumor-targeting peptide (RGD) engineered endogenous exosomes to obtain the dual-targeted nanomedicine (V2CPEG-TAT@Ex-RGD, Figs.3C and D).The targeted materials possess excellent immune escaping and tumor-nucleus targeting effect.Under 0.96 W/cm21064 nm laser irradiation, the tumor temperature was elevated to ~45 °C.The LTPTT mediated by V2CPEG-TAT@Ex-RGD completely inhibited the tumor growth in MCF-7 tumor-bearing mice without side effects.Nucleus-targeted LTPTT with NIR-II laser may be more effective, safe and suitable for deep tumor PTT.
In addition to the nucleus, mitochondria, the primary generation center of ROS, is also hypersensitive to heat.Delivery of PTAs into the mitochondria of cancer cells is another strategy for LTPTT.Nanomaterials are easier to accumulate into the mitochondria because they don’t need to cross the hurdles such as karyotheca[62].It is reported that AuNPs with small size have neglectable photothermal conversion effect, which was significantly elevated after aggregation due to the activation of interparticle plasmonic coupling effect [147].Such phenomena significantly stimulated the development of activatable PTT.Recently, Hanet al.realized effective LTPTT using triphenyl-phosphonium (TPP)-functionalized AuNPs [64].These NPs can specifically accumulate into the mitochondria of cancer cells to turn on the PTT effect.The cancer cellselective accumulation was attributed to the mitochondrial membrane potential of cancer cells are lower than that of normal cells.Upon NIR laser irradiation, highly efficient tumor ablation was realized.It has minimal damage effect on normal cells since the photothermal effect of non-aggregated AuNPs are much lower under laser irradiation.Tang’s group developed a dual-targeted selfassembled organic PTA for highly efficient cancer therapy (Fig.4)[65].The TPP and biotin dual-modified cyanine was co-assembled with FDA approved polymer F127 to afford the nanoPTA, which showed enhanced tumor-mitochondria accumulation.Under a single 0.5 W/cm2laser irradiation for 5 min, the 4T1 tumor was completely suppressed.
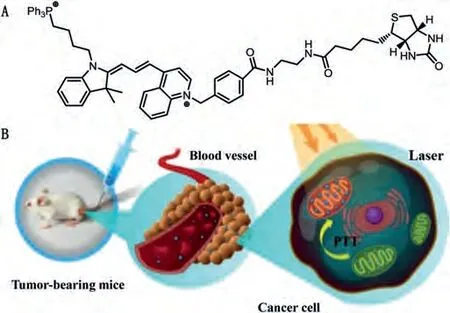
Fig.4.(A) The chemical structure of the TPP and biotin dual-modified cyanine and(B) the schematic illustration of its application in LTPTT.Reproduced with permission [65].Copyright 2019, Wiley-VCH Verlag GmbH & Co.KGaA, Weinheim.
Lysosomes and endoplasmic reticulum are also proven to be potential targets for LTPTT.Precisely targeting PTAs into these organelles have also made success for improved cancer treatment in recent years [62,148-151].
5.LTPTT by disturbing autophagy
Autophagy is another vital intracellular defense system.Cancer cells degrade and recycle the misfolded proteins and disordered organelles by autophagy to refresh the cell microenvironments [152].During PTT, the damaged proteins and organelles are lysed by autophagy, thus the injury degree during treatment can be reduced[60].Selectively modulation of the autophagy process which will hamper the vital defense pathway in cancer cells, is another reasonable route for sensitizing cancer cells to hyperthermia.To disrupt the autophagy homeostasis, inhibiting the pro-survival autophagy and inducing pro-death autophagy are developed recently[61,74].
PDA nanomaterials have received increasing interest in the past decade for biomedical applications like tissue engineering, drug delivery and PTT owing to their multifunctionality [153-158].Our group revealed PTT treatment of cancer cells will induce prosurvival autophagy [60].Autophagy inhibitor CQ loaded PEGylated PDA nanoparticles (PDA-PEG/CQ) could serve as an autophagy regulatable PTAs, hampering the pro-survival autophagy in cancer cells for enhanced tumor therapy under mild hyperthermia(Fig.5A).Thereafter, bismuth crystals embedded silica nanoparticles (Bi@SiO2) [59] and Prussian blue [126] were also employed for CQ loading for LTPTT.For bone tumors, it is recognized that the tumor can secrete cytokines to induce osteoclastogenesis, which could in turn adsorb bone matrix to release growth factors and further accelerate tumor growth.Such a vicious cycle between bone resorption and tumor progression can further facilitate tumor metastasis, and make bone tumors hard to treat [159].In this case, alendronate-PEG functionalized PDA loaded with CQ (PPA/CQ)was prepared for bone tumor therapy [138].PPA/CQ specifically targeted the osteolytic lesions near tumor tissues.CQ released from the targeted nanoparticles could simultaneously hinder the degradation of TNF receptor-associated receptor 3 to prevent osteoclastogenesis and hamper cancer cell apoptosis to elevate their thermal sensitivity.Excellent LTPTT effect was demonstrated with nanomedicine due to successful blockage of vicious cycle in the bone tumor microenvironment.
Recently, our group introduced beclin 1, an autophagy induction peptide onto RGD-functionalized PDA nanoparticles for LTPTT(Fig.5B) [61].The obtained nanoscale PTAs (PPBR) show enhanced tumor accumulation effect.After entering cancer cells, PPBR could induce overwhelming autophagy without obvious systematic toxicity.The activation of excess autophagy was demonstrated to be another feasible strategy to sensitize cancer cells to hyperthermia.Therefore, PPBR effectively suppressed the tumor growth in an MDA-MB-231 tumor model under 43 °C.
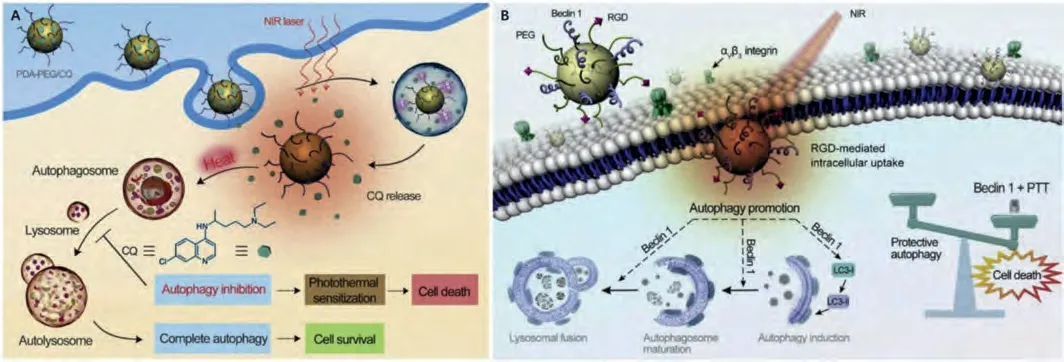
Fig.5.Schematic illustration of (A) autophagy inhibition and (B) autophagy induction sensitized LTPTT mediated by PDA-PEG/CQ and PPBR, respectively.Copied with permission [60,61].Copyright 2017 and 2019, Elsevier Ltd.
6.LTPTT by combination with other treatments
In addition to the above strategies, combining PTT with many other therapeutic modalities have also been investigated.The synergistic therapeutic effects of these therapies on PTT can also reduce the resistance of cancer cells to hyperthermia [117,119].Moreover, several synergistic approaches were proven to offer extra benefits [118,142,160,161].For instance, PTT may enhance the blood oxygenation by enhancing blood flow, reactive oxygen species (ROS)-based therapies combined with PTT would not only damage intracellular thermal defense systems, but also elevate the therapeutic effectviaenhanced oxygen content in the tumor [74,76].The past years have witnessed the combination of PTT with chemotherapy, photodynamic therapy, radiotherapy, immunotherapy, metabolic intervene, chemodynamic therapy (CDT)and multimodal therapy for enhanced cancer treatment under mild conditions.Benefiting from the supra-additive synergistic effect of“1 + 1>2”, the rationally designed strategies were generally more effective for tumor inhibition.
6.1.ROS-based treatments
ROS can oxidize diverse biomolecules such as proteins, nucleic acids, and lipids [162,163].The enhanced ROS levels in cancer cells can disrupt the redox homeostasis and induce oxidative stress to trigger cell damage and death.Strategies for elevating the intracellular ROS levels were developed for cancer treatment [164].
As discussed above, PDT mainly employs photosensitizers, laser and oxygen to induce oxidative stress in cancer cells.Combining PDT with PTT could synergistically resolve the disadvantages of the treatments.It should be noted that the excitation wavelength of photosensitizers and PTAs are usually different, the employment of different lasers to trigger PDT and PTT may result in unwanted normal tissue damage.Xia’s group reported the design of an ICGloaded AuNRs/MoS2dual plasmonic PTAs (Au/MoS2-ICG) for synergistic PDT and PTT combination therapy [165].Under a single laser irradiation, Au/MoS2effectively converts light to heat and triggers the release of absorbed ICG to activate PDT.Upon 5 min low power laser irradiation (808 nm, 0.2 W/cm2), PDT synergistically regressed tumorviaLTPTT.
Radiation therapy is the main strategy for clinical cancer treatment, which typically utilizes high energy ionizing radiation to induce oxidative stress and DNA damage in cancer cells [166,167].Liu’s group reported the development of a radionuclide131I-loaded PEGylated reduced graphene oxide nanoplatform (131I-RGO-PEG)for internal radiation sensitized PTT [74].The obtained nanoplatform could emit intense X-ray to inhibit tumor cells.Further irradiation by an 808 nm laser at a power density of 0.2 W/cm2for 20 min,131I-RGO-PEG completely inhibited tumor growth without obvious side effects within a 50-day observation period.
CDT employs catalysts to convert intratumoral overexpressed H2O2into ˙OH, which is a highly tumor-specific treatment without the requirement of external excitation.Combining CDT with PTT is another feasible tactic for reducing the therapeutic resistance of PTT.Ouet al.reported a BODIPY-Fe(III) crosslinked coordination NPs for CDT sensitized LTPTT (Fig.6) [141].The coordination NPs show enhanced NIR-II absorption and good catalytic activity towards H2O2, which completely eliminated the solid tumor after treatment.Recently, radical initiators have also been explored to induce intracellular radical accumulation for LTPTT [76].Very recently, Lin and Hou’s group developed a MOF-derived Pd single atom enzyme-loaded nanoPTA for ferroptosis promoted LTPTT[168].This nanoPTA exhibited peroxidase and GSH oxidase like activity, which could specifically generate hydroxyl radicals and eliminate GSH in cancer cells, and subsequently deactivate the intracellular GPX4 and induce lipid peroxidation.Finally, the ferroptosis as well as ROS inhibited HSP expression could facilitate NIR-II laser mediated LTPTT.
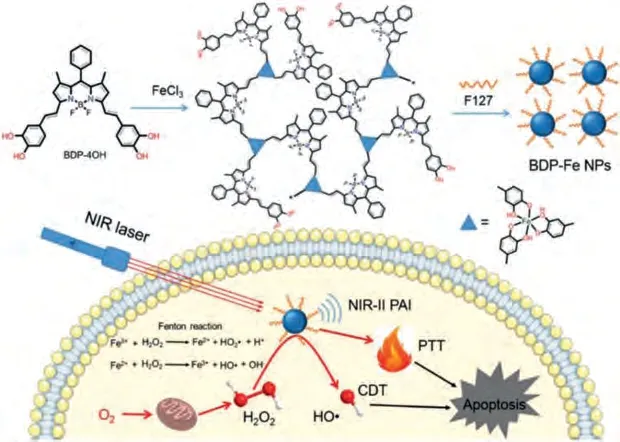
Fig.6.Schematic illustration of BODIPY-Fe(III) and its application for CDT sensitized PTT.Copied with permission [141].Copyright 2020, the Royal Society of Chemistry.
6.2.Gas sensitization
Gas therapy is an emerging cancer treatment strategy [137].Many gas molecules are proven to play decisive physiological roles in cellular signal transduction [162].Introducing gas signaling molecules into cancer cells may also break the homeostasis and trigger cell death, which has recently been explored as gas therapy[113,169-171].The successes in gas therapy inspired researchers to employ gas molecules to potentiate the efficiency of PTT, since selective delivering gas molecules such as NO, CO, H2and SO2into cancer cells can disturb the homeostasis in cancer cells, and reduce their thermal resistance.
NO is the earliest gas signal molecule identified by researchers which takes part in numerous physiological activities [162].Elevating the intracellular NO level has been developed for sensitizing cancer cells to chemotherapy, radiotherapy and so on.However,traditional NO donors are hard to realize controlled release.Zhao and Gu’s group prepared Bi2S3NPs loaded with bis-N-nitroso compounds (BNN) for on-demand NIR-responsive NO release (BNNBi2S3) (Figs.7A and B) [121].Under 808 nm laser irradiation, the high photothermal conversion effect of Bi2S3could effectively enhance the localized temperature to trigger the release of NO.The released NO can freely diffuse in the tumor tissues to inhibit the defensive process such as autophagic self-repairing to overcome the thermal resistance.As a result, a higher tumor inhibition effect was achieved for BNN-Bi2S3under mild NIR laser irradiation.Mesoporous silica nanoparticle (MSN) encapsulated Nb2C was used for S-nitrosothiol (SNO) delivery, and the NO release from the carrier can be triggered by a NIR-II laser for LTPTT [68].Wanget al.reported the use of MSN-coated Au nanorods as the carrier of SNO and PTA [67].These examples proved the potential of sensitizing cancer cells to hyperthermia by NO delivery.
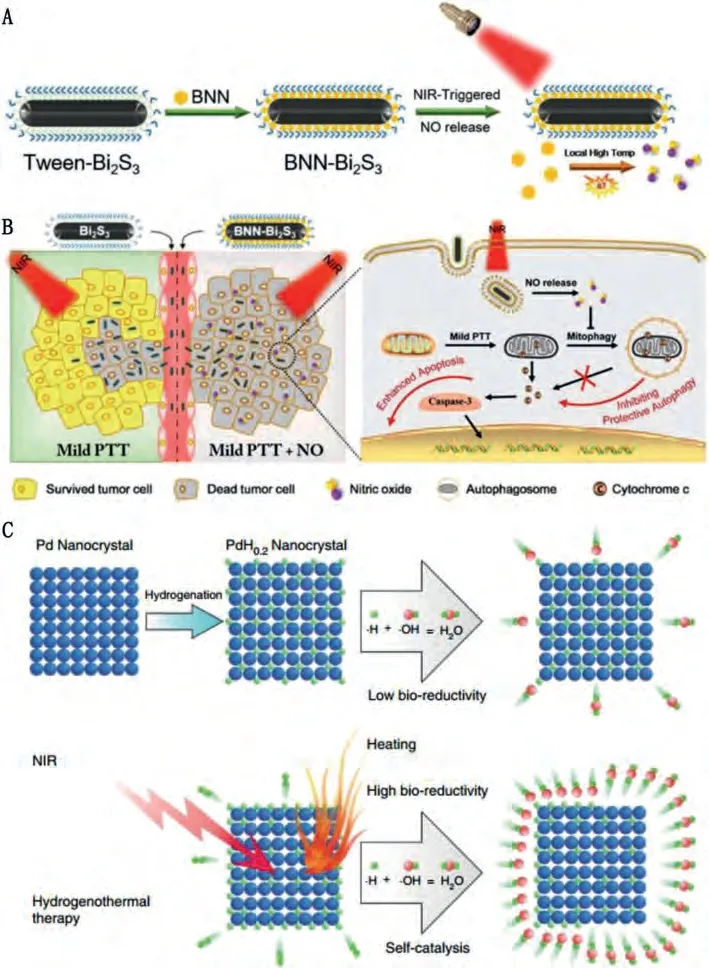
Fig.7.Schematic illustration of (A) the construction and (B) LTPTT application of BNN-Bi2S3.Copied with permission [121].Copyright 2018, Wiley-VCH Verlag GmbH & Co.KGaA, Weinheim.(C) Schematic illustration of the preparation of PdH0.2 and the NIR-responsive H2 release for LTPTT.Copied with permission [70].Copyright 2018, Springer Nature.
H2is an endogenous anti-oxidation gas molecule.It has been explored for the treatment of a series of major diseases like cancer,inflammation, diabetes, atherosclerosis, Alzheimer’s disease and so on [172].But the poor solubility of H2in physiological solutions restricted their applications by systematic injection.Inspired by the excellent H2storage effect of Pd nanocrystals, He and Gu’s groups developed Pd hydride (PdH0.2) for controlled H2delivery and release (Fig.7C) [70].After intravenous injection, PdH0.2with improved photothermal effect could effectively accumulate in tumor tissuesviathe EPR effect.Under 808 nm NIR laser irradiation, highly reductive H2was released to induce intracellular reductive stress, which effectively elevated the tumor inhibition effect by PTT.Interestingly, the side effects of PTT to the normal tissues can be reduced by the reductive H2molecules.
SO2, another important signaling molecule, has also been employed for sensitizing tumor therapy in recent years.However, efficient delivery of SO2prodrug and responsive release of SO2into deep tumor tissues remain challenging.Li’s group synthesized PDA encapsulated Au nanorods for the delivery of SO2prodrug benzothiazole sulfinate (BTS) [69].BTS could release SO2under both acidic microenvironment and hyperthermia conditions.External and internal stimuli can therefore trigger the release of SO2for LTPTT.Importantly, the released SO2can freely penetrate into the deeper tumor tissues to induce cell apoptosis, thus this platform could serve as a promising candidate for deep tumor PTT.
6.3.Chemotherapy
The non-specific distribution and resistance of chemotherapeutic drugs significantly restricted their clinical applications.NIR controlled on-demand drug release with nanoPTAs may not only resolve the side effects of chemotherapeutics but also effectively potentiate the PTT efficiency.Tang’s group developed an NIR controlled drug delivery system by coating ssDNA on MSN-coated gold nanorods (AuNRs@MS-DNA) [173].The negatively charged and flexible ssDNA chains were tightly coated on amino-modified MSN to prevent DOX leakage.Under mild 808 nm NIR irradiation, the localized hyperthermia mediated by AuNRs could disrupt the electrostatic interaction between ssDNA and MSN and trigger DOX release for cancer cell inhibition.When the laser was off, the valve was re-absorbed by MSN to stop drug release.This NIR controllable drug release nanoplatform offers a promising strategy for preventing unwanted drug leakage and sensitizing PTT by chemotherapy.In addition to nanocarriers, photothermal active hydrogels could also serve as promising platforms for drug loading and lowpower NIR triggered drug release.In 2017, our group developed PEG crosslinked PDA hydrogel for on-demand drug release [153].7-Ethyl-10-hydroxycamptothecin (SN38) was loaded on PDA NPsvia π-πstacking without obvious leakage.Under mild NIR irradiation, SN38 could be released on-demand due to the generation of mild hyperthermia (Fig.8).Thus, highly efficient photothermal chemotherapy was realized with this platform without obvious side effects.
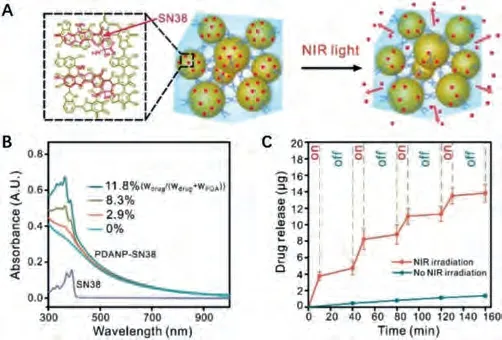
Fig.8.Schematic represents SN38 loaded on PDANPs via π-π stacking and released from PDA/PEG hydrogel upon NIR irradiation.Copied with permission [153].Copyright 2017, American Chemical Society.
6.4.Immunotherapy
Immunotherapy is a promising and effective strategy for cancer treatment, has made significant clinical success over the past decade [108,174-177].By inducing an intense immune response in living bodies, immune therapy can effectively kill tumor cells[175,178].PTT has been recognized as a useful method to induce immunogenic cell death (ICD) to enhance the expression of cancer-specific antigens [108], which could serve as an “Eat me”signal for antigen-presenting cells for cancer cell recognition and immune activation.Moreover, PTT can reduce the compact structure, improve intratumoral blood/oxygen supply, and reduce interstitial fluid pressure of solid tumors, which can be helpful for reconstructing tumor immunosuppressive microenvironment and enhancing the recruitment and infiltration of immune cell [175,179].Therefore, combining immunotherapy to enhance PTT has received increasing interest.Dotti and Guet al.found that ICG-loaded PLGA nanoparticles can synergy with CAR.CSPG4+T cells to effectively eliminate the WM115 tumors in mice (Fig.9) [47].Huanget al.loaded the anti-PD-L1 antibody and PTA IR820 in a phase change lipid gel, which could release the antibodies under mild 808 nm laser irradiation and improve the recruitment of systematic T cells to turn the “cold” tumors to “hot” ones for improved tumor regression [51].
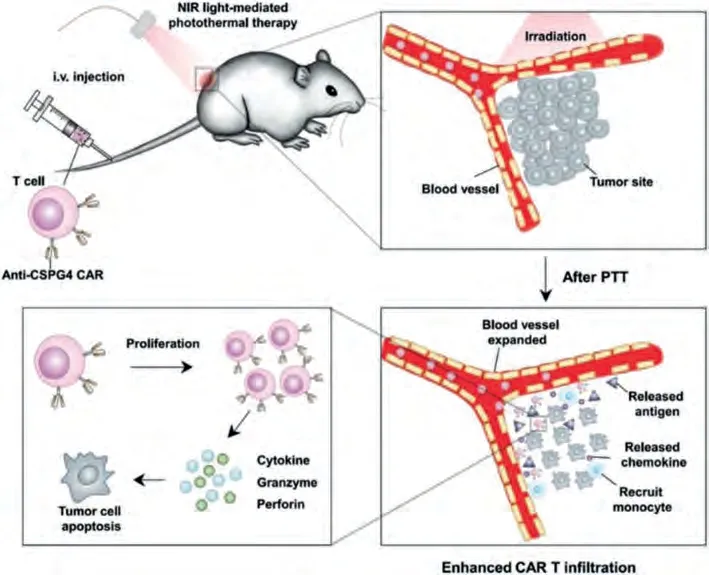
Fig.9.Schematic illustration of the PTT-immunotherapy of based on ICG@PLGA and CAR.CSPG4+ T cells.Copied with permission [47].Copyright 2019, Wiley-VCH Verlag GmbH & Co.KGaA, Weinheim.
6.5.Starvation therapy
Starvation therapy mainly induces cancer cell death by cutting off the nutrition supply.Blocking the blood vascular systems in tumors or inhibiting the cellular metabolism are the two major ways for starvation therapy [100,112].Recently, Cai’s group demonstrated glucose oxidase (GOx) mediated starvation therapy could effectively inhibit the intracellular HSPs and enhance the therapeutic effect of LTPTT (Figs.10A and B) [125].Hyaluronic acid-modified porous hollow Prussian blue NPs (PHPBNs) were employed as the PTAs and the carriers for GOx.Afteri.v.injection, the nanosystem could accumulate in tumor tissuesviaCD44 targeting acceptor.The GOx were further released to consume the glucose in tumor tissues when the disulfide bond between HA and PHPBNs was cleaved by GSH.Benefiting from the catalase-like activity of PHPBNs, the H2O2generated from GOx and glucose was decomposed into O2to relieve the hypoxia microenvironment.The excellent starvation effect of GOx could prohibit the expression of HSPs (HSP70 and HSP90),therefore, this nanosystem showed a superior tumor inhibition effect under mild temperature elevation.
6.6.Multi-modal therapy
In addition to combining with monotherapies, versatile photothermal nanoplatforms with multiple therapeutic effects were also widely developed for LTPTT.These multifunctional nanomedicines could effectively overcome the intrinsic limitations of PTT, and higher therapeutic effects can be realized by inducing cancer cell deathviamultiple pathways.During the past years,multimodal therapy sensitized LTPTT based on PDA [52,136,137],liposomes [72], layered double hydroxide [179], MOFs [132], Bi2Se3[122], CuS [120], and so on were widely developed [180,181].For example, NO, DOX sensitized LTPTT based on PDA NPs were proved to be more effective than NO or DOX sensitized PTT on multidrug resistance tumors [136,137].GOx, GA and ICG encapsulated liposomes (GOIGL) could effectively target tumor tissues, regress solid tumorviasynergistic starvation and HSP inhibition sensitized LTPTT (Fig.10C) [72].HSP70 siRNA loaded versatile zirconiumferriporphyrin MOF was reported with excellent therapeutic effectviaphotodynamic therapy, CDT and HSP inhibition enhanced PTT[132].
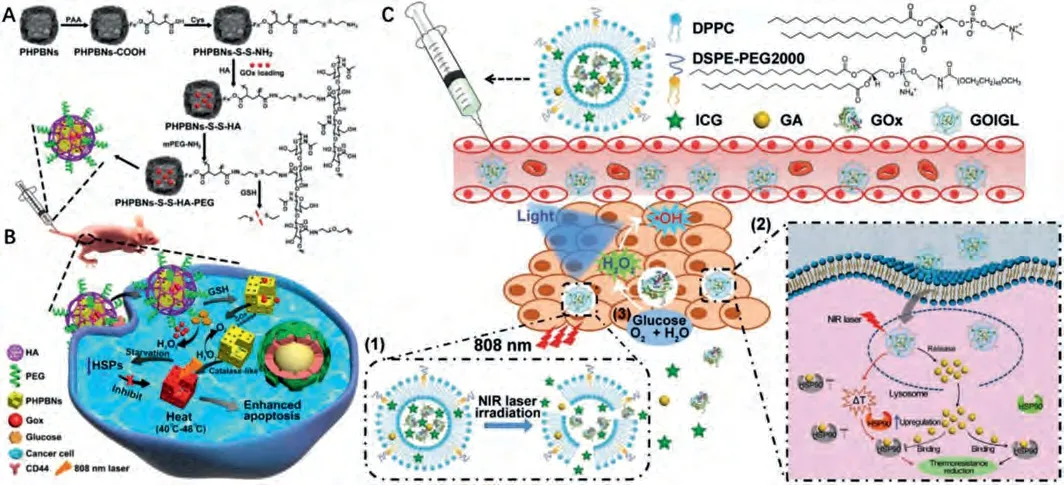
Fig.10.(A) Schematic illustration of the structure of PHPBNs-S-S-HA-PEG@GOx and (B) its application in starvation therapy sensitized LTPTT.Reproduced with permission[125].Copyright 2018, American Chemical Society.(C) Schematic illustration of GOIGL and its mechanism for enhanced LTPTT.Copied with permission [72].Copyright 2020,Wiley-VCH Verlag GmbH & Co.KGaA, Weinheim.
7.Summary and perspectives
In summary, PTT featured with excellent spatiotemporal controllability has become a promising method for cancer therapy.However, negative factors such as the pro-survival autophagy and the expression of HSPs in cancer cells, the poor tissue penetration of laser and the relatively low photothermal conversion efficiency of PTAs severely compromise the therapeutic outcome.To ensure a satisfied therapeutic effect, long-term and high-power density laser irradiations are required for traditional PTT, which will cause hyperthermia-induced inflammation and permanent damages to normal tissues due to inevitable thermal diffusion.Thus, LTPTT has been developed for efficient tumor inhibition under milder temperatures.Aiming at overcoming the defense systems in cancer cells, many strategies have been established for effective LTPTT.In this review, we systematically summarized the achievements of emerging tactics for LTPTT and discussed their intrinsic mechanisms.However, there are still many challenges that remain to be specified and overcome.
The imperative tasks for further investigations on LTPTT may include the following aspects: (1) Developing novel PTAs with higher photothermal conversion efficiency.The conversion efficiency of PTAs directly determines the therapeutic effect and required laser parameters for PTT, the values for most of the current PTAs are lower than 40%.Rational design of PTAs with higher photothermal conversion efficiencies may reduce the requirements for irradiation time and laser power density.(2) Enhancing the tumor targeting efficiency.The tumor accumulation for most molecular/nanodrugs is lower than 5%, and the latest research by Chan’s group revealed more than 97% of the nanoparticles entering solid tumorsviathe active process of endothelial cells, which calls for further investigations for enhancing the tumor-targeted delivery [182].In addition to targeting tumor tissues, delivery of nanodrugs into specific cell lines such as fibroblast and tumor stem cells as well as vital organelles will bring new insights for LTPTT.For superficial tumors, hydrogels [183] are ideal candidates.(3) Improving penetration depth of the laser.NIR-II laser has been proved with better tissue penetration capacity, and the maximum permissible exposure of laser at the NIR-II region is about 3-fold higher than that at the NIR-I region.LTPTT with laser in the NIR-II region should be more effective.As for other deep tumors such as liver cancer, employing ultrasound, magnetic field and therapeutic light guide fiber may be alternative choices for hyperthermia therapy.(4) Enhancing the specificity of LTPTT.Although LTPTT is highly controllable by the laser irradiation time and region, it may still cause damage to the nearby normal tissues with PTAs accumulation since the therapeutic functions of PTAs are always on.Developing more smart PTAs with tumor cell/microenvironment specificity, rendering them with imaging guiding, temperature/therapeutic effect monitoring functions will further pave the applications of LTPTT.However, the enhanced multifunctionality may increase the complexity and preparation cost of PTAs, developing PTAs with intrinsic multifunctionalities is highly desired.(5) Optimizing the synergistic enhancement of different treatments/therapeutic drugs on LTPTT.Though many treatments have been reported to synergize with PTT by overcoming the resistance of cancer cells, the intrinsic improvements still remained to be cleared.More specific parameters like drug loading strategy, loading amount can be optimized.Moreover, combining multimodal therapeutics with LTPTT may maximize the therapeutic outcome once the potential cumulative side effects can be resolved.(6) Evaluating the biosafety of PTAs for LTPTT.Considering the inorganic/non-biodegradable nature of most of the employed PTAs in PTT investigations, their biosafety is another critical concern hampering the further clinical translation of LTPTT.Moreover, combining these PTAs with other treatments may further complicate their biosafety.Systematically evaluating the biocompatibility of diverse PTAs before clinical testing is required.(7)Performing tests on more clinically relevant animal models.All the currently reported works were performed on tumor-bearing mice,which are still far from the features for clinical diseases.Performing experiments on more clinically relevant models such as orthotopic tumor-bearing large animals are thus highly recommended to further investigate the potential of LTPTT.Overall, we are confident that LTPTT will broaden the applications of PTT in cancer treatment after the challenges are well resolved under the significant efforts from clinical doctors and scientific researchers.
Declaration of competing interest
There are no conflicts to declare.
Acknowledgments
This work was financially supported by the Guangdong Provincial Key Laboratory of Functional and Intelligent Hybrid Materials and Devices (No.2019B121203003).
杂志排行
Chinese Chemical Letters的其它文章
- Comment on “Acid-induced tunable white light emission based on triphenylamine derivatives”
- Liposome-based delivery of biological drugs
- Macrophage-targeted nanomedicine for chronic diseases immunotherapy
- Advances, opportunities, and challenge for full-color emissive carbon dots
- Fluorine-containing agrochemicals in the last decade and approaches for fluorine incorporation
- Critical review of perovskites-based advanced oxidation processes for wastewater treatment: Operational parameters, reaction mechanisms,and prospects
