Studies on the biological activity of gem-difluorinated 3,3′-spirocyclic indole derivatives
2022-06-18QiangWangHongjianSongQingminWang
Qiang Wang, Hongjian Song, Qingmin Wang
a State Key Laboratory of Elemento-Organic Chemistry, Research Institute of Elemento-Organic Chemistry, College of Chemistry, Frontiers Science Center for New Organic Matter, Nankai University, Tianjin 300071, China
b Department of Organic Chemistry, Stockholm University, SE-106 91 Stockholm, Sweden
ABSTRACT The biological activities of a series of 3,3′-spirocyclic indole derivatives containing CF2, phosphine oxide,indole, and cyano functional groups were evaluated, and these derivatives were found to exhibit anti-TMV, fungicidal, and insecticidal activities.
Keywords:3,3′-Spirocyclic indole CF2 group Anti-TMV Fungicidal activity Insecticidal activity
Here we evaluated the biological activities of a series of designed 3,3′-spirocyclic indole derivatives, which contain privileged moieties and functional groups, such as CF2, phosphine oxide, indole, and cyano.The bioassay results show that the target compounds possess moderate to good anti-TMV activities, in which compound 5h exhibits the highest antiviral activity (51.2, 49.0,53.6 %, 500 mg/L)in vivo.This indicates compound 5h could be a promising candidate for anti-TMV development.3,3′-Spiroindoline derivatives bearing a cyano group have exhibited broad spectrum fungicidal activities against 14 kinds of phytopathogenic fungi and selective fungicidal activities againstSclerotinia sclerotiorum.Additionally, many target compounds have shown potent insecticidal activity againstMythimna separata.Some of them also show good activities againstHelicoverpa armigera, andPyrausta nubilalis,as well asCulex pipiens pallens.
Thegem-difluoromethylene group (CF2) has attracted significant attentions in medicinal [1] and agricultural chemistry [2] because of its specific steric and electronic properties [3-5].It can function as a mimic for hydroxyl [6] and carbonyl [7] groups, as well as replace oxygen atoms in phosphates [8,9], sulfates [10,11] and aryl ethers [12], thereby enhancing lipophilicity, bioavailability and binding affinity.Nevertheless, the application of CF2group in agrochemicals is actually far less than that of fluoro and trifluoromethyl functional groups [2].This is because of the limitations of synthetic method of CF2-containing compounds [13].In addition, for all existing difluoroalkylated agrochemicals, the CF2unit is always directly connected with aromatic rings or heteroatoms, such as oxygen and nitrogen, resulting in a lack of structural diversity [2].
The importance of nitrogen heterocycles in medicinal chemistry has been demonstrated by their presence in a considerable number of FDA-approved drugs [14].Rigid, three-dimensional molecular scaffolds have also attracted great attentions in recent research of drug discovery [15].Therefore, the 3,3′-spirocyclic indole derivatives, which fall into both of these categories, are particularly important.For example, 3,3′-spirooxindole and 3,3′-spiroindoline are both core skeleton widely existing in natural products [16].They have shown useful biological activities and have been extensively used for drug discovery (Fig.1a) [17,18].However, compared with their tremendous applications in the field of pharmaceuticals, there are few reports for their pesticide activities [19-22].Our group recently discovered that a series of 3,3′-spirooxindole and 3,3′-spiroindoline compounds exhibited potent antiviral, fungicidal and insecticidal activities (Fig.1b) [19-21].These studies mainly focused on the derivatization of the spiro ring at indole C-3 position.In contrast, the derivatization of the C-2 position of 3,3′-spirocyclic indole derivatives and the corresponding biological activities have not been extensively explored.As far as we know, only our group reported the fungicidal activities of a limited number of such compounds [23,24].
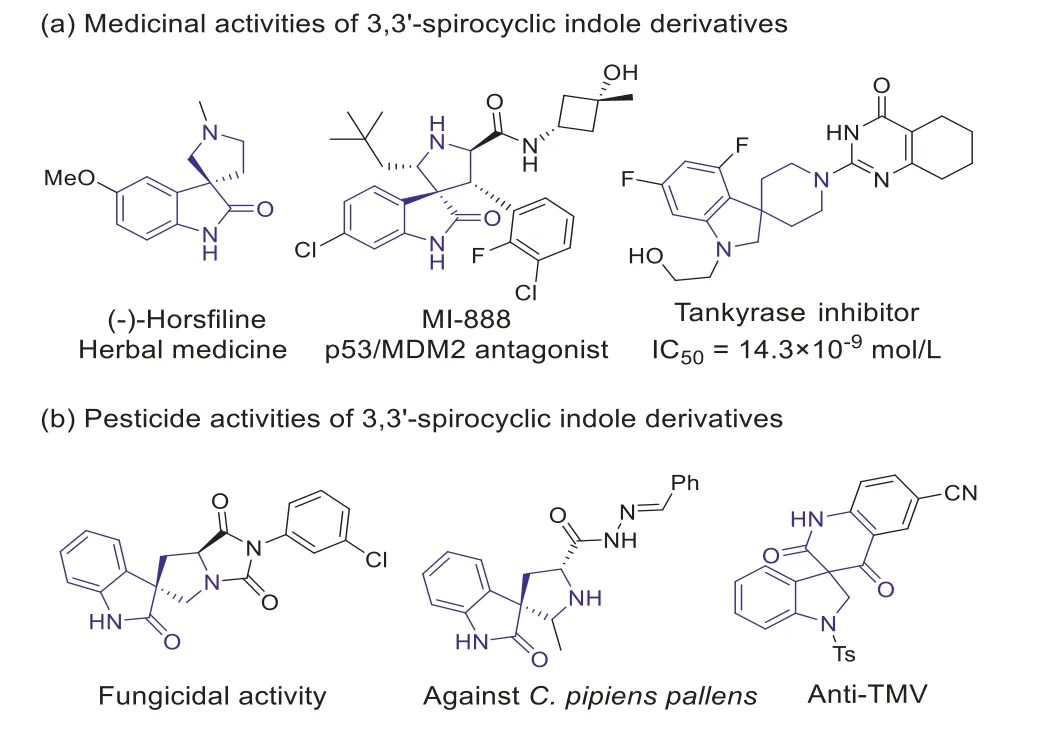
Fig.1.Examples of bioactive molecules containing 3,3′-spirooxindole and 3,3′-spiroindoline skeleton.
Considering of the importance of CF2group and 3,3′-spirocyclic indole skeleton, as well as their insufficient applications in the field of pesticides, we here decided to combine these two moieties and designed a series of multi-functionalized 3,3′-spirocyclic indole derivatives.In order to increase the diversity of the existence of CF2group in pesticide active molecules, we designed to install it into the spiro ring at indole C-3 position to obtain thegemdifluorinated lactam moiety (Fig.2).In addition, to further increase the molecular diversity and complexity, we designed to introduce other functional groups at the indole C-2 position (Fig.2), such as phosphine oxide, indole, and cyano groups.This kind of functional groups are also widely used in bioactive molecules [14,25,26].Then, we evaluated the anti-tobacco mosaic virus (TMV), fungicidal and insecticidal activities of these designed compounds.
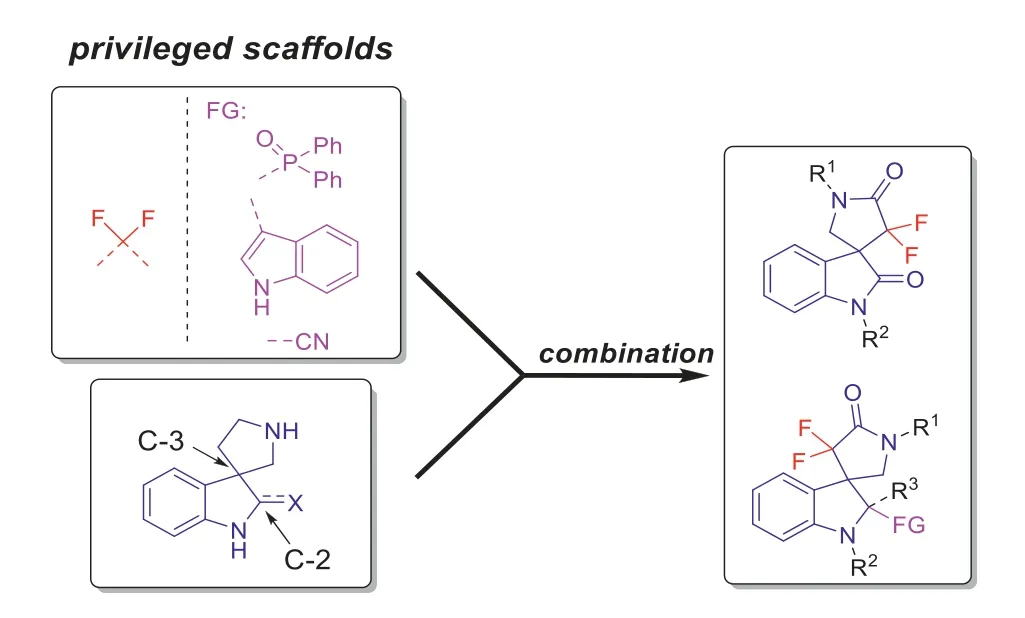
Fig.2.Design of target compounds.
We first synthesized four types of 3,3′-spirocyclic indole derivativesviavisible light mediated radical cascade reactions by using indole-derived bromodifluoroacetamide as starting material(Scheme 1) [23,24,27].The biological activities of these synthesized compounds were tested using previously reported method[19-21], Ribavirin, chlorothalonil, and rotenone were used as controls (Fig.3).The operating steps and physical data in detail of intermediates, target compounds and the detail biological assays methods were given in Supporting information.

Fig.3.Control drugs of biological activities tests.
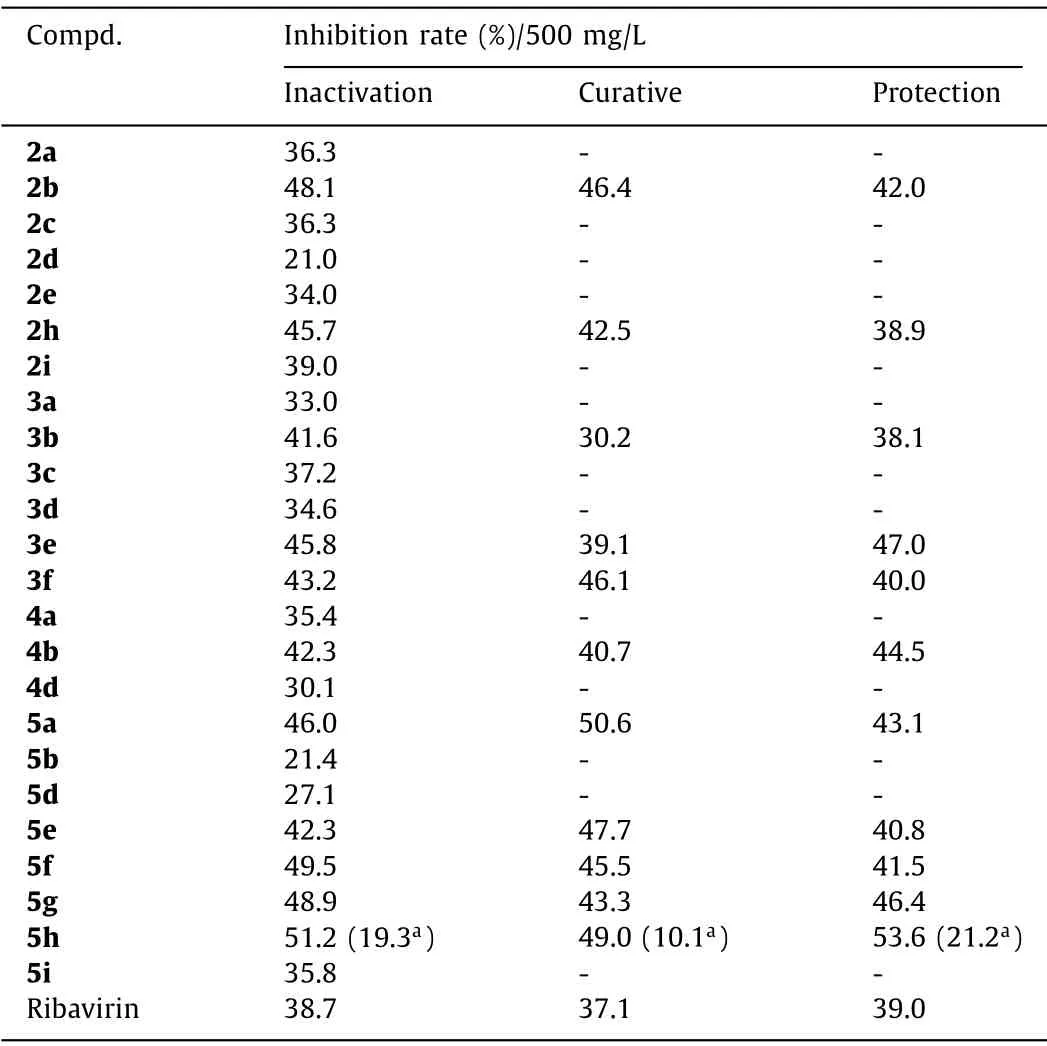
Table 1 In vivo anti-TMV activity of selected synthesized compounds.
As shown in Table 1, the anti-TMV activitiesin vivo, including inactivation, curative and protection effects were evaluated for selected compounds.As we can see, all four types of synthesized compounds exhibit moderate to good anti-TMV activities, which suggests that the idea of introducing CF2group and functional groups such as phosphine oxide, indole and cyano into the 3,3′-spirocyclic indole skeleton is successful.Part of the compounds,such as compounds 2b, 2h, 3e, 3f, 4b, 5a and 5e-5h, show significantly higher activities than that of commercialized anti-plan virus agent ribavirin.
For 3,3′-spirooxindole derivatives (class I), compound with a methyl substituent at C-4 position (2b) has significantly higher activity than compounds with the same substituent at other positions (2c-2e).Compound with a long carbon chain substituent on indole nitrogen (2h) has higher activity than compound with a methyl group (2a).The change of substituent on the amide group(2i) does not improve the activity.
For phosphine-oxide substituted 3,3′-spiroindoline derivatives(class II), the methyl substitution at indole C-6 position (3b) is beneficial for the anti-TMV activity.The study of electronic effects on the phosphine moiety shows that electron-withdrawing substituent (3e and 3f) on the phenyl ring improves the anti-TMV activity.
For indole substituted 3,3′-spiroindoline derivatives (class III),the indole C-6 bromo-substituted compound 4b give the best result.
For compounds containing a cyano group and two consecutive quaternary carbon centers (class IV), their structural modifications are most successful for anti-TMV activity.Compounds with alkyl substituents at indole C-2 position (5f-5h) all give higher anti-TMV activity than ribavirin.Especially for compound 5h, it exhibits the best activity (51.2%, 49.0%, 53.6%, 500 mg/L) among all the selected compounds.This indicates compound 5h could be a promising candidate for anti-TMV development.In addition, compound 5a exhibits better anti-TMV activity than its substituted analogues 5b and 5c.Interestingly, compound 5e with a thiophene moiety also shows good result.

Scheme 1.Synthesis of 3,3′-spirocyclic indole derivatives 2a-2i, 3a-3f, 4a-4d, 5a-5i.
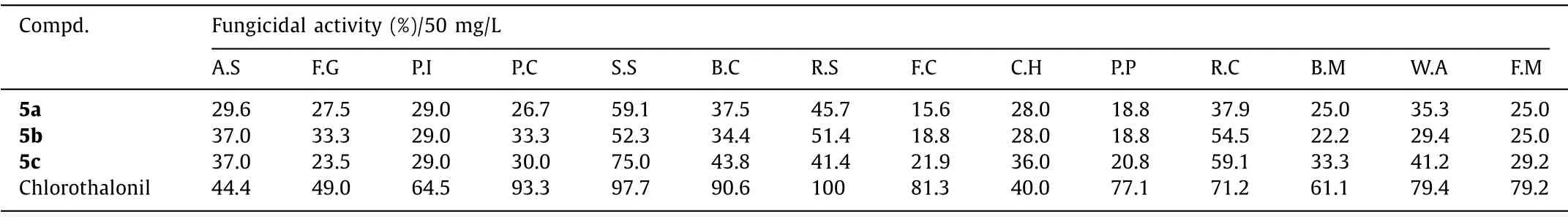
Table 2 Fungicidal activity of selected compounds 5a-5c.
Next, we investigated the fungicidal activities of our synthesized compounds against 14 kinds of phytopathogenic fungi by using chlorothalonil as a standard.As we mentioned above, the fungicidal activities of certain compounds have been reported[23,24], here we only focused on the cyano-substituted 3,3′-spiroindoline derivatives (Table 2).We found all the tested compounds exhibited broad-spectrum fungicidal activities, and the growth inhibitory rates againstSclerotinia sclerotiorumexceeded 50% for all compounds.In addition, compounds 5b and 5c also show good inhibitory activity againstRhizoctonia cerealis.
We also investigated the insecticidal activities of our synthesized compounds against Lepidoptera pests, such asMythimna sep-arata, Helicoverpa armigera, andPyrausta nubilalis, as well asCulex pipiens pallens(Table 3).In general, most of the selected compounds exhibit complete insecticidal activity againstM.separataat concentration of 600 mg/L.Especially for compounds 2d, 2a and 5a, they still show 100% inhibition rate at the concentration of 200 mg/L.In addition, the phosphine-oxide substituted compound 3a has shown potent activities against all three Lepidoptera pests.ForCulex pipiens pallens, the spirooxindole compounds 2g and 2h both exhibit 100% larvicidal activity at 10 mg/L (Table 3).
In summary, a series of 3,3′-spirocyclic indole derivatives containing CF2, phosphine oxide, indole, and cyano moieties were designed and synthesized using our previously reported methods onthe basis of the widely used privileged scaffolds in both drug and pesticide design.The bioassays results showed that the target compounds possessed moderate to good activities against TMV,among which compound 5h showed the highest antiviral activity.In the meantime, cyano-substituted 3,3′-spiroindoline derivatives have exhibited broad spectrum fungicidal activities against 14 kinds of phytopathogenic fungi and selective fungicidal activities againstSclerotinia sclerotiorum.In addition, many target compounds have shown potent insecticidal activity againstM.separata.Some of them also showed good activities againstH.armigera, andP.nubilalis, as well as mosquito.Further investigation on structural optimization and mode of action are in progress in our laboratory.
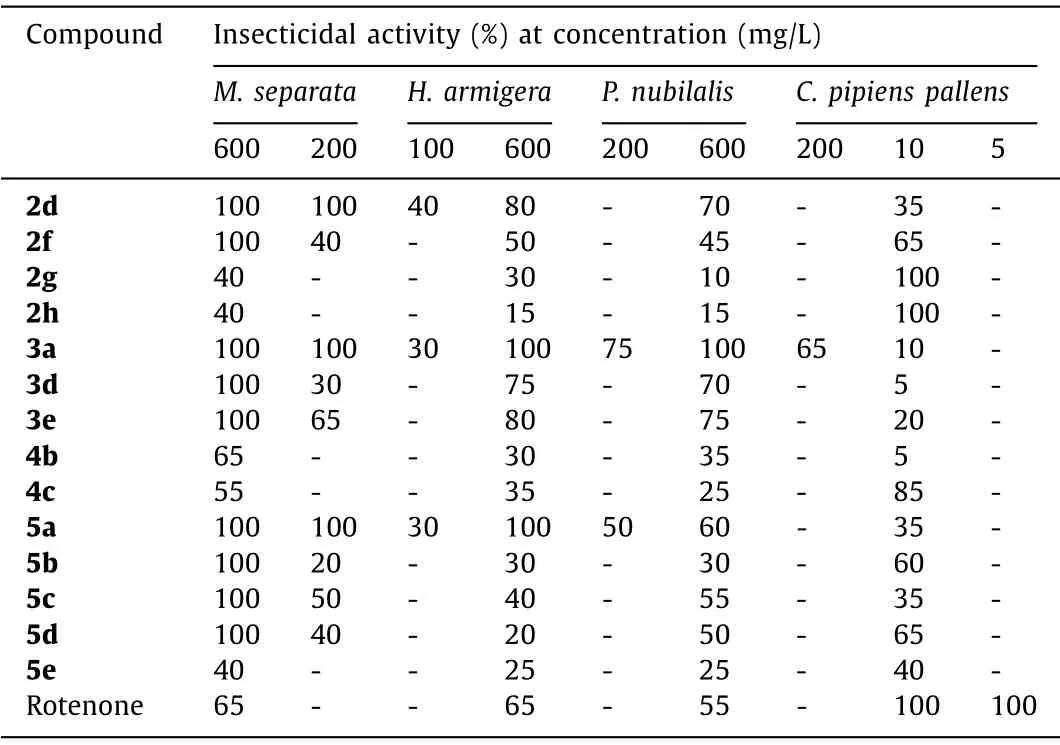
Table 3 Insecticidal activity of selected synthesized compounds.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We are grateful to the National Natural Science Foundation of China (Nos.21732002, 22077071, 21977056) and Frontiers Science Center for New Organic Matter, Nankai University (No.63181206)and the Fundamental Research Funds for the Central Universities,Nankai University (No.63201043) for generous financial support for our programs.This paper is also dedicated to the 100thanniversary of Chemistry at Nankai University.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.08.005.
杂志排行
Chinese Chemical Letters的其它文章
- Comment on “Acid-induced tunable white light emission based on triphenylamine derivatives”
- Strategies for efficient photothermal therapy at mild temperatures:Progresses and challenges
- Liposome-based delivery of biological drugs
- Macrophage-targeted nanomedicine for chronic diseases immunotherapy
- Advances, opportunities, and challenge for full-color emissive carbon dots
- Fluorine-containing agrochemicals in the last decade and approaches for fluorine incorporation
