Diuretic combinations in critically ill patients with respiratory failure:A systematic review and meta-analysis
2022-06-16JeanMaximeNadirGoulamhoussenBlaithinMcMahonPatrickMurray
Jean Maxime Côté, Nadir Goulamhoussen, Blaithin A McMahon, Patrick T Murray
Jean Maxime Côté, Nadir Goulamhoussen, Division of Nephrology, Department of Medicine,Centre Hospitalier de l'Université de Montréal, Montréal H2X0C1, Québec, Canada
Blaithin A McMahon, Division of Nephrology, Department of Medicine, Medical University of South Carolina, Charleston, SC 29425, United States
Patrick T Murray, Department of Medicine, School of Medicine, University College Dublin,Dublin D078NN, Ireland
Abstract BACKGROUND In patients with respiratory failure, loop diuretics remain the cornerstone of the treatment to maintain fluid balance, but resistance is common.AIM To determine the efficacy and safety of common diuretic combinations in critically ill patients with respiratory failure.METHODS We searched MEDLINE, Embase, Cochrane Library and PROSPERO for studies reporting the effects of a combination of a loop diuretic with another class of diuretic. A meta-analysis using mean differences (MD) with 95% confidence interval (CI) was performed for the 24-h fluid balance (primary outcome) and the 24-h urine output, while descriptive statistics were used for safety events.RESULTS Nine studies totalling 440 patients from a total of 6510 citations were included. When compared to loop diuretics alone, the addition of a second diuretic is associated with an improved negative fluid balance at 24 h [MD: -1.06 L (95%CI: -1.46; -0.65)], driven by the combination of a thiazide plus furosemide [MD: -1.25 L (95%CI: -1.68; -0.82)], while no difference was observed with the combination of a loop-diuretic plus acetazolamide [MD: -0.40 L (95%CI: -0.96; 0.16)] or spironolactone [MD: -0.65 L (95%CI: -1.66; 0.36)]. Heterogeneity was high and the report of clinical and safety endpoints varied across studies.CONCLUSION Based on limited evidence, the addition of a second diuretic to a loop diuretic may promote diuresis and negative fluid balance in patients with respiratory failure, but only when using a thiazide. Further larger trials to evaluate the safety and efficacy of such interventions in patients with respiratory failure are required.
Key Words: Respiratory failure; Diuretics; Fluid management; Furosemide; Thiazide; Systematic review
INTRODUCTION
Progressive fluid accumulation is a commonly encountered scenario in critically ill patients and in patients with acute kidney injury (AKI), acute heart failure, and other edematous states. Fluid overload is associated with increased mortality[1,2] and numerous systemic complications such as poor wound healing, AKI and pulmonary edema with acute hypoxemic respiratory failure (AHRF)[3]. Interpretation of studies evaluating the relationship between fluid balance and mortality in AHRF is complex, especially in the context of other organ outcomes[4]. Early observational studies of fluid management in the specific context of patients with AHRF have shown that a negative fluid balance is associated with improved survival, particularly in the context of acute respiratory distress syndrome (ARDS)[5,6]. Though, the definitive trial evaluating fluid management during ARDS showed that a conservative fluid balance achieved with diuretics did not statistically affect mortality but did increase the number of ventilator-free days and intensive care unit (ICU)-free days survival[7].
In the ICU, loop diuretics remain the most widely used class of diuretics, and are used in up to 49% of all ICU admissions[8]. However, prolonged use of loop diuretics may be associated with therapeutic resistance, which is a frequent observation in the ICU and associated with increased risk of mortality[9]. Combining multiple diuretics with different mechanisms of action may achieve a sequential nephron blockade, further limiting the kidney's ability to reabsorb fluid and electrolytes. These actions may further increase urine output, but also potentially lead to complications such as electrolyte and acid-base disorders and worsening kidney function[10,11]. Diuretic combinations are routinely used in the management of heart failure, and there is a significant body of evidence supporting that practice[12,13]. Both American and European Heart Failure Guidelines recommend that when diuresis remains inadequate with loop diuretic therapy despite dose escalation, the addition of thiazide diuretics may be considered[14,15]. Recent data have also shown that the addition of a second diuretic can help to mitigate loop-diuretic resistance in a broad cohort of patients hospitalised in the ICU[16].
However, in patients with AHRF, only few data exist on the additional efficacy of various diuretic regimens to promote diuresis in resistant edematous states, despite the use of this approach in up to 6% of all ICU admissions[8]. Instead of progressively escalate the dose in patients resistant to loop diuretics, a proactive administration of a second diuretic may help to quickly increase the urine output, and therefore minimize respiratory complications. On the other hand, as opposed to patients with heart failure where the extravascular fluid retention usually represents multiple liters, patients with AHRF may have a relatively small fluid retention but enough to significantly affect the perturbed pulmonary physiology. In these patients, the risks of quickly increasing the diuresis, and therefore having a substantial negative fluid balance, may be higher regarding renal function, electrolyte homeostasis or hypotension. To date, no systematic review has evaluated different protocols of diuretic combinations in this population regarding their efficacy but also their safety.
Scope
The aim of this systematic review was to determine the efficacy of common diuretic combinations to promote negative fluid balance in patients hospitalised in the ICU with AHRF. The objective was to compare the use of loop diuretics in monotherapy to the use of a loop diuretic with an adjunctive nonloop diuretic agent paying particular attention to rates of AKI and electrolyte disturbance.
MATERIALS AND METHODS
This systematic review with meta-analysis was reported following the Preferred Reporting Items for Systematic Reviews and Meta-analysis guidelines[17]. The protocol was registered on the PROSPERO international prospective register of systematic reviews (CRD42020218381).
Eligibility criteria
Inclusion criteria:Eligible studies compared diuretic combinations to loop diuretics alone in adult patients hospitalised in ICU with respiratory failure receiving diuretics for volume control. Respiratory failure was defined as receiving invasive or non-invasive positive ventilation for an acute hypoxemic or hypercapnic respiratory failure, or for severe pulmonary edema requiring oxygen therapy. Patients with non-primary pulmonary aetiology, such as acute decompensated heart failure, were included if signs of severe pulmonary edema requiring oxygen, with or without mechanical ventilation, were clearly reported. Studies evaluating a combination of diuretic agents without a comparison group were included in the systematic review if at least one efficacy clinical outcome of interest was reported, but were not included in the final meta-analysis. The following classes of non-loop diuretics in combination with a loop diuretic were included: Thiazide or thiazide-like agents, carbonic anhydrase inhibitors, Epithelial sodium channel (ENaC) inhibitors and mineralocorticoid antagonists. No study design, date or language limits were imposed on the literature search, although only studies in English, Spanish and French were included in the analysis.
Exclusion criteria:Studies reporting patients with peripheral edema only were excluded. Studies reporting patients with chronic kidney disease (CKD) treated with maintenance kidney replacement therapy (KRT) were also excluded. Studies of the use of loop diuretic agents in pediatric populations were excluded.
Literature search
According to the predetermined protocol, a systematic literature search of 4 databases (MEDLINE, Embase, Cochrane Library and PROSPERO) was performed from inception until May 5, 2021 in collaboration with a trained medical librarian (covering from 1946 to May 2021). The literature search strategy was developed using medical subject headings and text words related to all classes of diuretics included and their individual name, fluid balance, respiratory failure and hypoxemia, and critical care (Supplementary Table 1). We also scanned the reference lists of included studies and searched the grey literature for all abstracts listed into the annual meeting archives of theAmerican Society of Nephrology, theEuropean Society of Intensive Care Medicineand theSociety of Critical Care Medicine. Finally, a bibliography of all potentially included articles was circulated to all authors, to prompt consideration of any other relevant publications.
Study selection
Eligible studies were clinical trials, observational cohort studies, case-control studies and cross-sectional studies. Cases series with more than five patients and abstracts not yet published were also included when at least one outcome of interest was described quantitatively. Literature search results were uploaded and screened usingRayyan QCRIapplication. Two reviewers (JMC and NG) independently screened the titles and abstracts of all identified articles. These reviewers then screened the full-text reports for all potential studies and decided whether these met the inclusion criteria, reporting the reason(s) for exclusions. When necessary, the authors (JMC and BMcM) contacted the corresponding author of potential studies to obtain additional information. Once the final list of included articles was determined, there was no disagreements between authors.
Data extraction
RevMan(Version 5.4, The Cochrane Collaboration, 2020) was used to extract data from each eligible study. Data extracted included eligibility criteria, demographics, methodology, diuretic name, class and dosage, risk of bias and results. The prespecified primary efficacy outcome of interest was the cumulative fluid balance, and secondary outcomes were the 24-h urine output (diuresis), ventilationfree survival, number of days on mechanical ventilation, need of therapeutic paracentesis, hospital and ICU length-of-stay, in-hospital and 90-d mortality. Due to lack of data regarding the cumulative ICU fluid balance for all included studies, the 24-h fluid balance was therefore reported as primary outcome. Safety endpoints included AKI incidence and progression to KRT, electrolyte and acid-base abnormalities, creatinine and electrolyte changes from baseline (sodium, potassium, bicarbonate) and, finally, hypotensive events, arrythmias and ototoxicity occurrence. Reports of 24-h natriuresis, not planned in the original protocol, were also captured as this endpoint was considered clinically relevant.
The risk of bias was assessed using the Cochrane Collaboration tool for assessing the risk of bias for randomised controlled trials (RCTs) (RoB2)[18], and non-randomised trials (n-RCTs)(ROBINS-I)[19], and the Newcastle-Ottawa Scale for observational studies. These assessments were based on the reporting of fluid balance, the primary outcome of the current review. When insufficient details were reported, the risk of bias was judged as unclear.
Statistical analysis
A meta-analysis using mean differences (MD) with 95% confidence interval (CI) was performed for the primary outcome and for the 24-h urine output (secondary efficacy endpoint), while descriptive statistics were used for all other endpoints reported. The statistical heterogeneity for pooled results was reported usingI2. As the clinical heterogeneity of included studies was considered high, a randomeffects model was used for both meta-analyses. In studies reporting the endpoint using median and IQR, the statistical method described by Wanet al[20] was used to convert the reported value to mean ± SD allowing meta-analysis. None of the preplanned sub-analyses (dosage of loop diuretics and the type of respiratory failure) were performed due to limited data. All statistical analyses were performed onRevMan(Version 5.4, The Cochrane Collaboration, 2020) and SPSS (Version 26, IBM, Armonk NY).
RESULTS
Study selection
Study selection is depicted in Figure 1. After removal of duplicates, there were 6510 studies. Of these, 6476 were excluded after screening titles and abstracts. A total of 34 studies were assessed for eligibility, from which 25 were excluded for not meeting inclusion criteria (Supplementary Table 2). Therefore, a total of 9 studies were included[21-29], from which 8 presented quantitative results for endpoints metaanalysis[21-23,25-29].
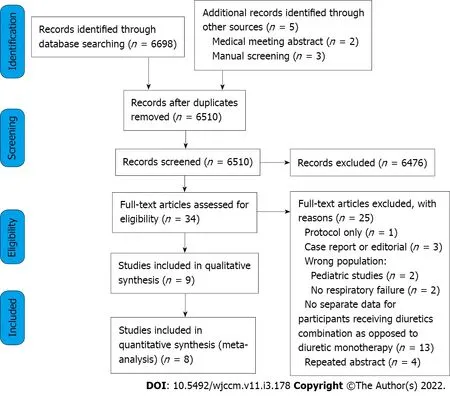
Figure 1 Flow chart of included studies.
Study characteristics
A detailed summary of each of the study characteristics is presented in Table 1. The included studies investigated the combination of furosemide with either spironolactone[21], indapamide[22], chlorothiazide[23,27,29], metolazone[23,27,28], acetazolamide[24,25] or a combination of hydrochloroth- iazide and amiloride[26] at various doses in patients with respiratory failure. These studies were published between 1997 and 2019, and included a total of 440 participants. Three studies were RCTs[21,22,25] and 5 were observational[23,24,27-29], and one was a prospective non-randomised interventional study[26].
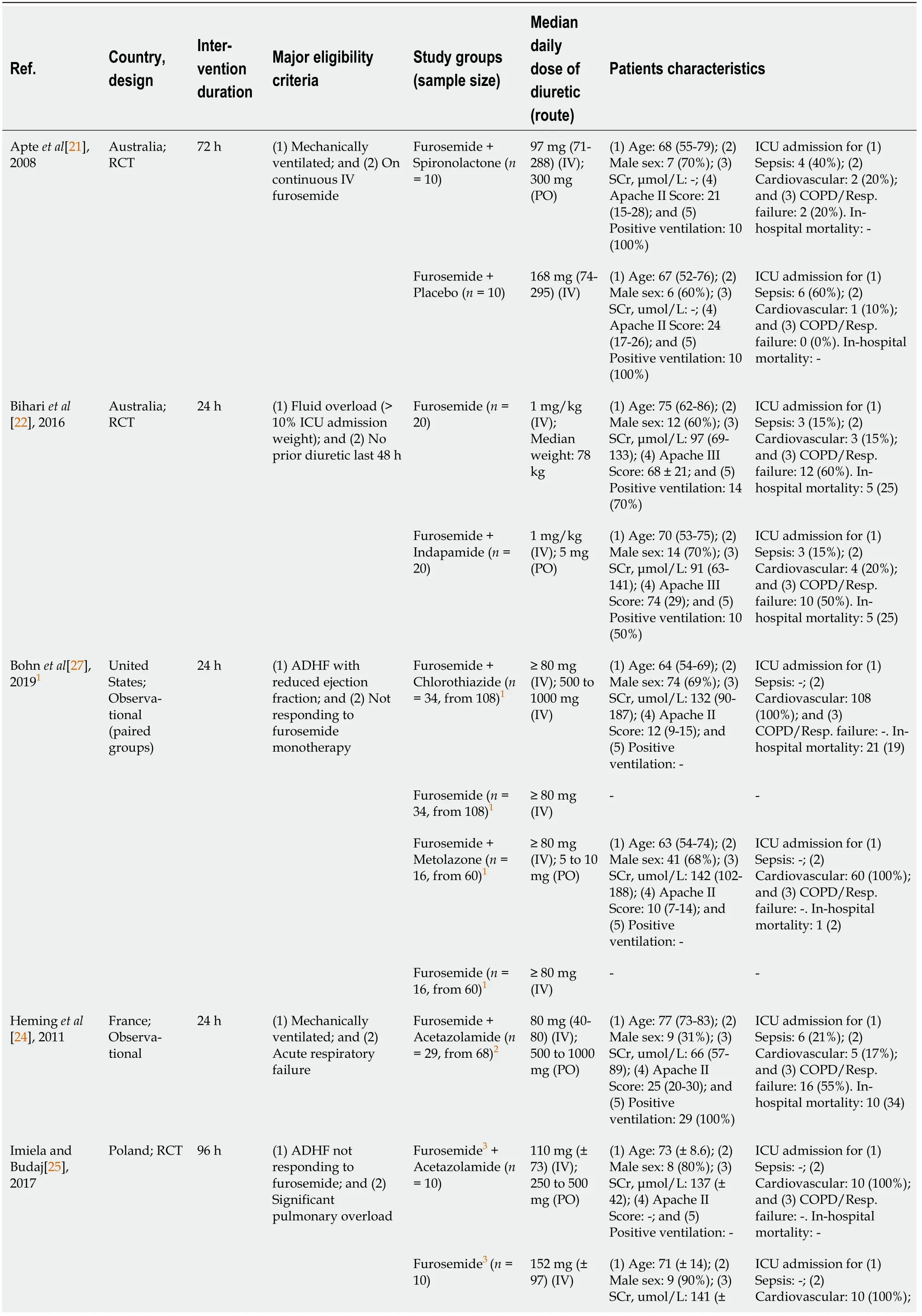
Table 1 Characteristics of included studies
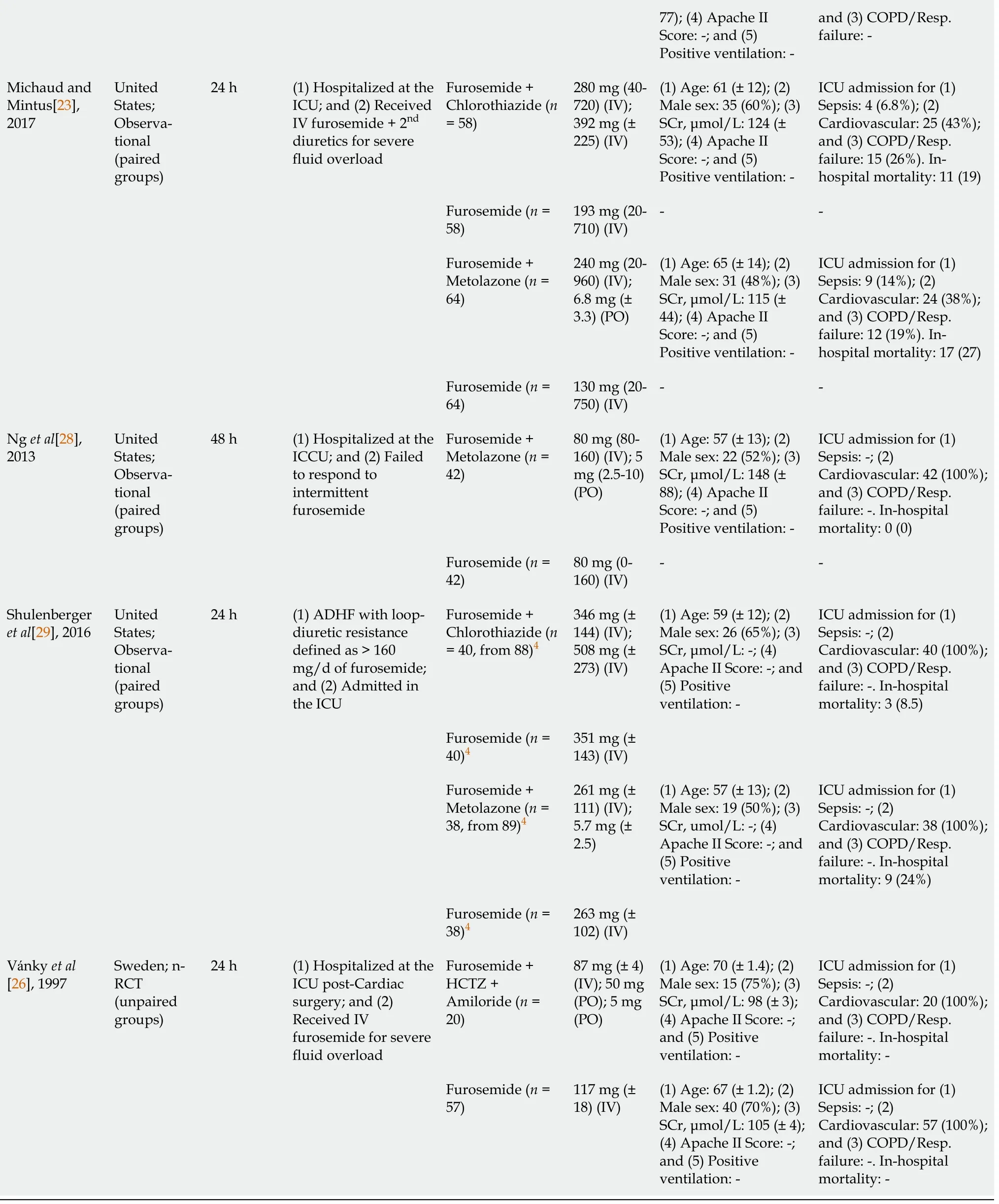
1Bohn et al[27]: Baseline characteristics reported are from the whole cohort. However, only critically ill patients receiving vasopressors (Chlorothiazide: 34, Metolazone: 16) were included in aggregated data.2Heming et al[24]: Only 29 participants from the whole cohort (n = 68) received a loop-diuretic in combination with acetazolamide. All aggregated data were re-analysed using the original dataset shared by the authors.3Some patients received torsemide. The dose was converted to furosemide equivalent.4Shulenberger et al[29]: Only intensive care unit patients (Chlorothiazide: 40, Metolazone: 38) were included in aggregated data, after re-analysis based on the original dataset shared by the authors.RCT: Randomized Controlled Trial; ADHF: Acute decompensated heart failure; SCr: Baseline Serum creatinine; ICU: Intensive care unit; ICCU: Intensive cardiac care unit.
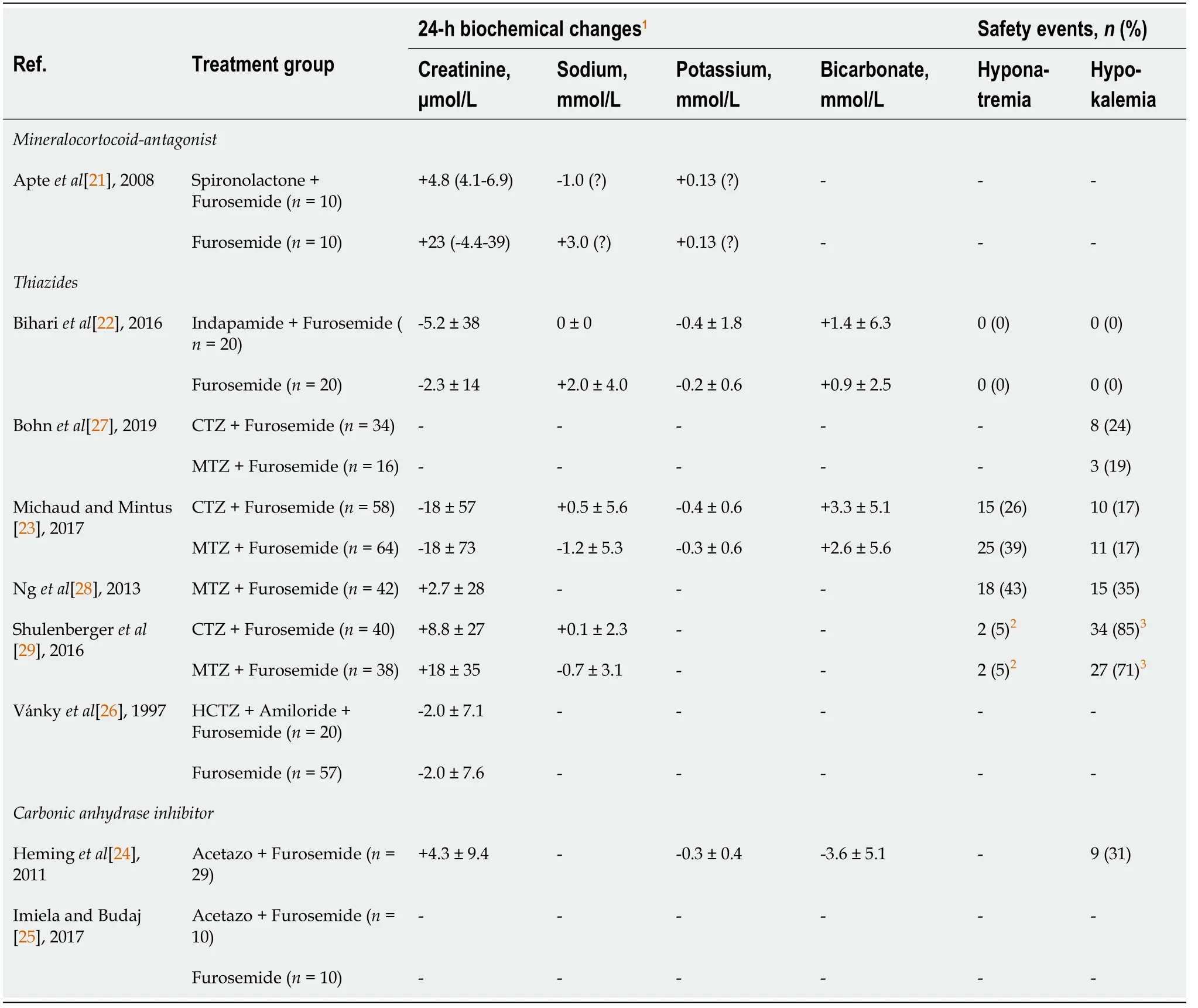
Table 2 Safety events and change in serum creatinine and electrolytes at 24-h for all included studies
For the study by Heminget al[24], only 29 from the 68 participants were receiving a loop diuretic in addition to acetazolamide. All results reported from this study were calculated using the subset of the entire cohort receiving that combination of diuretics based on the dataset shared by the authors. Similarly, only patients with confirmed ICU admission with respiratory failure from the Shulenbergeret al[29] study (n= 78, from 177 in total) were included in this review, after access to the original dataset. Overall, in this review, females were the minority and the median age ranged from 57 to 77 years. Most patients were admitted following cardiac surgery or acute decompensated heart failure. The duration of the diuretic combination intervention varied from 24 to 96 h, while the median furosemide dose (equivalent to intravenous furosemide) ranged from approximately 80 to 351 mgperday. The doses of the second diuretic are reported in Table 1.
Risk of bias
The quality assessment and risks of bias are presented in the Supplementary Material (Supplementary Table 3). All 3 RCTs included[21,22,25], despite limited sample size, were good quality with an overall low risk of bias. The non-randomised interventional trial was classified with an overall unclear risk of bias, due to missing data[26] and potential uncontrolled confounders. The observational cohort studies included were of good quality, where the risk of bias was adequately minimized for most components of the Newcastle-Ottawa Assessment Scale. No unpublished data was included in this review. Heterogeneity was substantial across all included studies, regarding study design, intervention duration and timing of administration, dose of loop-diuretics administered, baseline kidney function and safety endpoints reported. Notably, the intervention duration, defined as the period of diuretics administration during which clinical endpoints were measured, ranged between 24 h to 96 h. In addition, regarding the second diuretic, some studies reported a fixed dose for all patients, while other reported a titratable dose. The comparison group receiving only a loop-diuretic was an independent and parallel-group for 4 studies[21,22,25,26], and a sequential paired group-where clinical endpoints were compared before and after the addition of a second diuretic within the same group-for 4 studies[23,27-29 ].
Primary endpoint: Daily fluid balance
When combining all studies using various combinations of non-loop-diuretic plus loop-diuretic compared to loop-diuretics alone, a significant difference was observed in the primary outcome, with a MD in the 24-h fluid balance in favour of the combination group [overall MD: -1.06 L (95%CI: -1.46; -0.65),I2= 68%] (Figure 2A). However, when each combination diuretic class was analyzed separately, no significant difference was observed for the spironolactone-furosemide [MD: -0.65 L (95%CI: -1.66; 0.36),I2 = NA] or the acetazolamide-furosemide combination [MD: -0.40 L (95%CI: -0.96; 0.16),I2= NA]. Thus, the observed effect on the daily fluid balance was mainly driven by the thiazide-furosemide combinations [MD: -1.25 L (95%CI: -1.68; -0.82),I2= 60%]. Inspection of the funnel plot (Supplementary Figure 1) showed no substantial publication bias toward specific studies.
Secondary efficacy endpoints
Similar findings were reported for the 24-h urine output, where the addition of a second diuretic was associated with an increase in the urine output by 1.08 L (95%CI: 0.65; 1.52,I2= 73%). Once again, that effect was mainly attributed to the thiazide-furosemide combination [MD: 1.30 L (95%CI: 0.81-1.79),I2= 76%] as no difference was observed for other combinations (Figure 2B). Overall, while the addition of spironolactone or acetazolamide to furosemide had a limited effect on fluid and sodium balance (Supplementary Table 4), the addition of a thiazide was associated with an increase in urine output by 14% for indapamide, 31% for hydrochlorothiazide plus amiloride, ranged from 52%-101% for metolazone and, finally, from 89%-114% for chlorothiazide, with corresponding effects on the negative fluid balance. In-hospital mortality, ICU length-of-stay, and hospital length-of-stay are depicted in Supplementary Table 5. Due to limited data, no pooled analysis was performed for these outcomes. No study reported the 28-d or 90-d mortality, need of therapeutic paracentesis and ventilation free-survival.
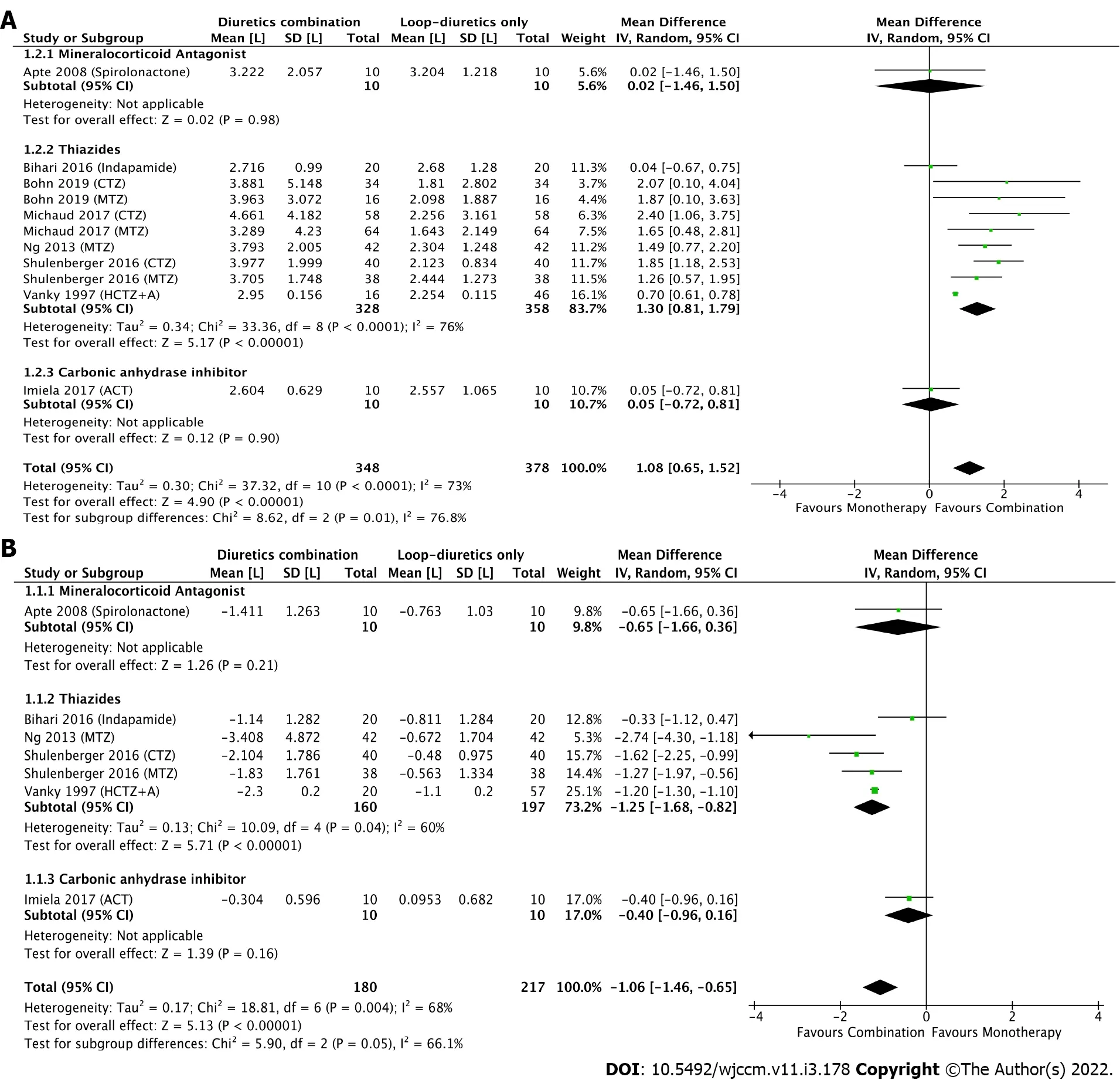
Figure 2 Forest plot. A: Daily fluid balance; B: Urine output. Comparing loop diuretic in monotherapy to three combinations of diuretics (mineralocorticoid antagonist, thiazides and carbonic anhydrase inhibitor). Mean difference and 95% confidence intervals are shown for each study and the pooled analysis using a random effects model and the Mantel-Haenszel method. Mean difference > 0 means that urine output is higher in the group receiving the combination of diuretics.
Safety endpoints
Available data on the physiological effects of these diuretic combinations on electrolytes and serum creatinine is shown in Table 2, but reporting was inconsistent. Due to significant heterogeneity across these studies, results for these endpoints were not pooled, but instead reported separately. No diuretic combination was associated with a substantial serum creatinine change at 24-h from baseline. According to the specific segment of the nephron targeted, varied impacts on electrolytes were observed for these three diuretic classes; for example, whereas a limited increase in serum potassium was observed with the spironolactone combination, a decrease in serum potassium was observed in all thiazide studies reporting this endpoint. Notably, as opposed to thiazide and loop-diuretic combinations, with which an increased in serum bicarbonate was observed, treatment with acetazolamide for 24-h reduced serum bicarbonate levels by 3.6 ± 5.1 mmol/L.
The risk of all other adverse (safety) events, where definitions and follow-up varied across included studies, are reported in Supplementary Table 6. Notably, hypokalemia was documented in 6 studies and ranged from 0% to 85%, while hyponatremia was documented in 4 studies and ranged from 0% to 43% when combining a thiazide with a loop-diuretic. No study reported arrythmia or ototoxicity events.
DISCUSSION
To our knowledge, this is the most comprehensive systematic review and meta-analysis to address the clinical efficacy and safety of various diuretic combinations in the context of patients hospitalised at the ICU with fluid overload and respiratory failure. A significant increase in the 24-h urine output leading to a negative fluid balance was observed in the pooled analyses, mainly attributed to the thiazidefurosemide combination. Reporting of other clinical endpoints including the efficacy, safety, and clinical outcomes of groups treated with each combination was inconsistent and generally incomplete.
Currently, strategies to manage fluid balance in critically ill patients with acute lung injury and other causes of respiratory failure include fluid restriction but this may be difficult given the requirement of fluid for carriers for vasopressors, antibiotics, and nutrition. A preferred option is augmenting urine output with diuretics. In addition, positive sodium balance specifically, rather than simple fluid balance, has recently been associated with respiratory dysfunction in mechanically ventilated patients[30,31], and with worsening prognosis in decompensated heart failure[32]. Ensuring adequate negative sodium balance along with increased urine output may be crucial to optimising extracellular fluid volume and outcomes. This approach is now endorsed by the European Society of Cardiology[33]. Also, as recently confirmed by the STARRT-AKI trial, delaying initiation of KRT based on a watchful waiting approach (in the absence of emergency indications for RRT initiation) can be beneficial by reducing RRT complications including prolonged KRT requirement[34]. Therefore, refining the ways to achieve a negative fluid balance with a diuretic combination strategy might potentially delay or avoid the need for RRT initiation (including ultrafiltration) to treat volume overload and control fluid balance in patients with loop-diuretic resistance.
The mechanisms of resistance to furosemide and other loop diuretics is multifactorial[35]. They include a decrease in sodium delivery to the site of action by systemic and renal hypoperfusion[36], as well as an increase in sodium and water retention due to neurohormonal, renin-angiotensin-aldosterone and antidiuretic hormone systems activation in critically ill patients. In addition, proximal tubular injury or loss in AKI or CKD results in diminished loop diuretic secretion into the tubular lumen and reduced effects more distally in the thick ascending limb of Henle’s loop, while in chronic exposure to furosemide, adaptive changes in the nephron occur with hypertrophy of the distal tubule leading to an increase of its reabsorptive capacity[37]. For patients who do not respond to an increasing dose of furosemide, sequential nephron blockade of sodium reabsorption with other classes of diuretics can overcome these resistance mechanisms[16], which was confirmed in the current review focusing on patients with AHRF.
In order to promote liberation from mechanical ventilation in patients with metabolic alkalosis and associated hypoventilation, normalisation of the acid-base state while improving fluid balance with acetazolamide has also been investigated[38-40]. Also, the combination of an aldosterone receptor antagonist with furosemide is recommended as first line therapy in cirrhotic patients with ascites[41], due to the efficacy of that combination to promote natriuresis while minimising the risk of hypokalemia. This combination is also widely recommended in the management of patients with chronic heart failure and has been shown to reduce morbidity and mortality in patients with reduced ejection fraction[42].
In this review, various factors may explain the limited efficacy of these combinations to promote diuresis and a negative fluid balance in some included studies. First, the dose of furosemide was not maximised for most studies, unlike recent RCTs on acute heart failure[12]. Indeed, the studies with higher median daily doses of furosemide were associated with higher and significant increases in urine output, even before addition of the second diuretic[23,29], which was also confirmed in previous cohorts[16]. On the other hand, the use of sub-maximal doses of multiple drugs in combination may additively or synergistically augment efficacy, while avoiding the adverse effects of higher doses of these drugs. Secondly, in the context of respiratory failure, the total negative fluid balance required to improve respiratory parameters may be less than the diuresis desired in patients with acute heart failure, in which the cumulative volume overload is usually greater[1]. As this review focused on the net fluid balance achieved instead of respiratory outcomes, it is still possible that the limited diuresis observed for these patients was judged as clinically sufficient to maintain an even fluid balance (rather than targeting negative fluid balance), as opposed to a fluid-liberal approach[7]. Also, none of these studies reported the use of an integrated tool, such as point-of-care ultrasound, bioimpedance, or other hemodynamic and volume measures[3], to evaluate the volume status of these patients, once again limiting the capacity to determine if the urine output achieved was adequate to optimise volume status.
All diuretic agents have a safety profile that varies according to their intrinsic mechanism of action. This review showed that combination of acetazolamide and furosemide may reduce serum bicarbonate and induce potassium loss, causing hypokalemia in up to 31% of patients[24] after only 24 h of treatment. In contrast, when furosemide is combined with thiazides, a trend toward an increase in bicarbonate and lower potassium levels was observed, reflecting the greater natriuretic and kaliuretic effects of reabsorption blockade in sequential nephron segments. The rate of hypokalemia was considerable, emphasizing the need to regularly monitor electrolyte levels, acid-base parameters, and kidney function (which is under-reported in this literature) when choosing such combinations. The role of potassium-sparing diuretics in the prevention of hypokalemia with aggressive diuretic regimens warrants further research.
In sum, this study brings new data on the use of diuretic combinations in the subgroup of ICU patients with AHRF, which has never been systematically reported before. The pooled analysis confirmed an increased efficacy regarding urine output and net fluid balance, which is interesting in a clinical setting, but also brings relevant data on the potential risk of substantial electrolyte disturbances in patients exposed to these combinations. Indeed, the study also confirms the need for additional lab monitoring when prescribing such combinations especially if no pre-emptive electrolytes administration is planned.
There are several limitations to the current systematic review. First, no study reported the preplanned endpoint of cumulative fluid balance, which required us to deviate from the original protocol and to use the daily fluid balance as primary outcome. Also, no study reported the use of ENaC inhibitors alone (e.g.triamterene, amiloride) in conjunction with furosemide, which did not allow this review to evaluate that combination. This highlights the importance of future studies using ENaC inhibitors in combination with loop-diuretics in the management of respiratory failure. In addition, the literature strategy was limited to generic name. The limited duration of these interventional periods, from 24 to 96 h, may not have substantially affected clinical outcomes such as in-hospital mortality, ICU length-of-stay and ventilation-free survival, which were only partially reported in these studies. Most importantly, the heterogeneity across all included studies was high, including for diuretics doses, renal function, reasons of ICU admission with notable inconsistencies in clinical endpoints reporting. We contacted corresponding authors of all included references to confirm eligibility criteria, but we cannot independently confirm with certainty that all included patients were on mechanical ventilation or required high oxygen volume as some did not respond. Finally, the risk of publication bias is significant, since only limited data has been published in the context of critically ill patients receiving such diuretic strategies.
CONCLUSION
In critically ill patients with respiratory failure receiving a loop diuretic, we showed that addition of another class of diuretic is associated with an increased 24-h urine output leading to a negative fluid balance, where the thiazide and loop-diuretic combination had the higher efficacy. However, given the significant heterogeneity, the risk of publication bias and the lack of adequately powered RCTs, no definitive evidence can be drawn, especially for non-thiazide combinations. In addition, electrolytes disturbance secondary to the use of these adjunctive diuretics in combination with a loop diuretic warrants additional monitoring to ensure their safety. This limited evidence emphasizes the need for further high-quality trials investigating the efficacy, safety profile and clinical outcomes of such therapeutic interventions for patients with respiratory failure requiring diuretics to control fluid balance.
ARTICLE HIGHLIGHTS
Research background
Diuretics are essential to maintai n fluid balance in patients admitted to intensive care units (ICUs).However, resistance to loop-diuretics is common and diuretic combinations are often used in order to mitigate this resistance.
Research motivation
As opposed to patients with heart failure where combinations of different classes of diuretics have been extensively studied and are now recommended, the body of evidence regarding diuretic combinations in ICU patients with hypoxemic respiratory failure is scarce.
Research objectives
This study systematically reviewed the efficacy and safety of common diuretics combinations in ICU patients with respiratory failure when compared to loop-diuretics in monotherapy.
Research methods
A systematic review and meta-analysis were performed. A pooled analysis of the mean difference for the 24-h urine output and the 24-h fluid balance between loop-diuretics in monotherapy and common diuretics combinations (thiazides, carbonic anhydrase inhibitors and mineralocorticoid antagonists) was performed. Descriptive statistics were used to report the occurrence of safety events, such as electrolyte disturbances, hypotension and acute kidney injury.
Research results
From 6510 citations, nine studies totalling 440 patients were included. When compared to loop diuretics alone, the addition of a second diuretic is associated with an improved negative fluid balance at 24 h mean differences (MD) of -1.06 L [95% confidence interval (CI): -1.46; -0.65], mainly driven by the combination of a thiazide plus furosemide [MD: -1.25 L (95%CI: -1.68; -0.82)]. The heterogeneity on the report of clinical and safety endpoints was high, but electrolytes anomalies were frequent and confirms the need for additional monitoring when prescribing such combinations.
Research conclusions
Larger trials are required to confirm the efficacy and safety of diuretic combinations in this population.However, based on limited evidence the combination of thiazide plus loop-diuretics is associated with an increase in urine output and negative fluid balance.
Research perspectives
The study has highlighted the paucity of data on the optimal strategy to optimise fluid balance in patients with respiratory failure and relative diuretics resistance.
ACKNOWLEDGEMENTS
The authors would like to thank Diarmuid Stokes, biomedical librarian, University College Dublin, for his assistance with the systematic review search. The authors would also like to thank Dr Nicholas Heming, Georges Pompidou Hospital, and his team to have agreed to share the data required for the reanalysis of the subgroup of patients receiving the combination of diuretics. The authors would also like to thank Dr. Brent N. Reed, University of Maryland, Baltimore, and his team to have agreed to share the data required for the reanalysis of the subgroup of patients admitted to the ICU.
FOOTNOTES
Author contributions:Côté JM, Goulamhoussen N, McMahon BA and Murray PT designed the research study and methodology; Côté JM and Goulamhoussen N performed the research and analyzed the data; Côté JM wrote the draft manuscript; all authors reviewed and approved the final manuscript.
Conflict-of-interest statement:No potential conflict of interest relevant to this article was reported.
PRISMA 2009 Checklist statement:The authors have read the PRISMA 2009 Checklist, and the manuscript was prepared and revised according to the PRISMA 2009 Checklist.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:Canada
ORCID number:Jean Maxime Côté 0000-0002-3487-2670; Nadir Goulamhoussen 0000-0002-7589-389X; Blaithin A McMahon 0000-0001-8585-2787; Patrick T Murray 0000-0002-0165-6672.
S-Editor:Fan JR
L-Editor:A
P-Editor:Fan JR
杂志排行
World Journal of Critical Care Medicine的其它文章
- Cough as a neurological sign: What a clinician should know
- Presentation and outcome of myocardial infarction with nonobstructive coronary arteries in coronavirus disease 2019
- Plasma D-dimer level in early and late-onset neonatal sepsis
- Stress cardiomyopathy in critical care: A case series of 109 patients
- Need for oxygen therapy and ventilatory support in premature infants in a hospital in Southern Brazil
- Critical care practices in the world: Results of the global intensive care unit need assessment survey 2020
