TIB for Micropropagation and the Relationship between Anthocyanins and Chlorophyll of Strawberry Seedlings
2022-06-18LIUShoumingNONGZhiwangCHENYuchenLIURenZHUXiwu
LIU Shou-ming, NONG Zhi-wang, CHEN Yu-chen, LIU Ren, ZHU Xi-wu,*
1. Hunan Academy of Agricultural Sciences, Changsha 410125, PRC;
2. Institute of Agriculture and Biotechnology, Hunan University of Humanities, Science and Technology, Loudi 417000, PRC;
3. Institute of Bioengineering, Zhejiang Sci-Tech University, Hangzhou 310018, PRC
Abstract In this study, we employed a temporary immersion bioreactor (TIB)system for the micropropagation of strawberry seedlings. The TIB method and the conventional method for the micropropagation of strawberry seedlings were compared in terms of explant propagation coefficient, seedling fresh weight,contents of anthocyanins and chlorophyll, and photosynthetic characteristics. The results showed that an inoculation density of 40 explants/L was suitable for the micropropagation of ‘Benihope’ strawberry seedlings in the TIB. The propagation coefficient, fresh weight, photosynthetic rate, stomatal conductance, transpiration coefficient, and the contents of total anthocyanins and chlorophyll of seedlings micropropagated in the TIB were significantly higher than those of the seedlings micropropagated by the conventional method. In conclusion, the TIB method was superior to the conventional method in the micropropagation of strawberry seedlings.The negative reciprocity did not occur between anthocyanins and chlorophyll in strawberry seedlings cultured under forced ventilation such as TIB.
Key words Strawberry seedlings; Micropropagation; Temporary immersion bioreactor; Anthocyanins; Photosynthetic characteristics
1. Introduction
Micropropagation is an approach for rapid proliferation or increasing the content of active ingredients of economic plants under artificial conditions[1]. Strawberry is a fruit crop with a remarkable economic value[2-3]. In China, transplanting is usually employed for the production of strawberry seedlings to reduce diseases and mitigate low yields.Therefore, a great number of strawberry seedlings are micropropagated every year.
In the micropropagation of strawberry seedlings, plant tissues are usually cultured in solid media (conventional method), which greatly increases the labor intensity and production costs[4]. These shortcomings necessitate a simple and economical method for producing strawberry seedlings. Temporary immersion culture bioreactor (TIB) systems have been used to propagate plant seedlings[4-12]as an alternative of the conventional method. The TIB method is timesaving in sterilization and medium dispensing and has low risk of contamination of explants (direct subcultured into TIBs and autocontrol of the TIB system), reducing labor intensity and production time[4,6].Some characteristics of the seedlings cultured by TIB,such as chlorophyll content and stomata density, have been reported[10].
In this study, we employed the TIB developed at Nanjing Tech University for the micropropagation of strawberry seedlings[13]and then compared the seedlings micropropagated by the TIB method and the conventional method. The findings will facilitate the development of micropropagation for strawberry seedlings.
2. Materials and Methods
‘Benihope’ strawberry seedlings were propagated by the conventional method and TIB method in our lab.
2.1. Conventional method for the micropropagation
The top of each stolon was successively treated with 75% ethanol for 40 s and mercuric chloride for 12 min. It was then transferred into a culture flask with MS medium and cultured for 15 d to induce the generation of callus and multiple shoot clumps.The multiple shoot clumps were cut into three buds and transplanted into a solid culture medium for cell differentiation over 25 d. The seedlings without roots were transplanted into a solid medium for rooting and seedling growth for 25 d. The seedlings were then transplanted into plug trays for hardening.
2.2. TIB for the micropropagation
The TIB (Fig. 1) was gifted by Professor CHEN Ji-shuan at Nanjing Tech University[13]. The TIB is a modified glass desiccator (4.5 L) integrated with a forced ventilation system and can be autocontrolled.
Under sterilized conditions, the cap of the TIB was opened for the addition of 1 L sterilized liquid medium, and then 20, 40 or 60 explants from the multiple shoot clumps were inoculated. After that, the TIB was sealed with vaseline, and air was pumped into the TIB at the aeration stage. Explants were immersed into the liquid medium for 10 min every 4 h. The TIB was placed on the same shelf as the culture flasks of the conventional method and operated in the plant tissue culture room under automatic temperature and light control [(25 ± 1)℃, 1 500~2 000 lx for 12 h each day]. The air flow of of the TIB was 15 L/min,and the explants were cultured for 50 d, during which the liquid medium in the TIB was replaced every 25 d under sterilized conditions. The experiment was conducted in triplicate.
The liquid medium for cell differentiation: MS:6-BA 0.3 mg/L, IBA 0.05 mg/L, GA 0.1 mg/L, sugar 3% (w/w), and pH 5.8. The liquid medium for rooting:1/2 MS: IBA 0.4 mg/L, AC 0.3 g/L, sugar 3%, and pH 6.0. The solid culture medium was prepared with the liquid medium and agar and adjusted to pH 5.8.
2.3. Measurement of strawberry seedling characteristics
After the strawberry seedlings were cultured by TIB or the conventional method for 50 d, the total fresh weight, number, leaf length, leaf width, and leafstalk length of the seedlings were measured. The average fresh weight propagated by each explant(MFWPEx) and the propagation coefficient of explants were calculated as follows:
MFWPEx=Total fresh weight/Number of explants in the TIB
Propagation coefficient of explants = Number of seedlings/Number of explants in the TIB.
The total anthocyanin content was measured according to the method of WANG S Yet al.[14-15].Specifically, anthocyanins were extracted with 25 mL of 80% acetone containing 0.2% formic acid from 5 g fresh leaves of the strawberry seedlings cultured for 50 d by TIB or the conventional method. The total anthocyanin content in leaf extract was determined using the pH differential method[14]. Absorbance (A)was measured by a microplate spectrophotometer(SPECTRA max PLUS384, Molecular Devices, USA)at 510 nm and 700 nm in the buffer solutions at pH 1.0 and 4.5.A= [(A510-A700)pH1.0-(A510-A700)pH4.5]. The results were expressed as milligrams of cyanidin -3-glucoside (molar extinction coefficient of 29 600)equivalent per 100 g of fresh weight (mg/100g).
Total chlorophyll content in the seedlings was measured according to the method reported by MISYURA Met al.[16]. Specifically, 200 mg freshfrozen leaf tissue sample was ground with chromesteel beads into pulp and then resuspended in 80%acetone by vortex, from which the pigments were extracted with 1.5 mL of acetone solution 2~3 times.A part (250 µL) of the extract was transferred to a cuvette along with 750 µL of fresh acetone solution,and the absorbance was measured at 645, 652, and 663 nm by a microplate spectrophotometer (SPECTRA max PLUS384, Molecular Devices, USA). The chlorophyll concentrations (mg/g) were calculated as follows:
Chlorophyll a=12.72×A663-2.58×A645;
Chlorophyll b=22.87×A645-4.67×A663;
Chlorophyll a+b=8.05×A663+20.29×A645.
After the seedlings cultured by TIB or the conventional method were grown for 30 d in plug trays, the photosynthetic rate (Pn), transpiration coefficient (Ek), stomatal conductance (Gs), and intercellular CO2concentration (Ci) of their leaves were measured by LC Pro+(ADC BioScientific) at the following conditions: PAR of 800 μmol/(m2·s);elevated CO2supply of (350±2) μmol/mol, chamber temperature of (25±1)℃, and RH of 65%±2%. Measurements were repeated 5 times for each treatment.
SPSS version19.0 (SPSS Inc., Chicago, USA)was used for data analysis.
3. Results
3.1. Effects of inoculation density on the total fresh weight, number, and average fresh weight of seedlings propagated from each explant and the propagation coefficient of explants in the TIB
To test whether inoculation density influenced the total fresh weight, number, and average fresh weight of seedlings propagated by each explant, as well as the propagation coefficient of explants in the TIB, we inoculated the explants at a density of 20 (D1),40 (D2) and 60 (D3) explants/L into the TIB under sterilized conditions. The growth of seedlings under different inoculation densities was shown in Fig. 1.The total fresh weight and seedling number of explants in the TIB under different inoculation densities were shown in Table 1. The total fresh weight and seedling number both showed the trend of D3>D2>D1 and had significant differences between D3 and D2, between D3 and D1, and between D2 and D1.
MFWPEx and propagation coefficient of explants under different inoculation densities were shown in Table 1. MFWPEx was ranked in the order of D2>D1>D3, which showed significant differences between D3 and D2 and between D3 and D1. D1 displayed the highest propagation coefficient of explants among the three treatments and had significant differences with D2 and D3.
3.2. Effects of inoculation density on the leaf growth of seedlings in the TIB
We tested whether inoculation density influenced the leaf growth of seedlings propagated in the TIB.As shown in Table 2, the leaf length, leaf width, and leafstalk length all followed the trend of D2>D1>D3.The three leaf indexes showed significant differences between D2 and D3 and between D1 and D3.
3.3. Effects of different micropropagation methods on the growth of strawberry seedlings
To test whether different methods influenced the micropropagation of strawberry seedlings, we compared the propagation coefficients of the explants and the leaf growth of strawberry seedlings between the TIB method and the conventional method. As shown in Table 3, both MFWPEx and propagation coefficients of the TIB method were significantly higher than those of the conventional method.
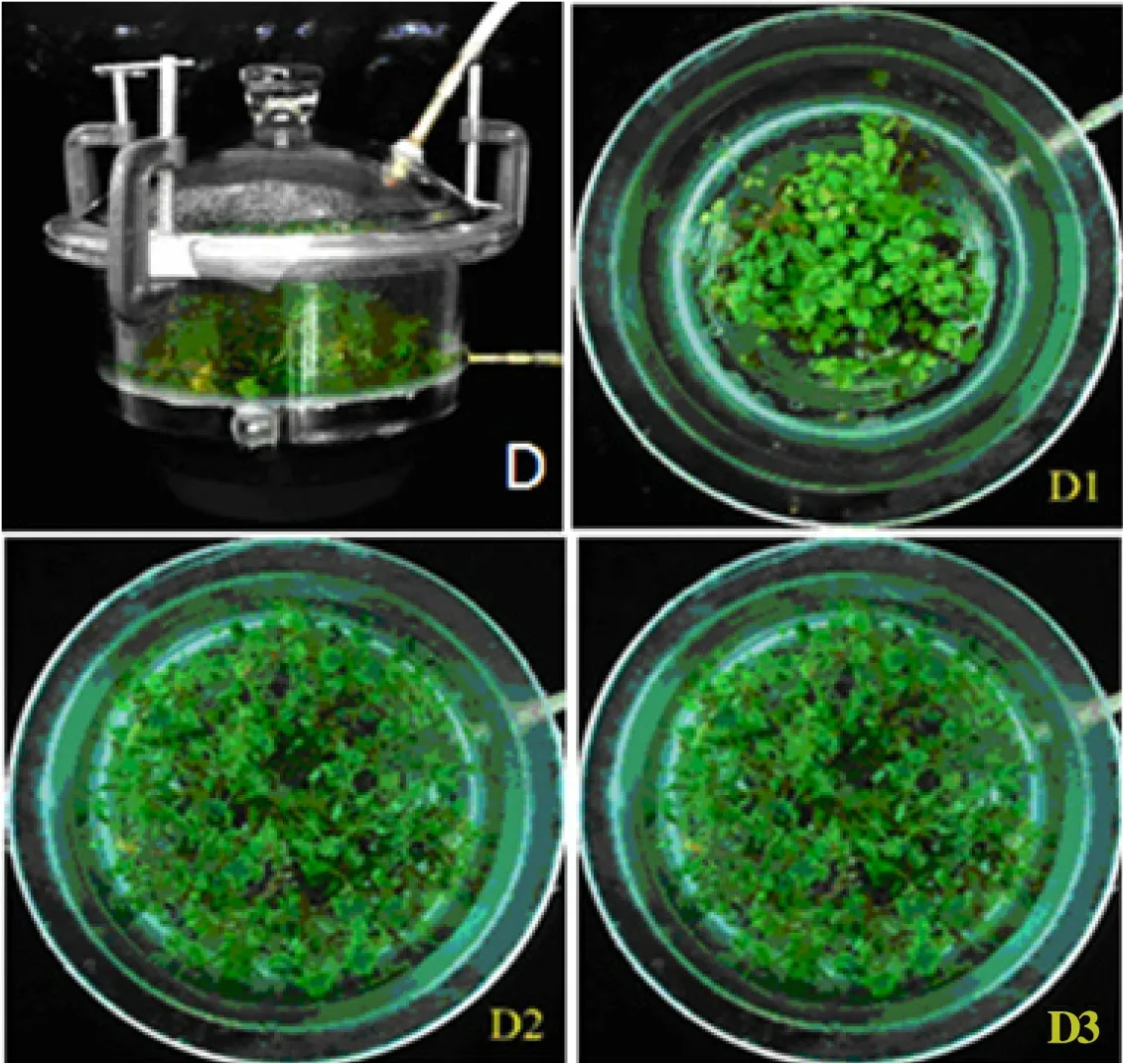
Fig. 1 Growth of seedlings in the TIB with different inoculation densities

Table 1 The total fresh weight, seedling number, and propagation coefficient of explant in the TIB with different inoculation densities
The leaf length, leaf width, and leafstalk length showed no significant differences between the two micropropagation methods, though the leaf length and leaf width of the TIB method were lower than those of the conventional method (data not shown). The seedlings produced by both methods grew well in plug trays and the greenhouse (data not shown).
3.4. Effects of different micropropagation methods on the total anthocyanin and chlorophyll contents of seedlings
The total anthocyanin and chlorophyll contents in strawberry seedlings propagated with TIB or conventional method were compared. As shown in Table 4, the contents of total anthocyanins and chlorophyll in the seedlings propagated in the TIB was significantly higher than that in the seedlings propagated by the conventional method.
3.5. Effects of different micropropagation methods on the photosynthetic characteristics of seedlings
The photosynthetic characteristics of seedlings were measured after seedlings had grown in plug trays for 30 d. As shown in Table 5, the photosynthetic rate,stomatal conductance, and transpiration coefficient of seedlings propagated in the TIB were significantly higher than those of the seedlings propagated by the conventional method. The intercellular CO2concentration showed an opposite trend, while it had no significant difference between the two methods.
4. Discussion
We compared the total fresh weight, number,leaf length, leaf width, leafstalk length, and MFWPEx of strawberry seedlings as well as the propagation coefficients of explants in the TIB among different inoculation densities. The inoculation density D1 was not suitable for micropropagation in the TIB becauseof the low total fresh weight and seedling number.D3 was not suitable for micropropagation in the TIB due to the low propagation coefficients and short and narrow leaf. D2 was suitable for micropropagation in the TIB considering the high MFWPEx, propagation coefficient of explants, seedling number, and leaf length and width. Therefore, we suggested that inoculation density D2 (40 explants/L liquid medium)was suitable for the micropropagation of ‘Benihope’strawberry seedlings by TIB.

Table 2 Leaf length, leaf width and leafstalk length of seedlings cultured at different inoculation densities
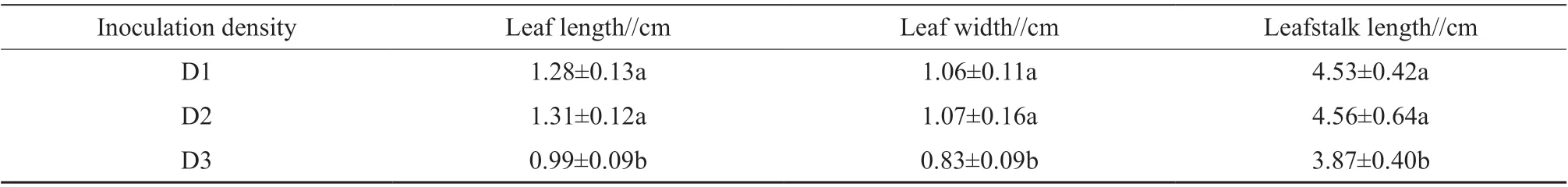
Table 3 Comparison of MFWPEx and propagation coefficient of explant between different micropropagation methodsTable 4 Total anthocyanin and chlorophyll contents of seedlings produced with different methods

Table 4 Total anthocyanin and chlorophyll contents of seedlings produced with different methods

Table 5 Photosynthetic characteristics of seedlings produced with different methods
The strawberry seedlings micropropagated by the TIB method and the conventional method demonstrated significant differences. Specifically,TIB surpassed the conventional method in terms of propagation coefficient, total fresh weight, MFWPEx,and photosynthetic characteristics of the seedlings.
Interestingly, we found that the total anthocyanin and chlorophyll contents of strawberry seedlings propagated in the TIB was significantly higher than that of seedlings propagated by the conventional method.The higher chlorophyll content in the seedlings propagated in the TIB may be attributed to the forced ventilation in TIB, while the glass bottles of the conventional method lacked forced ventilation. This result was consistent with the findings of ZHAO Yet al.that forced ventilation increased chlorophyll content[11].The higher total anthocyanin content of seedlings propagated in the TIB may be ascribed to the exposure of the seedlings propagated in the TIB to more light which can promote anthocyanin synthesis[17-20],whereas the culture flasks of the conventional method had plastic lids. The relationship between total anthocyanin content and chlorophyll content of strawberry seedlings may be associated with an integrated effect between light and forced ventilation.Generally, at the early growth stage when seedlings were most susceptible to light stress, negative reciprocity occurred between pathways, which ensured the synthesis of anthocyanins and inhibition of greening[21]. Our result showed that this negative reciprocity did not occur between anthocyanins and chlorophyll in strawberry seedlings, which were cultured under forced ventilation.
Photosynthetic characteristics such as photosynthetic rate, stomatal conductance, and transpiration coefficient of the strawberry seedlings propagated in the TIB were better than those of the seedlings propagated by the conventional method, indicating that propagation method influenced the photosynthetic characteristics of seedlings. Our result was similar to the findings of YUE Det al.[22]and ZHAO Yet al.[11]who reported that seedlings under forced ventilation had higher photosynthetic rate than the controls.Additionally, ZHAO Yet al.[11]suggested that forced ventilation increased chlorophyll content. Therefore,we suggested that the higher chlorophyll content of the seedlings produced by TIB may be an important reason for the higher photosynthetic rate. The higher stomatal conductance of the TIB method than the conventional method was consistent with the results of MAJADA J Pet al.[23]and ZHAO Yet al.[11].MAJADA J Pet al.[23]pointed out that stomatal function in leaves ofDianthus caryophylluswere improved in ventilated vessels and ZHAO Yet al.[11]found that forced ventilation enhanced stomatal density. Our study for the first time demonstrated that the transpiration coefficient of seedlings propagated by TIB was significantly higher than that of the seedlings produced by the conventional method. The intercellular CO2concentration showed no significant difference between the TIB method and the conventional method,which implied that micropropagation method did not influence the intercellular CO2concentration of seedlings.
5. Conclusion
Inoculation density D2 (40 explants/L liquid medium) was suitable for the micropropagation of ‘Benihope’ strawberry seedlings in the TIB.TIB outperformed the conventional method in the micropropagation of strawberry seedlings. Negative reciprocity did not occur between anthocyanins and chlorophyll in strawberry seedlings cultured under forced ventilation.
杂志排行
Agricultural Science & Technology的其它文章
- Effects of Different Bagging Treatments on Fruit Quality of Jinlan Pomelo
- Effects of Different Intercropping Patterns on Population Yield and Benefit of Fresh Maize and Mung Bean
- Analysis on Interaction Effects Between Variety and Site of Silage Maize Regional Test in Guizhou Province
- Mechanism of Solid State Fermentation in Reducing Free Gossypol in Cottonseed Meal and the Effects on the Growth of Broiler Chickens
- Control Effects of Mixture of Metamifop and Cyhalofopbutyl on Annual Weeds Barnyard Grass in Directseeding Paddy Field
- Study on Weed Control and Safety of Tembotrione-Atrazine Tank Mixture in Spring Maize Fields
