Mechanism of Solid State Fermentation in Reducing Free Gossypol in Cottonseed Meal and the Effects on the Growth of Broiler Chickens
2022-06-18ZHAOJieyuMEIJiajiaQINFeiWEITao
ZHAO Jie-yu, MEI Jia-jia, QIN Fei, WEI Tao
College of Biochemical Engineering, Beijing Union University, Beijing 100023, PRC
Abstract [Objective] This study aims to reduce the free gossypol (FG) and improve utilization rate of cottonseed meal (CSM) by solid state fermentation (SSF). [Method]Bacillus subtilis GJ00141 and Saccharomyces cerevisiae GJ00079 were applied for the SSF and the optimal carbon source, nitrogen source, inorganic salt, moisture content, inoculum level, fermentation temperature, and fermentation time were investigated. The detoxifying effects of different products of GJ00141 were examined with gossypol as substrate. A total of 90 one-day-old broilers were randomized into group A [control, basal diet with 36% soybean meal (SM)], group B (basal diet with 18% SM and 18% CSM), and group C [basal diet with 18% SM and 18% fermented CSM (FCSM)] and thereby the influence of FCSM on the growth of broilers was explored. [Results] The maximum reduction rate (59%) of FG was achieved under the following fermentation conditions: solid medium composed of 96% CSM, 1%glucose, 1% ammonium sulfate, and 2% corn grits, 45% moisture content, 20%inoculum, fermentation at 30 °C for 60 h. Both the viable and inactivated cells of GJ00141 can reduce the content of gossypol, but the reduction rates were only about 20% after 72 h of incubation. Cellular contents and supernatant demonstrated strong detoxifying activity, which achieved the reduction rates of about 95% after 48 h, and the removal was free from the influence of proteinase K, heat, or EDTA. In the 42 d feeding experiment on broilers, the ratios of feed to gain were insignificantly different between the group C and group A. [Conclusion] This method achieved high rate of removing FG in CSM. The reason was the likelihood that the stable compounds in the cellular contents and supernatant of GJ00141 adsorb or bind to FG. Broilers grew well with the FCSM. Thus, it was an efficient detoxifying method for CSM.
Key words Cottonseed meal; Solid state fermentation; Free gossypol; Bacillus subtilis; Mechanism
1. Introduction
Cottonseed meal (CSM), a by-product of oil industry, is rich in crude protein and amino acids[1-3]. It is largely available especially in well-known cotton-producing countries such as China, India, and the US.Despite a major protein resource, CSM is inferior to soybean meal and rapeseed meal and only a small part of it has been used as a feed for animals due to the main culprit, the toxic component gossypol[4-6].
Gossypol is a yellow phenolic compound produced by pigment glands in cottonseeds and other parts of cotton plants. It presents in two forms:free gossypol (FG) and bound gossypol (BG)[7]. It is generally recognized that FG is more toxic than BG because the latter is blocked by reacting with the epsilon-amino groups from lysine and arginine[8].High intake of feeds containing FG will cause liver damage, immunotoxicity, growth depression,reproductive diseases, and other internal organ abnormalities in animals[9-13].
A number of methods, such as solvent extraction,ferrous sulfate/calcium hydroxide treatment, and microbial fermentation, have been developed for the reduction of FG and improvement of CSM utilization rate in animal feeds[14-17]. Among them, microbial fermentation is preferred, as it decreases the content of FG while improving the nutrients in CSM. As a result, there has been an explosion of research on the strain screening and optimization of the fermentation process. A lot of species, such asSaccharomyces cerevisiae,Aspergillus niger,Aspergillus oryzae,Pleurotus sajor-caju, andCandida tropicalis, have been applied for removing the gossypol in CSM[18-20]. Nevertheless,heat-sterilized CSM was mainly used in the previous studies on microbial fermentation of CSM, which led to nutrient loss and color intensification. The mechanism of detoxification by microbial fermentation is unclear, and some studies argue that the FG decrease is attributed to the binding of FG to protein and/or amino acids secreted by microorganisms, degradation by microbial exoenzyme, or both[21]. Compared with the microbial fermentation of CSM, there are few studies on the mechanism of detoxification.
In our previous study, a strain with gossypolreducing activity was isolated and identified asBacillus subtilisGJ00141. In an attempt to reduce FG and improve utilization of CSM, we used GJ00141 andSaccharomyces cerevisiaeGJ00079 for solid state fermentation (SSF) with unsterilized CSM as substrate and optimized the SSF process. Moreover,we preliminarily explored the functional mechanism of GJ00141, hoping to provide a method for effective utilization of CSM.
2. Materials and Methods
2.1. Materials
GJ00141 and GJ00079 were part of strains collected by Academy of State Administration of Grain. GJ00141 was cultured in nutrient broth at 37 °C for 12 h, and GJ00079 in PDA at 30°C for 36 h.CSM was obtained from TYCOON Co., Ltd. (Xinjiang,China), which was mixed with glucose, wheat bran, and ammonium sulfate as basal substrate(CSM ∶glucose ∶wheat bran ∶ammonium sulfate =95 ∶2 ∶2 ∶1). No sterilization of the basal substrate was carried out before use.
2.2. Methods
2.2.1. Effect of solid medium composition on FG reduction
The influence of solid medium composition on FG reduction was investigated (Table 1). The static experiments were conducted in 300 mL conical flasks containing 70 g basal substrate, 10% (V/W) of GJ00079 (107CFU/mL), and 10% (V/W) of GJ00141(108CFU/mL), with moisture content of 50% and natural pH value. Then the mixture was incubated at 30°C for 48 h, during which it was mixed twice.Triplicate flasks were set up for each experiment. The yielded product was dried in an oven at 45°C for 6 h and weight loss was determined. Subsequently, the samples were powdered for further analysis.
2.2.2. Effect of fermentation temperature on FG reduction
The fermentation was carried out at 25, 27, 30,33, 37, and 40°C, respectively, with other parameters at the optimum levels, to explore the influence of temperature on FG reduction. The yielded optimal temperature was recorded for further experiment.
2.2.3. Effect of initial moisture content on FG reduction
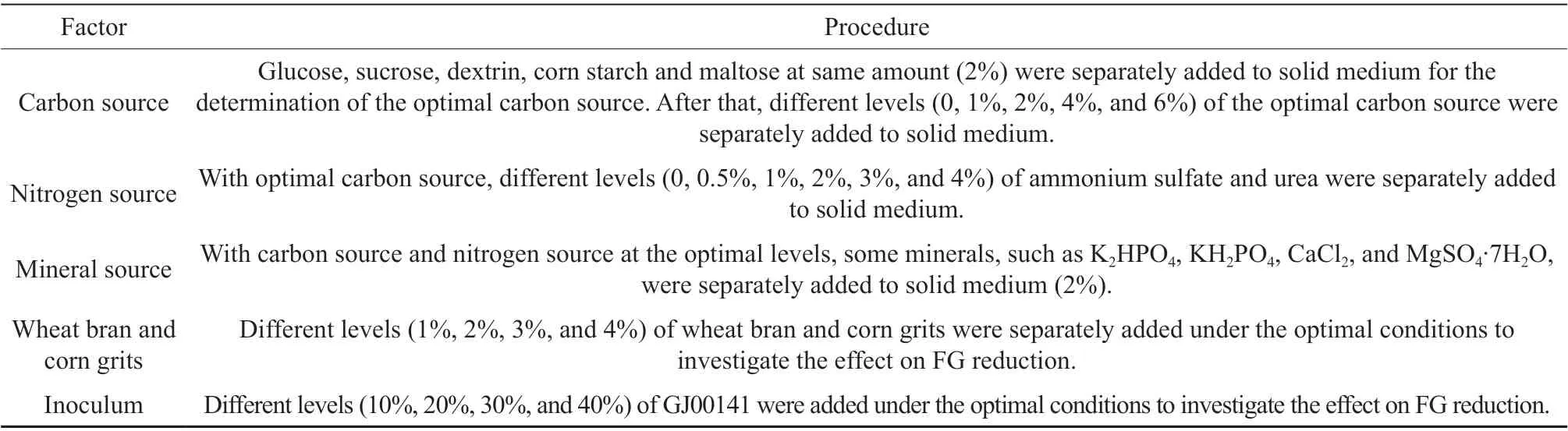
Table 1 Optimization of solid medium for reducing FG
With other parameters at the optimal levels,different moisture content (35%, 40%, 45%, 50%,55%, and 60%) was designed by using distilled water to examine the influence of initial moisture content on FG reduction. The optimal moisture content was recorded for the following experiment.
2.2.4. Effect of fermentation time on FG reduction
With other parameters at the optimal levels,the fermentation time was changed from 12 to 108 h to examine the influence on FG reduction, and the optimal fermentation time was recorded for further analysis.
2.2.5. Determination of gossypol by HPLC
HPLC was employed for gossypol determination under the following conditions: Agilent ZORBAX Eclipse XDB-C18 column, samples which had been filtered through a 0.22 μm polypropylene filter,acetonitrile-water (8 ∶2, V/V) as mobile phase with pH 3.0 (adjusted by phosphoric acid), which was degassed in an ultrasonic bath before use, detection wavelength of 238 nm, injection volume of 10 μL, column temperature of 25 °C, and flow rate of 1 mL/min.
2.2.6. Measurement of FG in CSM
FG in CSM was measured by phloroglucinol colorimetry. The fermented sample was extracted with 70% acetone (1 ∶15, W/V) by sonication for 90 min in an ice bath, and the extract was centrifuged at 10 000 × g for 5 min. The mixture of hydrochloric acid and phloroglucinol solution (30 g/L) (5 ∶1, V/V)was used as the chromogenic agent, and 1 mL of the extract was mixed with 2 mL chromogenic agent.After 25 min of incubation at room temperature, 7 mL of 95% ethanol was added to the mixture and FG was detected by monitoring the absorbance at 550 nm. The concentration of FG was calculated based on comparison with an FG standard curve (0~0.08 mg/mL).
2.2.7. FG-reducing mechanism of GJ00141
GJ00141 was inoculated in nutrient broth at 37°C for 12 h and then centrifuged at 10 000 × g for 5 min. The cells, cellular contents, and supernatant were collected separately for the following analysis.
Cells were harvested from the nutrient broth and rinsed with phosphate-buffered saline (PBS, 10 mmol/L,pH 7.5) 3 times. Then they were suspended in PBS and divided into two parts. One was sterilized at 121°C for 30 min, while the other was left untreated.Gossypol solution was added to the samples with initial concentration calculated to be 0.5 g/L. The mixtures were incubated at 37 °C and sampled at different time points (12, 24, 36, 48, 60, and 72 h) to detect the reduction rate of FG by HPLC.
For the cellular contents, cells were suspended in PBS and disrupted by sonication for 20 min in an ice bath. The suspension was centrifuged at 12 000×g for 20 min at 4°C and filtered through a 0.22 μm polypropylene filter. The filtrate was divided into two parts. One was heated at 100°C for 2 h, while the other was left untreated. Gossypol solution was added to the samples with initial concentration calculated to be 0.5 g/L. The mixtures were incubated at 37°C for 48 h and then the reduction rate of FG was calculated by HPLC.
The supernatant was collected and filtered through a 0.22 μm polypropylene filter. Then it was divided into four parts and treated as follows.Proteinase K solution was added to the first part with initial concentration calculated to be 5 U/mL and the mixture was incubated at 65°C for 2 h. The second part was heated at 100°C for 2 h. EDTA solution was added to the third part with initial concentration calculated to be 0.5 mg/mL. The last part was left untreated. Afterward, gossypol solution was added to the samples with initial concentration of 0.5 g/L.The mixtures were incubated at 37 °C and sampled at different time points (6, 18, 24, 36, and 48 h) to detect the reduction rate of FG by HPLC.
2.2.8. Feeding experiment
Arbor Acres (AA) broilers (n = 90; 1 day old;males and females) were housed in cages (40 cm × 60 cm × 40 cm) in an environmentally controlled room with continuous light. They were randomized into three groups, with 6 replicates for each group and 5 broilers each replicate: group A [basal diet added with 36% soybean meal (SM)], group B (basal diet added with 18% SM and 18% CSM), and group C[basal diet added with 18% SM and 18% fermented CSM (FCSM)]. The basal diet was prepared to meet the nutrient requirements of NRC (National Research Council, 1994). Body weight and feed weight were determined every 7 d. Average daily gain (ADG),average daily feed intake, and ratio of feed to gain (F/G) were calculated for 42 d. All procedures had been approved by the Animal Care and Use Committee of China Agricultural University.
2.3. Statistical analysis
The data were expressed as the mean ± standard deviation. SPSS was employed for ANOVA and LSD test of data in all the experiments.
3. Results and Discussion
3.1. Effect of solid medium composition on FG reduction
Since CSM contained the only carbon source of cellulose, extra carbon source, such as glucose,sucrose, corn starch, maltose, and dextrin, was added to the basal substrate to stimulate cell growth and then increase the reduction rate of FG. Both 2% glucose and 2% sucrose can improve the reduction rate of FG,especially glucose. Hence, glucose was selected as the carbon source and the optimal level (1%~6%) was investigated. The result showed that groups with 1%and 2% glucose had nearly identical removal rates (Fig.1A). Considering the cost, 1% glucose was selected as carbon source.
For the nitrogen source, ammonium sulfate and urea were supplemented at different levels. Results indicated that 1% ammonium sulfate improved the reduction rate of FG compared with the control, with the maximum removal rate of about 45% (Fig. 1B).
With carbon source and nitrogen source at the optimal levels, we investigated the influence of minerals on FG reduction. The result suggested no significant difference in the removal rate between groups with and without additional minerals. Besides crude protein and amino acids, CSM offered a variety of vitamins and minerals. Therefore, it was unnecessary to supplement minerals to the medium.
Some loose materials, such as wheat bran,rice bran, and corn grits, were often added to the medium for SSF in order to relieve the agglomeration of the substrate and stimulate cell growth. Results indicated that wheat bran and corn grits can both enhance the reduction of FG, particularly corn grits(1%~4%). The maximum removal rate (about 48%)was achieved with 2% corn grits, and no significant differences were observed among levels of 2%~4%(Fig. 1C). Therefore, the 2% corn grits was selected in consideration of the cost.
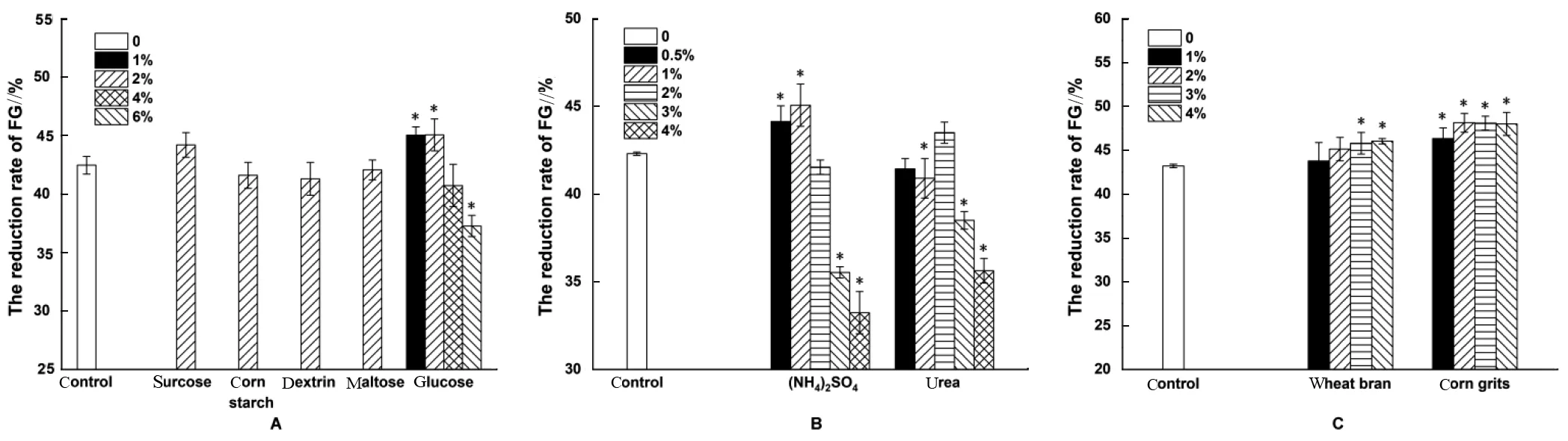
Fig. 1 Effects of carbon source (A), nitrogen source (B), and loose materials (C) on FG reduction
In terms of inoculum level, we found that GJ00079 could decrease the pH value and improve the flavor of CSM but almost had no influence on FG.Therefore, only the level of GJ00141was investigated.The fermentation with 20% GJ00141 (108CFU/mL)achieved the maximal removal rate of 52%, and further increasing the inoculum failed to improve the removal rate.
3.2. Effect of fermentation temperature on FG reduction
At 30°C~33°C, the maximal reduction rate(53%) was achieved. Lower or higher temperature would have adverse effects on the removal (Fig. 2A).Therefore, the optimal temperature was determined tobe 30°C.

Fig. 2 Effects of temperature (A), initial moisture content (B), and fermentation time (C) on FG reduction.
3.3. Effect of initial moisture content on FG reduction
SSF is a fermentation mode carried out in the absence of free water, and moisture content in substrate is the key to the fermentation as it affects the solubility of nutrients, degree of swelling,agglomeration of the substrate, transfer of heat and oxygen,etc. The removal rate kept increasing as the moisture content rose from 35% to 45%, and the maximal removal rate (about 55%) was achieved at 45% moisture content. The rate then decreased with further elevation of moisture content (Fig. 2B). This can be explained by the fact that high moisture content leads to substrate agglomeration and further restricts the diffusion of air and nutrients.
3.4. Effect of fermentation time on FG reduction
Fermentation time is pivotal for SSF. As shown in Fig. 2C, long time (from 12 h to 60 h) of fermentation facilitated the removal, and the reduction rate of FG peaked (about 59%) at 60 h. However,longer time (> 60 h) failed to significantly elevate the reduction rate. Therefore, the optimal fermentation time was 60 h.
3.5. Mechanism of GJ00141
GJ00141 was cultured and the cells and supernatant were collected separately to explore the reduction mechanism. The results showed that both viable cells and inactivated cells can reduce the content of gossypol, and inactivated treatment posed negative influence on the detoxifying capability. The reduction rates of viable cells were significantly higher than those of inactivated cells within 48 h, while the two groups exhibited similar rates after 60 h of incubation,perhaps because of cellular aging and the toxicity of gossypol to viable cells. These results indicated that the cells exerted the detoxifying function by adsorption, binding, and metabolic activity.However, their reduction rates were not high and after 72 h of incubation, which were only about 20%(Fig. 3).

Fig. 3 Reduction rates of gossypol by inactivated cells and viable cells
The supernatant which was treated with proteinase K, heat, or EDTA was also investigated for the reduction mechanism. Compared with the control, no treatment mentioned above dramatically affected the detoxifying ability of the supernatant and about 95% gossypol was reduced after 48 h of incubation (Fig. 4). The cellular contents had the same performance with supernatant and after 48 h of incubation and the reduction rate stood at about 95% after heat treatment (data not shown). The above findings suggested that the cellular contents and supernatant may exert the detoxifying function by adsorbing and binding with some stable compounds such as small molecullar peptides and polysaccharides secreted by cells rather than enzyme reaction.
3.6. Growth of AA broilers
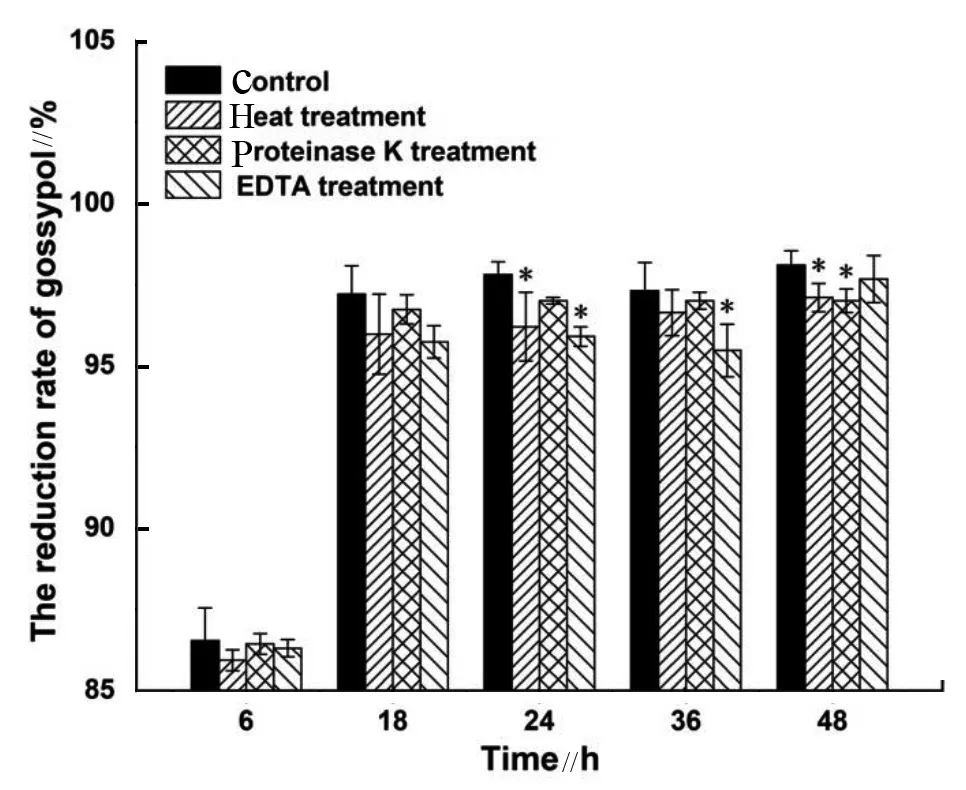
Fig. 4 Reduction rates of gossypol by supernatant under different treatments
The growth of broilers in three groups did not differ at the early stage of the experiment but showed differences at the late stage (Table 2). Over the 42 d of experiment, ADG in groups B and C was not significantly higher (P> 0.05) than that in group A.The F/G in groups A and C was respectively 8.3% and 5.5% lower than that in group B (P< 0.05).
4. Conclusions
This paper proved that SSF can effectively reduce FG in un-sterilized CSM. Medium components and fermentation parameters played a crucial part in increasing the reduction rate of FG. Some stable compounds secreted by cells may exert the main detoxifying function by adsorbing and binding to FG.Owing to low energy consumption and high nutrient maintenance, this method enabled the large-scale useof CSM in feed. Moreover, the findings suggested that FCSM can improve the growth of AA broilers.
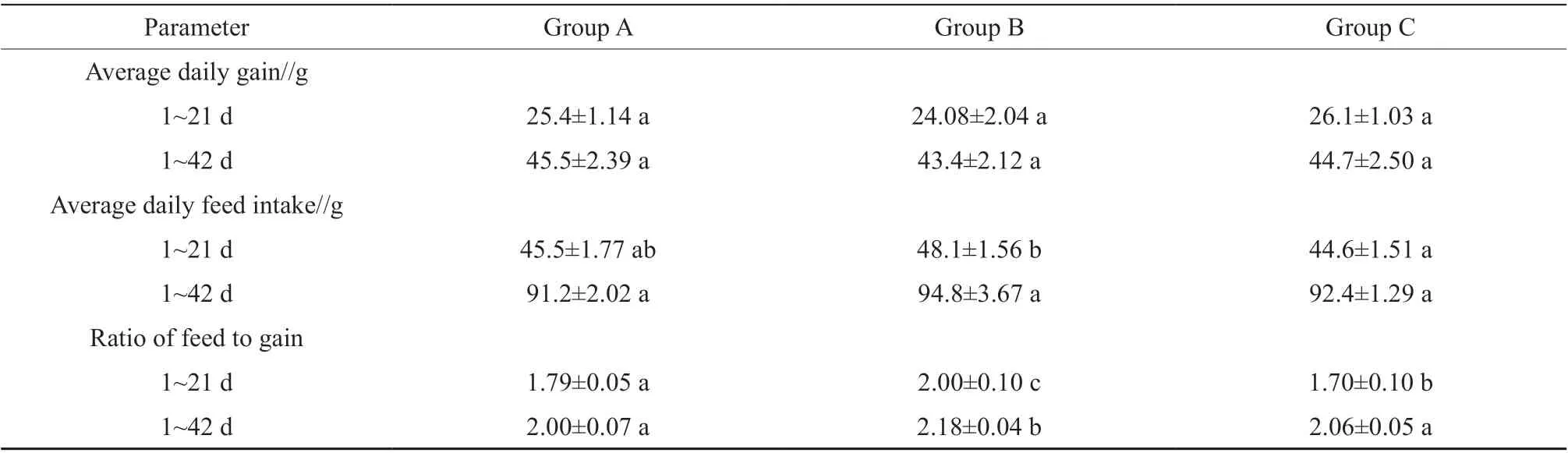
Table 2 Effects of the FCSM supplementation on growth performance of broilers
杂志排行
Agricultural Science & Technology的其它文章
- Effects of Different Cultivation Media and Periods on the Content of Main Active Components of Cordyceps militaris Strain QC04
- TIB for Micropropagation and the Relationship between Anthocyanins and Chlorophyll of Strawberry Seedlings
- Study on Weed Control and Safety of Tembotrione-Atrazine Tank Mixture in Spring Maize Fields
- Control Effects of Mixture of Metamifop and Cyhalofopbutyl on Annual Weeds Barnyard Grass in Directseeding Paddy Field
- Analysis on Interaction Effects Between Variety and Site of Silage Maize Regional Test in Guizhou Province
- Effects of Different Intercropping Patterns on Population Yield and Benefit of Fresh Maize and Mung Bean
