实验室模拟研究硫酸铵和甘氨酸对乙醇醛与胺反应体系生成棕碳的影响
2022-06-16丁喆正易雅谊陈彦奇薛丽坤王文兴周学华
孙 娟,丁喆正,易雅谊,陈彦奇,薛丽坤,王文兴,周学华*
1.山东大学 环境研究院,青岛 266237
2.青岛众瑞智能仪器股份有限公司,青岛 266237
Brown carbon (BrC) is becoming a concern in atmospheric research because organic carbon (OC)only scatters solar radiation with negative forcing.Atmospheric BrC is known to have multiple sources,including primary emissions from biomass burning(Kirchstetter et al, 2004; Hecobian et al, 2010;Lack et al, 2012; Kawamura et al, 2013), fossil fuel combustion (Bond, 2001; Zhang et al, 2011), and vehicle exhaust and secondary formation via various mechanisms such as the ozonolysis of terpenes,subsequent aging in the presence of ammonium ions(Bones et al, 2010) or reactions of carbonyls / aromatic compounds with nitrogenous substances (Laskin et al,2015).Among these reactions, water-soluble carbonyl compounds with ammonium salts, amino acids, such as glycine (Gly), or primary amines, such as methylamine(MA), can undergo Maillard-type browning or aldol condensation reactions to form secondary organic aerosols (SOA) (Hastings et al, 2005; de Haan et al,2009; de Haan, 2011).Moreover, other researchers observed that the Maillard reaction in the atmosphere confirms the presence of the actual atmospheric environment (Chen et al, 2021a; Chen et al,2021b; Tang et al, 2021).Based on constraints on the global deposition of OC, Goldstein and Galbally(2007) estimated that >175 Tg · a-1(in carbon) is deposited on the Earth’s surface as SOA.The global emission of SOA from the irreversible uptake of dicarbonyls is 11 Tg · a-1(in carbon), 90% of which occurs in clouds (Fu et al, 2008).Note that theseSOA products have considerable influence on aerosol optical properties because of the production of BrC(Powelson et al, 2014).Shapiro et al (2009) proposed that the aldol condensation and oligomerization of glyoxal were responsible for forming light-absorbing products in the reactions of glyoxal / ammonium sulfate (AS) solution, and Bones et al (2010) examined the formation of strong absorbers and fluorophores in the products of limonene-O3SOA.Currently, multiple carbonyl compounds (such as glyoxal, methylglyoxal,acetaldehyde, and hydroxyacetone) have been reported in reactions with either ammonium or amine(Galloway et al, 2009; Shapiro et al, 2009; Schwier et al, 2010; Yu et al, 2011; Yi et al, 2018a; de Haan et al, 2020; Jimenez et al, 2022).Nitrogen in amine(MA, Gly), as a nucleophile, is more active in attacking the carbenium in carbonyl compounds(Galano et al, 2004; de Haan et al, 2018; Yi et al,2018a).What happens when AS or Gly is added in the reaction of carbonyl compounds and amine? This question inevitably arises in the real atmospheric environment and is rarely investigated.
In atmospheric chemistry, glycolaldehyde(GAld), the simplest hydroxycarbonyl, is an important compound, which can be directly emitted by biomass burning or formed by the oxidation of volatile organic compounds (Bacher et al, 2001; Kua et al, 2013).In the atmosphere, GAld was reported at mid-partper-trillion to low-part-per-billion mixing ratios in the gas and atmospheric aqueous phases such as clouds, fog, and aqueous aerosol (Zhou et al, 2009).In the gas phase, hydroxyl (OH) radical oxidation and photolysis are the dominant loss processes of GAld, causing an atmospheric lifetime of only several hours to a day (Bacher et al, 2001; Spaulding et al,2003).The photolysis of GAld in the condensed phase is considerably less relevant than the aqueous phase because about 90% of GAld is reported in the hydrated, existing gem-diol form rather than the more reactive aldehyde form (Sørensen, 1972; Bacher et al,2001).The aqueous phase oxidation of GAld by OH radicals can form glyoxal and oxidized acids (e.g.,oxalic, glyoxylic, and glycolic) (Warneck, 2003; Perri et al, 2009).GAld has the potential to form BrC with ammonium salts/amines via nonoxidative liquid-phase reactions (Powelson et al, 2014).In BrC formation,the reaction of GAld with amines had far faster rates than that of GAld with AS (Yi et al, 2018a).However,the influences of AS or Gly on the reactions of GAld with amine are not understood well.
In this study, under dark conditions, the variations of light-absorbing secondary OC (SOC)products from the reactions of GAld with MA/Gly in the aqueous phase after AS was added and from the reaction of GAld with MA after Gly was added were investigated at (275 ± 2) K.The ultravioletvisible (UV-vis) absorbance spectra of products were monitored for reaction kinetics and optical characteristics.The solution pH was inspected before and after AS or Gly was added in the reaction of GAld with amines.Moreover, time-of-flight and ion trap mass spectrometry (LCMS-IT-TOF) was employed to determine the reaction products and confirm the reaction mechanisms.Finally, the produced SOC masses were evaluated.
1 Materials and methods
1.1 Sample preparation
All chemicals were purchased from Aladdin unless otherwise designated, including GAld dimer(Sigma-Aldrich), AS (AR, ≥99.0%), Gly (AR, 98%),and MA (Aladdin, 40% in water).The reactions of 0.2 mol · L-1GAld + 0.25 mol · L-1MA + 0.25 mol · L-1AS, 0.2 mol · L-1GAld + 0.25 mol · L-1Gly + 0.25 mol · L-1AS, and 0.2 mol · L-1GAld + 0.25 mol · L-1MA +0.25 mol · L-1Gly were performed at a constant temperature and in a humidity chamber at (275 ± 2) K(Powelson et al, 2014).Moreover, the contrasting experiments, the reactions of 0.2 mol · L-1GAld +0.25 mol · L-1AS, 0.2 mol · L-1GAld + 0.25 mol · L-1MA,and 0.2 mol · L-1GAld + 0.25 mol · L-1Gly were performed under the same condition.
1.2 Analytical methods
The experiments were performed in glass vials wrapped with Al foil to avoid exposure to light and then placed in a constant temperature and humiditychamber (BPHS-060A, Shanghai Yiheng Technology Instrument Co., Ltd.,) with the temperature at (275 ±2) K.A UV-vis spectrophotometer (TU-1900, Beijing Purkinje General Instrument Co., Ltd.,) recorded changes in absorbance between 190 nm and 900 nm with 1-mm-sized cuvettes of quartz.
Absorbance data were converted to mass absorption coefficients (MAC, in cm2· g-1) using the following equation (Nozière and Esteve, 2007):

where Abseis base e absorbance at wavelengthλ,bis the path length in cm, andCmassis the total concentration of reactants in g · cm-3.
Online electrospray ionization tandem mass spectroscopy (ESI-MS) was performed on an ion traptime of flight hybrid mass spectrometer (Shimadzu,Japan).ESI-MS analysis was set in the negative or positive ion mode (Yi et al, 2018a; Yi et al, 2018b).The mass acquisition range was 50 — 500.
A thermal-optical transmittance carbon analyzer(Sunset Laboratory Tigard, OR, USA) was used to determine the OC under a modified version of the NIOSH protocol (Birch and Cary, 1996).The reaction solution (10 μL) was introduced into preheated quartz filters (1×1.5 cm2, Pallflex, Tissuquartz 2500 QATUP) using a syringe.In the first stage, the oven temperature was increased to 1143 K in pure He to thermally volatilize OC; then, the temperature was reduced to 823 K, and oxygen / helium (10∶90) was introduced, and finally the oven was heated to 1143 K for thermal oxidization of element carbon (EC).
A pH meter (Five Easy Plus, FE28-Standard,Mettler-Toledo Instruments, Shanghai) was employed to monitor the solution pH.
1.3 Reaction kinetics
Kinetic data were obtained based on the Beer-Lambert law.The sample absorbance (Ab(λ)) was converted in extinction coefficient (ελ) (Eq.(2)), and the absorption indices (Aλ) were calculated using Eq.(3) for direct comparison with the optical properties of atmospheric aerosols.


Reactions obeying the first-order kinetics were fitted using Eq.(4), and the corresponding rate constants (kI(s-1)) were obtained from the slope of Eq.(5).

whereλis the wavelength,lis the optical path length(l= 0.1 cm), andis the reaction mixture absorption after a long time (i.e., at a high degree of conversion)(Nozière and Esteve, 2007).
2 Results and Discussion
2.1 UV-vis absorption of products
Fig.1 shows the UV-vis absorption spectra of background solution GAld(a), AS(b), Gly(c), and MA(d).Fig.2a shows the absorption of products from GAld + MA + AS, GAld + MA, and GAld + AS reactions.From the figure, in the GAld + MA reaction,in addition to the absorption at about 216 nm, there was another peak at about 330 nm, attributable to imine and carbonyl group-containing in the production(Yu et al, 2011).However, there was an obvious decrease for absorption at >300 nm after AS was added, indicating that AS had an inhibitory effect on the BrC formation in the GAld + MA reaction.In Fig.2b, the GAld + Gly reaction demonstrated similar absorption peaks at about 216 nm and 330 nm to that from GAld + MA.After AS was added in the GAld + Gly reaction, the absorption peak at 330 nm was slightly lower than that of the GAld + Gly reaction, indicating that AS had an inconspicuous inhibitory effect on the BrC production in the GAld + Gly reaction.Unlike AS, after Gly was added in the GAld + MA reaction, the absorption peak at about 330 nm was far higher than that of the GAld reaction with either MA or Gly (Fig.2c).Moreover,the GAld + MA + Gly reaction exceeded the UV-vis spectral range after 9 h, which indicates that the addition of Gly into the GAld + MA reaction caused an accelerated formation of BrC, thus indicating a synergistic effect of Gly and MA.
Fig.1 UV-vis absorption spectra of background solutions GAld (a), AS (b), Gly (c) and MA (d)
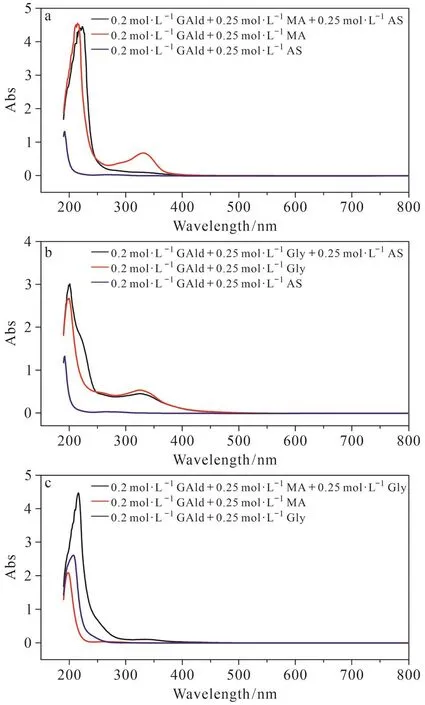
Fig.2 The comparison of UV-vis absorption after AS was added into the GAld + MA reaction of in 7 d (a), after AS was added into the GAld +Gly reaction in 7 d (b), and after Gly was added into the GAld+MA reaction in 4 h (c)
2.2 Reaction kinetics
The reaction kinetics was analyzed based on the absorbance of reaction products measured by the UV-vis spectra.The GAld + MA + AS, GAld + MA,GAld + Gly + AS, GAld + Gly, and GAld + MA + Gly reactions were first-order reactions, which agreed with a previous study (Yi et al, 2018a).The rate constants at about 216 nm and 330 nm are listed in Tab.1.After AS was added into the GAld + MA reaction, the rate constant of GAld + MA + AS had the same order of magnitude as that of GAld + MA but lower values with factors of 0.71 and 0.61 at 216 nm and 330 nm,respectively, although the reactant (MA + AS)concentrations increased because of the AS added compared with GAld + MA.This suggested that AS could decrease the reaction rate of GAld + MA.The pH of AS is (5.45 ± 0.06) based on Reaction (1), and the solution of MA has a high pH of (12.0 ± 0.20)because of Reaction (2).Fig.3 shows the pH of the GAld + MA + AS and GAld + MA solutions.From the figure, after AS was added to the GAld + MA reaction,according to Reaction (1),was ionized in NH3and H, decreasing the pH of the solution.The decreased pH might reduce the reaction of GAld with MA (Yi et al, 2018b).The rate constants of GAld + Gly + AS at 216 nm and 330 nm were 0.76 and 0.77 times those of GAld + Gly, respectively.The AS and GAld + Gly solutions had near pH values.AfterAS was added into the GAld + Gly solution, the pH was almost unchanged (Fig.3); Reactions (1) and (3)can ionize H+; therefore, the influence of AS on the GAld + Gly reaction was minute.In comparison, the rate constant of the GAld + MA + Gly reaction was higher than that of GAld + MA or GAld + Gly at both 216 nm and 330 nm, even 32 and 19 times the sums at the two wavelengths, respectively.This showed that the mixtures of Gly and MA solutions significantly accelerated the reaction and favored the BrC formation.From Fig.3, after Gly was added in the GAld +MA solution, the pH of the solution decreased because of Reaction (3).The decreased pH might decrease the reaction of a carbonyl compound with MA (Yi et al,2018b).However, for the GAld + Gly solution, the pH increased when it was mixed with MA (Reaction (3)),particularly with a considerably jump from weak acidity to strong alkalinity, which might yield an explosive BrC formation.In the alkaline solution, the GAld + Gly reaction could produce BrC with high absorbance (Fig.4).

Fig.3 The pH variations of GAld + MA + AS, GAld + Gly + AS,GAld + MA + Gly, GAld + MA, and GAld + Gly over time

Tab.1 The first-order reaction rate constants (k) of GAld and MA, Gly, the mixture of two types of species among AS, MA,and Gly at 216 nm and 330 nm
The pH of the 0.25 mol · L-1GAld + 0.25 mol · L-1Gly reaction was adjusted to 9.43 with NaOH, close to the value of (9.47 ± 0.10) in the 0.25 mol · L-1GAld + 0.25 mol · L-1MA + 0.25 mol · L-1Gly reaction,which were both alkaline conditions (Fig.4).The color of the GAld + Gly solution changed from colorless to bright yellow and finally to dark brown.A drastic BrC formation could be observed (Fig.5), which was similar to that of the GAld + MA + Gly reaction.


Fig.4 The changes of UV-vis absorption from the reaction productions of 0.2 mol · L-1 GAld + 0.25 mol · L-1 Gly (a) and 0.2 mol · L-1 GAld + 0.25 mol · L-1 MA + 0.25 mol · L-1 Gly (b)

Fig.5 The variations of solution color in the reaction of 0.2 mol · L-1 GAld + 0.25 mol · L-1 Gly at pH = 9.43
2.3 The reaction mechanisms
Fig.6 shows the LCMS-IT-TOF spectra of the reaction products of GAld + MA + AS, GAld + Gly + AS,and GAld + MA + Gly.In the GAld + MA + AS reaction,carbonium ion on the aldehyde group was nucleophilically attacked by the nitrogen atom on the amine group to form a Schiff base (Fig.7a) (de Haan et al,2009), which then underwent oligomerization reactions to form another product (m/z170).Moreover, the primary productm/z202 [M + H]was produced by the product (m/z170) combined with one molecule of MA.Furthermore, two molecules of GAld could undergo the hemiacetal reaction, then couple with one molecule of NH3, and lose two molecules of H2O to form an imine withm/z101.The primary product withm/z272 [M + H]was produced by the product (m/z170)reacting with imine (m/z101).The primary product withm/z217 [M + K]was produced by the acetal of three molecules of GAld coupled with two molecules of NH3, and then one molecule of H2O was lost.Indeed, NH3was a significant reactant in the GAld + MA + AS reaction.However, the GAld + NH3products had no absorption at 330 nm (Fig.2a), which would cause the reduction in the peak at 330 nm for GAld + MA because of the consumption of GAld as the reactant in the GAld + NH3reaction.Moreover, AS could decrease the pH of the solution from(10.81 ± 0.16) to (8.60 ± 0.13) after it was added in GAld + MA (Fig.3), which shifted Reaction (2) to the right and MA concentration decreased.It is easier for nitrogen in MA than that into attack the carbonium because of higher electronegativity.The reduction in MA concentration might decline the reaction rate of GAld + MA and the reduction for the absorption peak at >300 nm.Fig.7b shows the reaction mechanisms of GAld + Gly + AS.The product detected atm/z201 [M + H]was attributed to the product(m/z219) reacting with NH3and then losing two molecules of H2O.Here, the reaction mechanisms demonstrated that AS in the mixture could react with the GAld + Gly reaction products.

Fig.6 LCMS-IT-TOF spectra of solutions containing GAld + MA + AS (a), GAld + Gly + AS (b),and GAld + MA + Gly (c) under positive mode
For the GAld + MA + Gly reaction, the primary product withm/z202 [M + H]was produced by the acetal of three molecules of GAld coupled with one molecule of Gly and then two molecules of H2O were lost (Fig.7c).Moreover, three molecules of GAld coupled with MA and lost one or two molecules of H2O to form the products withm/z175 andm/z157.Furthermore, one molecule of Gly reacted with one molecule of MA to form the product withm/z88,which coupled with the products withm/z175 andm/z157, and then one molecule of H2O was lost,respectively, to form the products withm/z246[M + H]and 228 [M + H].The product withm/z259[M + H]was produced by the product withm/z227 coupled with one molecule of MA.The product detected atm/z303 [M + H]was attributed to the product (m/z227) reacting with Gly (m/z75).The product withm/z259 [M + H]was produced through another pathway in which four molecules of GAld underwent acetal reaction and then coupled with two molecules of H2O.Obviously, Gly participated in the reaction.When Gly was added to the GAld + MA solution, for the GAld + Gly reaction system, Reaction (3)shifted to the right because of higher pH (Fig.3)and the H2NCH2COO-concentration increased.There is a greater electronegativity for nitrogen in H2NCH2COO-than that in H2NCH2COOH.Therefore,it is easier to attack carbonium ion for H2NCH2COO-than Gly.With the addition of H2NCH2COO-, the GAld + H2NCH2COO-reaction might contribute high absorption at >300 nm even a huge absorption because of the sharp pH increase.
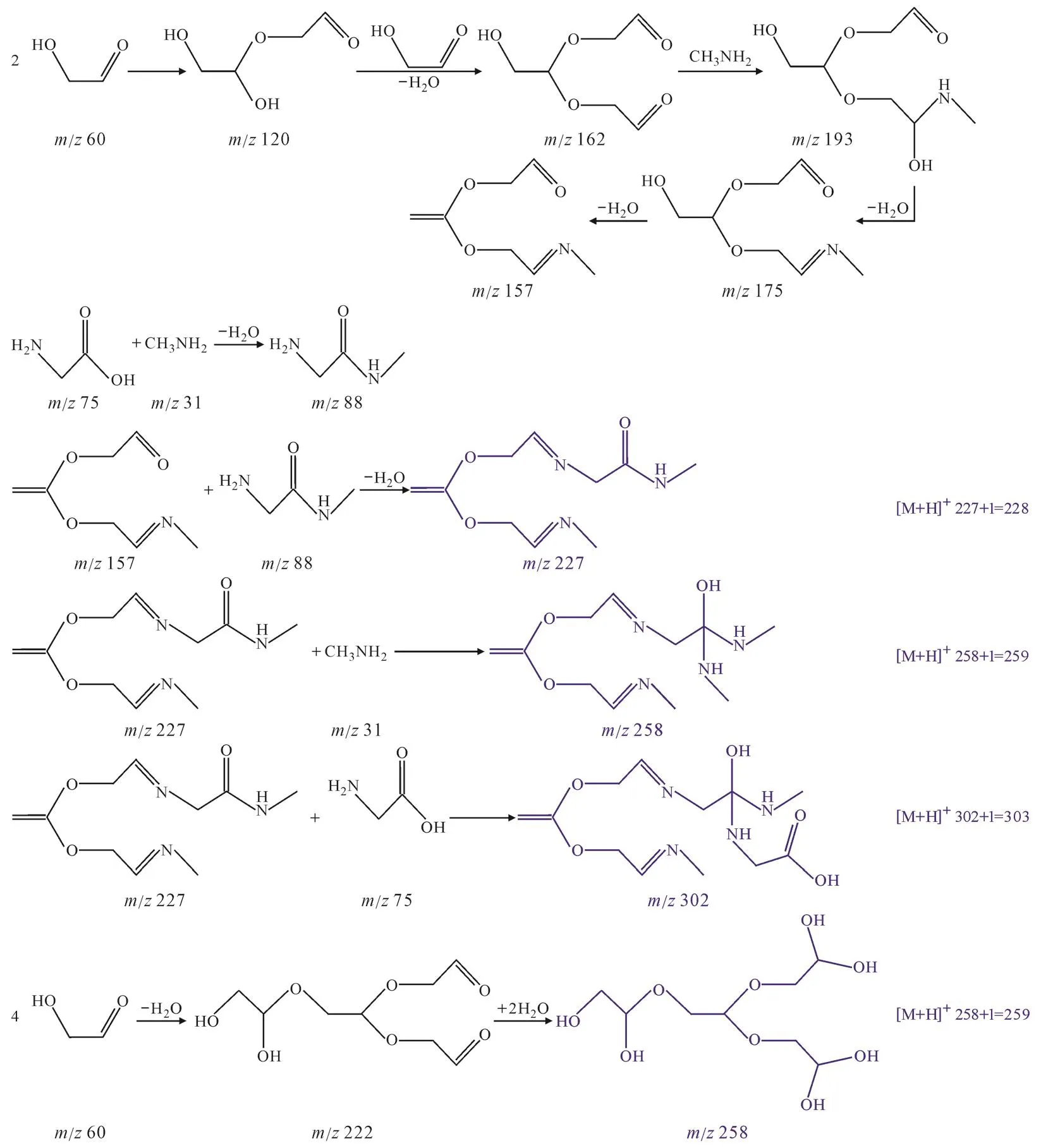
Fig.7 Possible reactions occurring in the GAld + MA + AS (a), GAld + Gly + AS (b) and GAld + MA + Gly (c) in 7 d(blue colored compounds were identified in this study)
2.4 Formed aqueous SOC evaluation
Thermal fractions of OC differ by sources(Watson et al, 1994; Chow et al, 2004; Niu et al, 2013)and have been used for the source apportionment of carbonaceous aerosols (Kim et al, 2003; Cao et al,2005).OC is divided into Pyrol C, Pk1 C, Pk2 C,Pk3 C, and Pk4 C in the thermal-optical carbon analyzer with an increase in temperature.The highmolecular-weight compounds with are harder to decompose; therefore, Pk4 C usually represents compounds with the highest molecular weights(MW) in five fractions.In this study, Pyrol C was derived from small molecules in the reactions ofGAld with either AS, MA, or Gly and GAld with MA + AS, Gly + AS, or MA + Gly.Tab.2 shows the OC mass distributions of the GAld + AS, GAld + MA,GAld + Gly, GAld + MA + AS, GAld + Gly + AS, and GAld + MA + Gly reaction products in 7 d.From the table, GAld, MA, and Gly were primarily distributed in Pk1 C.Therefore, in these reactions, the OC masses in Pk2 C, Pk3 C, Pk4 C, and Pyrol C might be considered to be from the secondary formation.They were the lowest amount of SOC formed in the reactions when an assumption was made that Pk1 C in the reactions comes from the reactants.
Based on Tab.2, compared with the SOC mass of GAld + MA (1.24 g · L-1in 7 d), after AS was added into the GAld + MA reaction, there was a higher SOC mass (3.69 g · L-1in 7 d).MA is a species with high volatility in the aqueous phase.MA in the GAld + MA +AS mixed solution was considerably less volatile than that in GAld + MA because of additionalin the aqueous phase with decrease in pH after AS was added.This might cause the SOC mass of GAld +MA + AS to be higher than that of GAld + MA.The relative contribution of each fraction SOC in the GAld + MA + AS reaction followed an order of Pyrol C (58.3%) > Pk4 C (27.6%) > Pk2 C (7.6%) > Pk3 C(6.8%) in 7 d, indicating that most reaction productshad small molecule weight.In the GAld + Gly + AS reaction, there was an increased SOC mass (6.60 g · L-1in 7 d), contrary to that from the GAld + Gly reaction(4.52 g · L-1in 7 d).The SOC mass distribution of the products from the GAld + Gly + AS reaction followed an order of Pyrol C (52.1%) > Pk4 C (27.4%) > Pk2 C(11.8%) > Pk3 C (8.8%) in 7 d, indicating that the low-molecular-weight reaction products were dominant.The GAld + MA + Gly mixture (5.77 g · L-1in 7 d) demonstrated higher SOC mass than those from the reactions of GAld + MA (1.24 g · L-1in 7 d) and GAld + Gly (4.52 g · L-1in 7 d).In the GAld + MA + Gly reaction, compared with the SOC mass of GAld + MA or GAld + Gly reaction products,there was a significant increase in SOC formation.The SOC mass distribution followed an order of Pk2 C(36.6%) > Pyrol C (32.9%) > Pk4 C (20.1%) > Pk3 C(10.4%) in 7 d.From the abovementioned results, after AS was added into GAld + MA or GAld + Gly and after Gly was added into GAld + MA, there was higher SOC mass in the aqueous phase than those from GAld + MA or GAld + Gly.
Note that, from Tab.2, the ratio of SOC with the relatively high MW (Pk3 C + Pk4 C) of the GAld +MA + AS reaction was 34.4%, higher than that of the GAld + MA reaction (31.8%).Similarly, the high MW SOC (Pk3 C + Pk4 C) occupied 36.1% in the produced SOC from the GAld + Gly + AS reaction, which was greater than that from the GAld + Gly reaction(32.3%).In these two reactions, AS participated in the reactions as reactants.Different from them, in the GAld + MA + Gly reaction, higher SOC mass was produced than in the GAld + MA and GAld + Gly reactions; however, the percentage of SOC mass with high MW was relatively low.Interestingly,in this study, the highest percentage of SOC mass occurred with high MW in produced SOC from the GAld + AS reaction among all reactions.Therefore,it could be speculated that in the reaction of GAld with ammonium/amine, after AS participated in the reaction, high MW products tended to form.

Tab.2 The OC mass and distribution of separate 0.2 mol · L-1 GAld, 0.25 mol · L-1 AS, 0.25 mol · L-1 MA, and 0.25 mol · L-1 Gly and the reactions of 0.2 mol · L-1 GAld + 0.25 mol · L-1 AS, 0.2 mol · L-1 GAld + 0.25 mol · L-1 MA, 0.2 mol · L-1 GAld +0.25 mol · L-1 Gly, 0.2 mol · L-1 GAld + 0.25 mol · L-1 MA + 0.25 mol · L-1 AS, 0.2 mol · L-1 GAld + 0.25 mol · L-1 Gly +0.25 mol · L-1 AS, and 0.2 mol · L-1 GAld + 0.25 mol · L-1 MA + 0.25 mol · L-1 Gly in 7 d
2.5 Atmospheric implications
GAld is a sufficiently water-soluble carbonyl compound with an effective Henry’s constant (H* =0.41 × e[4.6×103(1/T-1/298)]mol · L-1· Pa-1).AS is an important component of global anthropogenic aerosols with a concentration of 3200 — 7700 pmol · m-3in the aerosols(Matsumoto and Uematsu, 2005).MA, the smallest alkyl amine in the atmosphere, has the highest emission in the globe, which has been detected in aerosols with a concentration of (56 ± 52) pmol · m-3.Gly is a representative dissolved free amino acid with the simplest structure of all dissolved free amino acids.The Gly concentration ranges from 0.2 to 170 pmol · m-3in aerosols (Zhang and Anastasio, 2003; Matsumoto and Uematsu, 2005).GAld in aerosol particles could react with two types of species among AS, MA, and Gly and produced BrC.Fig.8 shows the MAC of reactions of GAld and single MA and Gly and GAld with MA + AS,Gly + AS, and MA + Gly.From the figure, the MAC at 330 nm (MAC330) with the GAld + MA + AS and GAld + Gly + AS reactions were 38.4 cm2· g-1and 147 cm2· g-1after 7 d, respectively, whereas that of GAld + MA + Gly after 7 d was 5.98 ×103cm2· g-1.In comparison, the MAC330of products from the GAld + MA and GAld + Gly reactions were 716 cm2· g-1and 356 cm2· g-1, respectively.Obviously, the MAC of products reduced when AS was added in the GAld + MA or GAld + Gly reaction, whereas, when Gly was added into the GAld + MA reaction, the MAC of products increased, suggesting the inhibitory effect of AS and the synergistic effect of Gly in the reactions of GAld with amines to form BrC.Therefore, where there were high concentrations of carbonyl compounds and amines, e.g., in the urban and forest areas (Ortiz et al,2006; Volkamer et al, 2007), and in rural and marine areas (O’Neill and Phillips, 1992; Schade and Crutzen,1995; Calderón et al, 2007; Dai et al, 2012), there would be additional BrC in the atmospheric particles,which will result in additional radiative forcing.While where there was a high concentration AS, such as in the urban areas in North China Plain (Cao et al, 2017), BrC formation from GAld with amines would be inhibited.These results significant implications on BrC formation in the atmosphere, the contribution of GAld to BrC formation in chemical models, and the predictions of the behaviors of other carbonyls in the atmosphere.

Fig.8 The MAC of the GAld + MA, GAld + MA + AS,GAld + Gly, GAld + Gly + AS, and GAld + MA + Gly reaction products in 7 d

3 Conclusions
The influences of AS and Gly on reactions of GAld with amine were examined in the aqueous phase.The absorbance followed the orders of GAld + MA +AS < GAld + MA, GAld + Gly + AS < GAld + Gly,GAld + MA + Gly > GAld + MA or GAld + Gly.The reaction rate constants demonstrated the same patterns as those of the absorbance, suggesting AS played an inhibitory role, and the mixture of MA and Gly together had a synergistic effect on BrC formation.The primary reaction mechanisms were hemiacetal/acetal, nucleophilic attack, and additional complex high MW substance production from the polymerization of the formed Schiff imine.Moreover,AS participated in the GAld + MA and GAld + Gly reactions.After AS or Gly was added, additional SOC mass in the mixed reaction was produced than those of GAld with single MA and Gly.AS favored the production of high MW SOC in the reactions of GAld with amines.
