Solid-phase impregnation promotes Ce doping in TiO2 for boosted denitration of CeO2/TiO2 catalysts
2022-06-18WngSongJiwiJiKiGuoXinWngXioqinWiYniCiWiTnLuluLiJingfngSunChngjinTngLinDong
Wng Song, Jiwi Ji, Ki Guo, Xin Wng, Xioqin Wi, Yni Ci, Wi Tn,Lulu Li, Jingfng Sun, Chngjin Tng, Lin Dong,,
a School of Chemistry and Chemical Engineering, Nanjing University, Nanjing 210093, China
b Key Laboratory of Vehicles Emission Control of Jiangsu Province, Center of Modern Analysis, Nanjing University, Nanjing 210093, China
c School of Environment, Nanjing Normal University, Nanjing 210023, China
d School of the Environment, Nanjing University, Nanjing 210093, China
e School of Environmental and Chemical Engineering, Jiangsu University of Science and Technology, Zhenjiang 212003, China
ABSTRACT CeO2/TiO2 (denoted as CeTi) catalysts obtained by solid-phase impregnation behaved better in lowtemperature selective catalytic reduction of NOx with NH3 (NH3-SCR) than that by conventional wet impregnation.To explore the main factors for activity distinction, the texture property, CeO2 dispersion and structure changes of TiO2 were comprehensively analyzed.It was found that surface changes of TiO2 had a significant impact on the improved activity.From results of inductively coupled plasma atomic emission spectrometer (ICP-AES), diffuse reflectance UV–vis spectroscopy (UV–vis-DRS) and Raman, it was inferred that Ce ions were partially incorporated into TiO2 lattice, accompanied with the formation of defects and vacancies during solid-phase impregnation.Accordingly, CeTi catalysts from solid-phase impregnation exhibited superiority in adsorption and activation of reactants.Further result from monitoring the preparation process indicated that the evolved NO played an important role in promoting Ce doping through depriving oxygen atoms on TiO2 surface.The interaction between Ce and Ti was enhanced.The catalyst performed better in NH3-SCR, especially at low temperature, which testified the solid-phase impregnation could be an effective method to modulate interface structure for designing efficient catalyst.
Keywords:CeO2/TiO2 NH3-SCR Solid-phase impregnation Interfacial interaction NO induced incorporation
Air pollution has been severe in China and nitrogen oxide (NOx)is one of the major contaminants.NOxemission elicits problems such as ozone holes, acid rain, photochemical smog, human respiratory diseases [1-3].Till now, catalytic reduction of NOxwith ammonia (NH3-SCR) is the dominant technology for nitrogen oxide elimination [2,3].Currently, the commercial catalysts for NH3-SCR are V2O5-WO3(MoO3)/TiO2.They show excellent activity at 300–420 °C with admirable SO2tolerance.Nevertheless, several drawbacks are also confronted, such as narrow operating temperature window, poor N2selectivity at high temperature, and toxicity of V2O5[4,5].To overcome these obstacles, tremendous attention has been paid to improving V-based catalysts or developing novel catalysts like Ce, Mn, Fe, Cu-based catalysts,etc.[6-11].As a typical Ce-based catalyst, CeTi has been widely investigated in recent decades due to its excellent performance in the mid temperature and environmental-friendly feature [12].
Conventional preparation methods of CeTi catalyst, such as wet impregnation, co-precipitation and sol-gel method [13], are ubiquitously operated in solution, involving evaporation and drying.Except for high energy consumption, evaporation of solvent can also lead to the redispersion of active components and phase segregation, which are not conducive to the improvement of catalytic activity [14].We previously reported an approach called solid-phase impregnation to synthesize supported catalysts [15,16].During the process, the active component precursor (nitrate) and supports mechanically are mixed and then calcined.In addition to avoiding these disadvantages, the interaction between active components and carriers can also be enhanced [17,18].Herein, the CeTi catalysts were rationally constructed by solid-phase impregnation(CeTi-G) and compared to the counterparts from wet impregnation(CeTi-I).The detailed preparation procedure and activity test condition are shown in the supporting information.The activity results showed that CeTi-G samples exhibited superior performance, especially at low temperature.Furthermore,viavarious characterization techniques and tracing the catalyst preparation process, the driving force for the enhanced activity of CeTi-G was revealed.
The NH3-SCR activity test result is shown in Fig.1.It is obvious that irrespective of ceria loading, the sample prepared by the solidphase impregnation method showed better activity, especially at low temperature range (≤300 °C).For instance, at 300 °C the NO conversion of CeTi-G0.1 was 81%, which was much higher than that of CeTi-I0.1 (59%).There was almost no N2O observed (less than 20 ppm) during the reaction for all samples, which demonstrated excellent N2selectivity of CeTi based catalyst (Fig.S1 in Supporting information) [19,20].
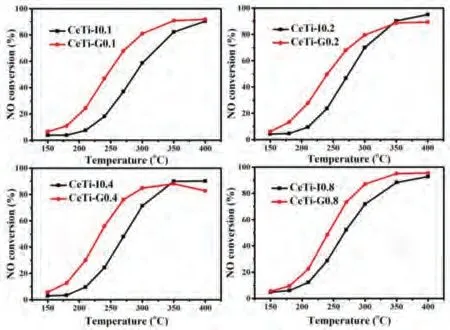
Fig.1.NO conversion of CeTi catalysts.Reaction conditions: [NO] = [NH3] = 500 ppm, [O2] = 5 vol%, Ar balance and WHSV = 60,000 mL g-1 h-1.
Previous studies showed that catalytic activity of supported catalysts is dependent on the particle size and dispersion of active component over supports [21-23].As shown in the Table S1 and Fig.S2 (Supporting information), no conspicuous difference could be observed for the specific surface area, pore volume, pore size and particle size.Given that, it is concluded that texture properties had limited impact on performance of both samples.Furthermore, the dispersion of active phase over support was measured by X-ray diffraction (Fig.2a).When the loading amount is below 0.4 mmol CeO2/g TiO2, only peaks attributed to anatase(PDF# 21-1272) emerged, suggesting that CeO2was in a well dispersion state.Further increasing loading amount, CeO2(PDF# 34-0394) crystal phase peak did appear in the CeTi-I0.4, while did not exist in CeTi-G0.4.Besides, both CeTi-I0.8 and CeTi-G0.8 obviously showed the CeO2phase.Convincing results could be found in the HR-TEM images (Fig.S2).For CeTi-G0.4, lattice fringe belonging to CeO2(111) was absent but clearly shown in CeTi-I0.4.No CeO2lattice fringe was observed for CeTi-0.1 samples.Combined with XRD results, it could be concluded that CeO2was well dispersed over TiO2in both CeTi-G0.1 and CeTi-I0.1, with no distinct difference in the surface dispersion of CeO2.Nevertheless, a discrepancy in NO conversion still existed between them, indicating that there was other pivotal factor to determine the catalytic performance instead of the dispersion of active components.
In literature report, it has been well established that the catalytic activity of CeO2-TiO2based catalysts has a close relation with the degree of interaction between CeO2and TiO2support[19,24].As reported by several works on CeTi mixed oxides [19,24],the strong interaction originated from the incorporation into each other’s lattice.It is noteworthy that 0.4 mmol/g is exactly the single-layer dispersion capacity of CeO2over TiO2surface in this work [25].The absence of CeO2crystal phase in the CeTi-G0.4 hinted that Ce ions might be incorporated into support TiO2surface lattice oxide, enhancing the interaction between Ce and Ti.Consequently, the activity of CeTi catalysts was improved.
In order to verify the assumption above, we investigated surface properties of CeTi catalysts.According to the literature [26],Ce ions embedded in the lattice of TiO2are difficult to be dissolved out.To find out whether Ce ions entered into TiO2lattice, samples were dissolved in a mixture solution of H2O2and aqua regia, then cerium content in solution was analyzedviaICP-AES, and results are listed in Table 1.Except for CeTi-G0.1, series of CeTi-G specimens showed a lower Ce concentration than corresponding CeTi-I ones, which proved the speculation of incorporation.Measurement errors from low Ce concentration might account for the exception.To keep samples with similar dispersion state for better comparison, we briefly chose CeTi-G0.1 and CeTi-I0.1 as representatives for further characterizations.
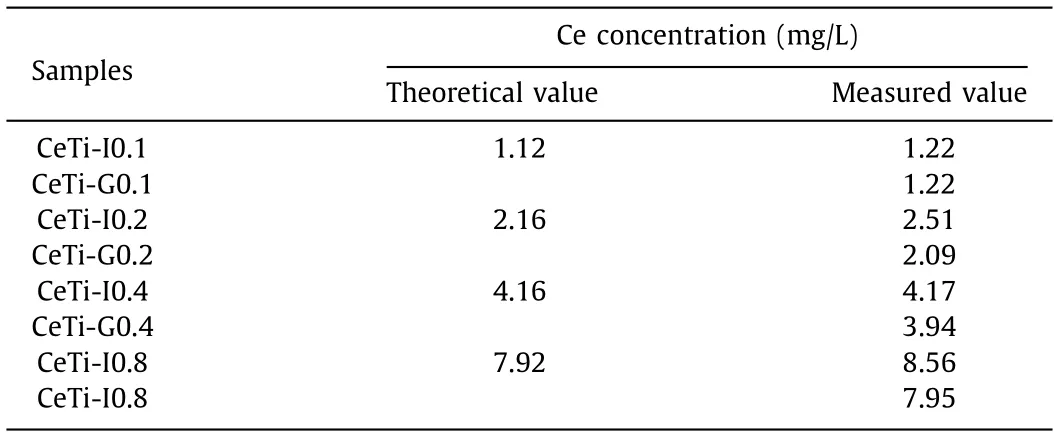
Table 1 ICP-AES results of CeTi samples.
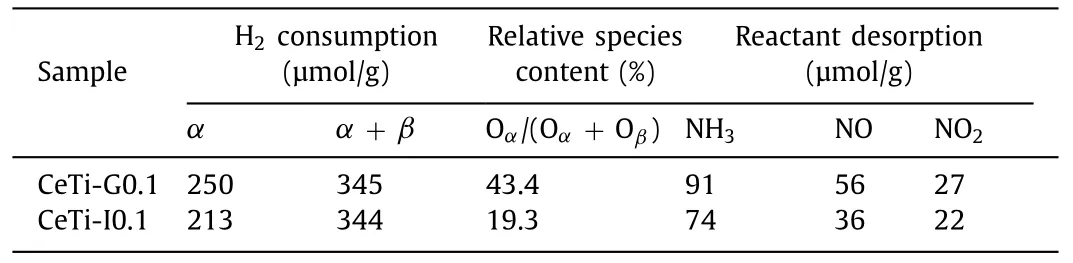
Table 2 H2 consumption, surface atomic ratio, quantitative amount of NH3 and NOx of TPD profiles over CeTi catalysts.
Apparently, CeTi-G0.1 is yellow colored while CeTi-I0.1 was pale close to TiO2(Fig.2b), which reflects their different surface states[27].In this regard, we characterized the two catalysts together with TiO2by diffuse reflectance UV–vis spectroscopy.Compared with CeTi-I0.1, the absorption wavelength of CeTi-G0.1 showed an obvious red shift from 400 nm to 500 nm, indicating the generation of defects on TiO2surface [28,29].As shown in Fig.2c,all samples show four typical Raman bands at around 142, 394,514, and 638 cm-1, which can be attributed to modes of anatase phase with the symmetries of Eg, B1g, A1g, and Eg, respectively[30].Conspicuously, the dominated peak, shifted to 142.7 cm-1and broadened, which could be attributed to localized defects or lattice disorder (non-stoichiometry) related to surface oxygen vacancies [31,32].Considering the analyses above, we suppose surface defects or oxygen vacancies might be induced by the incorporation of Ce into surface TiO2lattice.Thereby, the interaction between Ce and Ti species was enhanced, which was a prerequisite for the improvement of activity.
Changes of TiO2surface affected the redox properties and surface oxygen species of samples, hence H2-temperature programmed reduction (H2-TPR) experiment was conducted.In Fig.2d, for CeTi-G0.1 and CeTi-I0.1, two reduction peaks appeared at around 450 °C and above 650 °C, assigned to the reduction of surface oxygen species (α) and lattice oxygen (β), respectively[33,34].The H2consumption of CeTi-G0.1 at low temperature was higher than that of CeTi-I0.1 (Table 2), manifesting that CeTi-G0.1 has more surface oxygen species.This could be corroborated by the result of O 1s by X-ray photoelectron spectroscopy (XPS).As shown in Fig.2e and Table 2, it could be obtained that CeTi-G0.1 displayed a higher content of Oα(43.4%) in comparison with CeTi-I0.1(19.3%).Abundant surface defects or vacancies led to the enhanced surface oxygen adsorption.Thus, more NO could be oxidized to NO2, which was demonstrated by NO oxidation test (Fig.S3 in Supporting information) and NO + O2-temperature programmed desorption (NO+O2-TPD) below.This process promoted the “fast-SCR”reaction that was beneficial for improving the low-temperature catalytic activity [35].
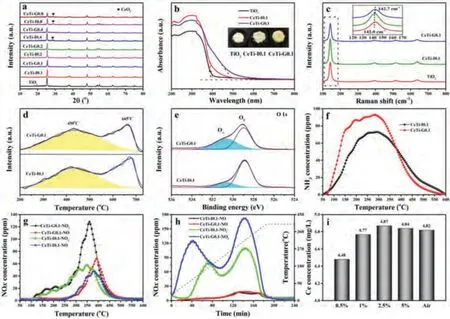
Fig.2.(a) The XRD patterns of samples.(b) Photographs and diffuse reflectance UV–vis spectra of samples.(c) Raman spectra of catalysts.(d) H2-TPR results of CeTi.(e) XPS O 1s spectra of CeTi-G0.1 and CeTi-I0.1.(f) NH3-TPD, (g) NO + O2-TPD profiles of CeTi-G0.1 and CeTi-I0.1.(h) NOx released from samples during heating in Ar flow.(i) Ce concentration by ICP-AES from CeTi-I0.4 precursor heated in different atmosphere.Calcination condition: 0.5-5 vol% NO, balance He, 30 mL/min (air as a contrast).
Surface changes also influenced adsorption property of catalysts[36-38].To investigate the impact further, NH3-TPD and NO + O2-TPD experiments were carried out.The results were depicted in Figs.2f and g, and quantitative desorption amount was listed in Table 2.It could be found that ammonia desorption capacity of CeTi-G0.1 (91 μmol/g) was higher than that of CeTi-I0.1 (74 μmol/g), especially at low temperature.This evidenced that CeTi-G0.1 exhibited the superiority to absorb and activate NH3, which were vital to SCR reaction.It is reported that ammonia desorption peak at low and medium temperature was assigned to NH4+absorbed on Brønsted acid sites [20,39].Surface defects mentioned above could participate in the formation of Brønsted acid sites through H2O dissociation to form hydroxyl groups [40].In Fig.2g,two kinds of NOxspecies desorbed over samples, NO2dominated quantitatively and the initial desorption temperature was lower than that of NO.For CeTi-G0.1, NO and NO2desorbed was 27 and 56 μmol/g, which was higher than that for CeTi-I0.1 (22 and 36 μmol/g, respectively).More NO was adsorbed and then oxidized into NO2over CeTi-G0.1 due to abundant surface oxygen species from surface defects, which agreed with the outcome of NO oxidation experiment (Fig.S3).NO2was conducive to the "fast SCR"reaction, resulting in improved low-temperature performance [35].As such, CeTi-G0.1 exhibited preponderance in activation and desorption of reactants due to the existence of surface defects and vacancies, which originated from Ce doping into TiO2.
The above characterization results give clear evidence that solid-phase impregnation has the advantage of incorporation more Ce into the lattice of TiO2.As a result, the interface structure of CeO2/TiO2changed, which influenced the catalytic performance[41,42].So, the question arises, what is the driving force for the solid-phase impregnation to alter the interface structure of CeTi catalyst? According to literature study [22,23], we propose here for the first time that the main transformation of CeO2from TiO2surface to bulk is related to the decomposition property of nitrate,which plays a significant role in constructing the interface structure.Cerium nitrates decompose according to the general reaction[43]:
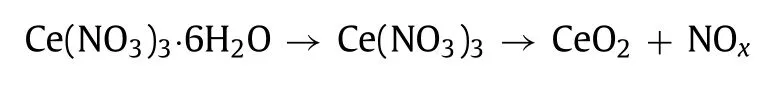
It could be seen that vast of nitrogen oxides would release during the transformation.To distinguish the kinds of nitrogen oxides and probe their respective impacts on catalyst structure, the decomposition was tracedviadetecting the exhaust gas composition from heating uncalcined precursors in Ar atmosphere.As shown in Fig.2h and Fig.S4 (Supporting information), products of nitrate decomposition were NO, NO2and negligible N2O.As the main product, amount of NO2differed apparently, while that of NO resembled for two samples.In Fig.2h, it was found that once heating, NO2was let off concomitantly for CeTi-G0.1, which was much earlier than CeTi-I0.1.Meantime, NO2released from CeTi-G0.1 was much more than that from CeTi-I0.1.Besides loss of part nitrate species during evaporation in wet impregnation, the discrepancy of NO2emission might result from that more NO transformed into NO2through snatching one oxygen atom of TiO2over CeTi-G0.1 precursor.This might also provide an opportunity for Ce ions to enter into the TiO2surface lattice, inducing more defects or vacancies on the surface of anatase, which was borne out by characterizations above.Furthermore, the presence of NO could prevent sintering and redistribution during nitrate decomposition, and then ensure good dispersion of active species [22,23].In order to confirm whether NO could promote Ce to intercalate into TiO2lattice,CeTi-I0.4 sample before calcination was heated in a flow containing different NO concentrations, and Ce content was measured by ICPAES mentioned above.The corresponding result was presented in Fig.2i.In contrast to that heated in air, less Ce was dissolved out for samples heated in low NO concentration (≤1%) atmosphere,indicating that the promotion of NO did exist.Interestingly, such an effect was not evident further increasing NO concentration in atmosphere.Hence, more NO evolved during decomposition of nitrate precursor indeed played a non-negligible role in facilitating the incorporation of Ce into TiO2surface lattice.
In summary, the supported CeTi catalysts prepared by solidphase impregnation exhibited better performance than that by wet impregnation in NH3-SCR.The texture properties and dispersion of CeO2had a limited impact on the activity.It could be found that Ce might be embedded into TiO2surface lattice, and more defects and vacancies were created through ICP-AES, UV–vis-DRS and Raman analyses.Moreover, CeTi-G-0.1 behaved better in redox, adsorption and activation of reactants because of defects and vacancies over supports.The traced results of preparation process showed that NO might scavenge atomic oxygen of TiO2and made it possible for Ce to incorporate into TiO2which was affirmed by ICP-AES outcomes.We expected that such solid-phase impregnation would be an effective approach to developing high-performed NH3-SCR catalysts.
Declaration of competing interest
He missed his family. His mom raised four kids by herself on a forty?acre farm in Missouri but no matter how scarce money was, she d always made sure they had a good Christmas. He thought about his box of gifts in the truck.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
We gratefully acknowledge the financial supports from the National Natural Science Foundation of China (Nos.21976081,21773106).
Supplementary materials
杂志排行
Chinese Chemical Letters的其它文章
- Comment on “Acid-induced tunable white light emission based on triphenylamine derivatives”
- Strategies for efficient photothermal therapy at mild temperatures:Progresses and challenges
- Liposome-based delivery of biological drugs
- Macrophage-targeted nanomedicine for chronic diseases immunotherapy
- Advances, opportunities, and challenge for full-color emissive carbon dots
- Fluorine-containing agrochemicals in the last decade and approaches for fluorine incorporation
