Abnormal lipid synthesis as a therapeutic target for cancer stem cells
2022-06-02SiYuWangQinChaoHuTongWuJuanXiaXiaoAnTaoBinCheng
Si-Yu Wang,Qin-Chao Hu,Tong Wu,Juan Xia,Xiao-An Tao,Bin Cheng
Si-Yu Wang,Qin-Chao Hu,Tong Wu,Juan Xia,Xiao-An Tao,Bin Cheng,Department of Oral Medicine,Hospital of Stomatology,Sun Yat-sen University,Guangzhou 510000,Guangdong Province,China
Si-Yu Wang,Qin-Chao Hu,Tong Wu,Juan Xia,Xiao-An Tao,Bin Cheng,Guangdong Provincial Key Laboratory of Stomatology,Guanghua School of Stomatology,Sun Yat-sen University,Guangzhou 510000,Guangdong Province,China
Abstract Cancer stem cells(CSCs)comprise a subpopulation of cancer cells with stem cell properties,which exhibit the characteristics of high tumorigenicity,self-renewal,and tumor initiation and are associated with the occurrence,metastasis,therapy resistance,and relapse of cancer.Compared with differentiated cells,CSCs have unique metabolic characteristics,and metabolic reprogramming contributes to the self-renewal and maintenance of stem cells.It has been reported that CSCs are highly dependent on lipid metabolism to maintain stemness and satisfy the requirements of biosynthesis and energy metabolism.In this review,we demonstrate that lipid anabolism alterations promote the survival of CSCs,including de novo lipogenesis,lipid desaturation,and cholesterol synthesis.In addition,we also emphasize the molecular mechanism underlying the relationship between lipid synthesis and stem cell survival,the signal transduction pathways involved,and the application prospect of lipid synthesis reprogramming in CSC therapy.It is demonstrated that the dependence on lipid synthesis makes targeting of lipid synthesis metabolism a promising therapeutic strategy for eliminating CSCs.Targeting key molecules in lipid synthesis will play an important role in anti-CSC therapy.
Key Words: Lipid synthesis;Cancer stem cells;Anti-cancer therapy;Stem cell survival;Lipid anabolism
lNTRODUCTlON
Cancer stem cells(CSCs)comprise a subpopulation of cancer cells with stem cell properties,which exhibit the characteristics of high tumorigenicity,self-renewal,and tumor initiation.They may be responsible for cancer occurrence,metastasis,therapy resistance,and relapse of cancer[1,2].CSCs are able to differentiate into diverse cancer cell progenies to maintain the hierarchical organization of a tumor[3].
In solid tumors,the expression of the CSC markers,including CD133,CD44,and aldehyde dehydrogenase(ALDH)1,is similar to that in normal human embryonic stem cells,thus transformed adult stem cells are one possible source of CSCs.Another possibility is differentiated cells under long-term stress conditions,which transform into CSCs through reprogramming due to genetic instability and epigenetic abnormalities[4-6](Figure 1).
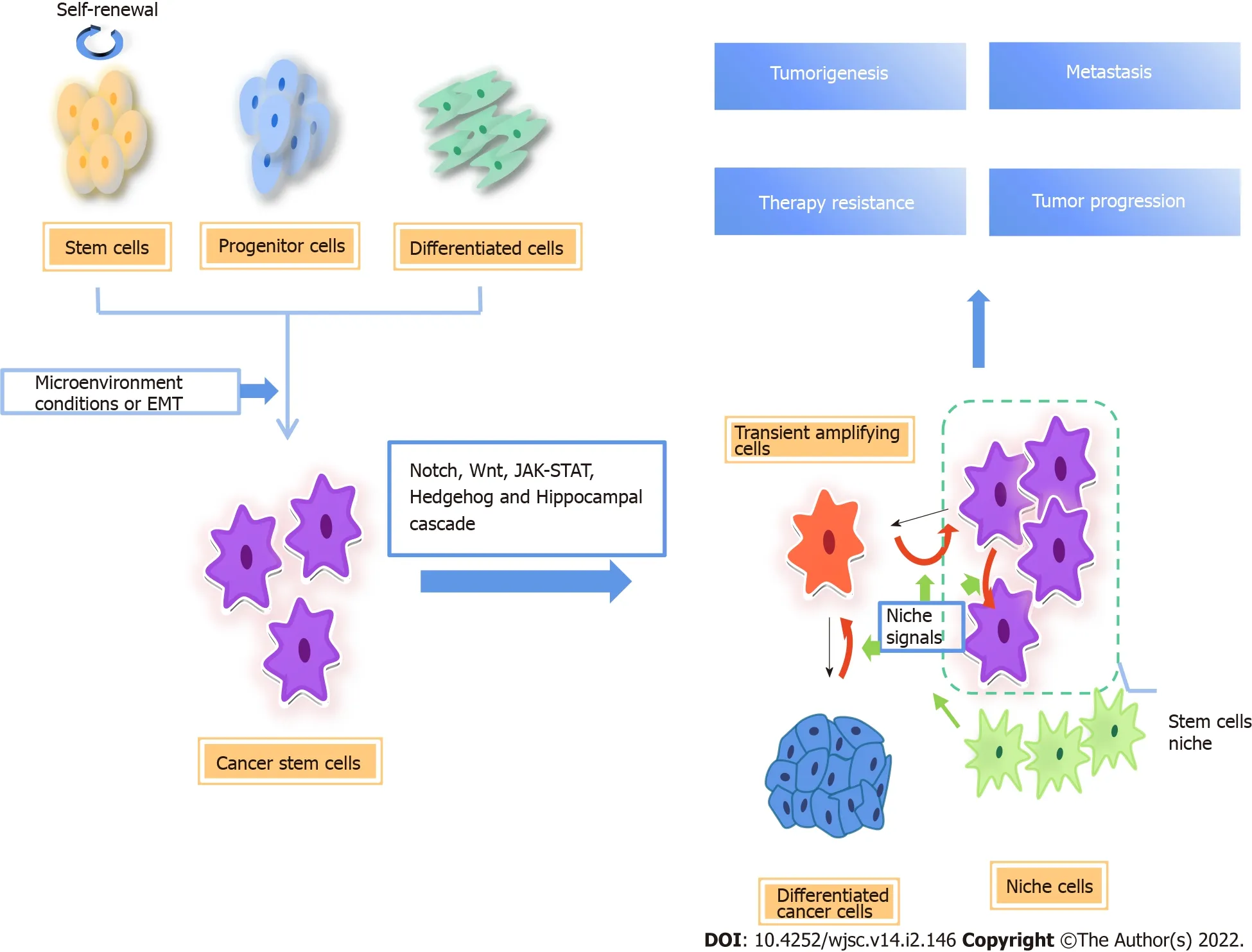
Figure 1 Origin of cancer stem cells and regulatory pathways involved.There are two possible origins of cancer stem cells(CSCs),one is normal stem cells/progenitor cells,and the other is fully differentiated cells.CSCs are closely related to tumor microenvironmental factors.In the process of epithelialmesenchymal transformation,cancer cells acquire stem cell-like characteristics.The differentiation direction of CSC progeny is determined by niche signal,and the available niche space determines the number of progeny stem cells.When there is no space available in the niche,the stem cells divide into transient amplifying(TA)cells,which divide and differentiate rapidly.At the same time,niche cells reprogram TA cells and differentiated cells into CSCs by niche signals[7].CSCs are important subsets of tumor cells,which are regulated by a variety of signal pathways,including Notch,Wnt/β-catenin,Hippo,and Hedgehog signaling,which are the main causes of cancer initiation,progression,metastasis,therapy resistance,and relapse.EMT:Epithelial-to-mesenchymal transition.
Various studies have shown that both CSC and non-CSC are plastic,and the interconversion between them may be a common phenomenon.Epithelial-to-mesenchymal transition(EMT)is the process by which epithelial cancer cells acquire a mesenchymal gene program that promotes migration and invasion.Many studies suggest that EMT promotes the transition from non-CSCs to CSC[7].During EMT,cancer cells obtain stem cell-like properties to migrate and grow into distant tissues[8-10].In a human model,the EMT major transcription factor Snail was elevated in cancer cells that displayed enhanced oncogenic capability and metastatic potential and was tightly associated with a CSC phenotype[11].The plasticity of CSCs is also closely related to microenvironment.Angiogenesis,the hypoxic niche,and extracellular matrix are essential for maintaining the stemness of glioblastoma stem cells[12].In addition,there is evidence that,in colon cancer,myofibroblasts enhance Wnt signaling through secreted factors,establishing a CSC niche and restoring the stemness of highly differentiated cancer cells[13].In non-CSCs,the promoter of zinc-finger E-box-binding(ZEB)1,the key regulator of EMT,maintains the bivalent chromatin configuration,making non-CSCs respond readily to microenvironmental signals.When the promoter converts to active chromatin configuration,ZEB1 transcription increases and non-CSCs convert to the CSC state.
Independent of the origin,CSCs are important cancer cell subsets.The existence of CSCs is clearly demonstrated in different types of cancer,including leukemia[14,15],tongue squamous cell carcinoma[16],breast cancer[17],glioblastoma[18],lung cancer[19,20],and osteosarcoma[21].They actuate tumorigenesis and progression,and promote therapy resistance,metastasis,and recurrence of cancers.A growing number of studies have shown that metabolic reprogramming of cancer cells caused by changes in the microenvironment exerts a marked effect on the properties of stem cells.
METABOLlC REPROGRAMMlNG lN CSCS
The interaction between CSCs and the tumor microenvironment(TME)is related to tumorigenesis and disease progression[22].Due to the rapid proliferation of tumor cells and insufficient angiogenesis,the TME has the characteristics of hypoxic,acidic,and nutrient-poor conditions;therefore,tumor cells must adjust energy metabolism to deal with this adverse microenvironment,and maintain the rapid growth and proliferation of tumor cells[23-25],a process called metabolic reprogramming.The metabolic phenotype of CSCs may depend on the microenvironment to a great extent.
Several studies have been conducted on a variety of cancer types,such as nasopharyngeal carcinoma[26],leukemia[27],osteosarcoma[28],breast cancer[29],and ovarian cancer[30],which suggest that CSCs show a greater reliance on glycolysis for energy supply compared with other differentiated cancer cellsin vitroandin vivo.Evidence suggests that paracrine hepatocyte growth factor/c-MET enhances the expression of hexokinase 2 and promotes glycolysis by activating Yes-associated protein(YAP)/hypoxia-inducible factor-1α in pancreatic cancer,which may facilitate CSC-like properties[31].
However,there is also growing evidence that mitochondrial oxidative metabolism is the preferred form of energy production in CSCs,including CD133+ colon cancer cells[32],CD44+ and CD117+ovarian cancer cells[33],cholangiocarcinoma cells[34],brain tumor cells[35],and leukemia cells[36].In addition,it is found that pancreatic CSCs(PaCSCs)are enriched in the oxidative phosphorylation(OXPHOS)promotion system using galactose instead of glucose as carbon sourcein vitro.And significant CSC features are present,such as the expression of multiple CSC biomarkers,the overexpression of stem-related pathways,the enhancement of self-renewal ability,and the significant improvement of tumorigenicityin vivo.Meanwhile,OXPHOS promoted the immune escape properties of PaCSCs[37].
A large number of the above studies have shown that CSC metabolism is highly heterogeneous.CSCs exhibit a metabolic phenotype dependent on glycolysis or OXPHOS,which mainly depends on the heterogeneity of tumor origin and surrounding microenvironmental conditions.
In addition to glucose metabolism,alterations in lipid metabolism also modulate tumor development and progression.Lipid metabolism is related to the stem cell properties in cancers.A growing body of evidence suggests that alterations in metabolic pathways associated with lipids,including fatty acids(FA)and cholesterol,are crucial for maintaining the stemness of CSCs.Lipid synthesis and catabolism are strictly regulated by CSCs to maintain self-renewal,proliferation,and chemotherapy resistance of the CSCs.Increasedde novolipid biosynthesis and lipid storage,as well as enhanced lipid oxidation,are unique features of many CSCs.It has been reported that fatty acid oxidation(FAO)can support selfrenewal and drug resistance of breast CSCs.The Leptin-LEPR-JAK-STAT3-dependent FAO pathway plays an important role in the self-renewal of breast cancer stem cell(BCSC)associated with chemotherapy resistance in breast cancer.Blocking FAO and/or Leptin re-sensitize them to chemotherapy and inhibit breast CSCsin vivo[38].Furthermore,targeting FAO enhances the chemotherapy efficacy of cytarabine(AraC)in AraC-resistant acute myeloid leukemia enriched in leukemic stem cells[39].Mesenchymal stem cells promoted stemness and chemoresistance in gastric cancer cells through FAOin vitroandin vivo[40].Lipid droplets(LDs),organelles that store neutral lipids,are accumulated in CSCs in numerous types of cancer[41,42].LDs are more abundant in pancreatic and colorectal CSCs than in isogenic non-CSCs[43].
Lipid synthesis has been shown to play a significant role in maintaining the characteristics of CSCs during tumorigenesis.De novolipid biosynthesis is one of the most targetable features of CSCs[44].We will highlight the important role of lipid synthesis in CSCs,including the pathways involved and promising therapeutic targets(Figure 2).
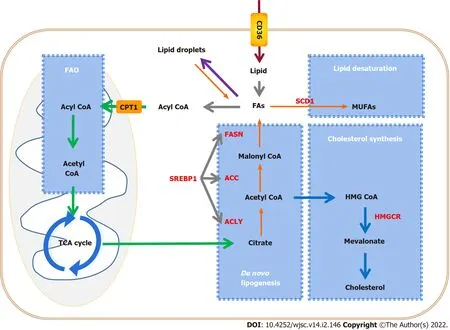
Figure 2 Alteration of lipid metabolic pathways in tumors and cancer stem cells.Cancer stem cells(CSCs)enhance lipid metabolic activities,such as fatty acid synthesis,fatty acid oxidation,and lipid storage,to promote self-renewal and proliferation.Key enzymes that control lipid metabolism(red letters)are considered to be ideal therapeutic targets for CSCs.CPT1:Carnitine palmitoyl-transferase 1;FAO:Fatty acid oxidation;TCA cycle:Tricarboxylic acid cycle;CD36:Cluster of differentiation 36;FA:Fatty acid;FASN:Fatty acid synthase;ACC:Acetyl-CoA carboxylase;ACLY:ATP citrate lyase;SREBP1:Sterol-regulatory element binding protein 1;SCD1:Stearoyl-CoA desaturase 1;MUFA:Monounsaturated fatty acid;HMGCR:3-hydroxy-3-methylglutaryl coenzyme A reductase.
ALTERATlONS AND KEY MODULATORS lN LlPlD SYNTHESlS lN CSCS
Lipid synthesis includesde novolipid biosynthesis,lipid desaturation,and cholesterol synthesis.Metabonomic analysis demonstrated that FA and cholesterol synthesis displays high activity in triplenegative breast CSCs(TNBCSCs).Cholesterol synthesis is essential for the survival and migration of CSCs,and inhibition of cholesterol synthesis induces cytotoxic effects on CSCs.For instance,pyridine pamoate(PP)can induce a cell killing effect on CSCs and prevent tumor metastasis by inhibiting cholesterol anabolic flux.By supplementing cholesterol to restore the level of free and bound cholesterol,the cytotoxicity induced by PP is effectively limited[45].Compared with non-CSCs,the rates of lipid unsaturation in the CSCs were further increased[46,47].In addition,in various cancers such as ovarian cancer,glioblastoma multiforme,and colon cancer,more monounsaturated FAs(MUFAs)are demanded by CSCs,which indicates that MUFAs may be involved in mediating various signaling pathways in CSCs and associated with stemness,and lipid desaturation may be an ideal and specific therapeutic target for CSCs[48,49].
FA synthesis in CSCs
Experimental investigation indicated thatde novoFA synthesis is more active in CSCs than in differentiated cells,suggesting that it is essential for CSCs to maintain stemness.In CSCs,the key rate-limiting enzymes ofde novoFA synthesis,including ATP-citrate lyase(ACLY),acetyl-CoA carboxylase(ACC),and fatty acid synthase(FASN),as well as sterol regulatory element-binding proteins(SREBPs),which regulate the expression level of lipid synthesis genes,are highly expressed.
ACLY
ACLY is principally located in the cytoplasm,which catalyzes the conversion of citrate to acetyl-CoA.Acetyl-CoA is not only an important substrate for the synthesis of FAs and cholesterol,but it is also necessary for protein acetylation reactions.Therefore,ACLY is a key enzyme of lipid synthesis that links catabolic pathways to biosynthesis.In many types of cancer,ACLY is upregulated or activated[50-52].ACLY upregulation contributes to stemness maintenance and tumorigenesis[53,54].ACLY overexpression increased the expression of Snail,which is known to promote EMT and stemness[55].ACLY inhibition decreased the invasiveness of breast cancer cells,and targeting ACLY attenuated the proliferation potential and cisplatin resistance in ovarian cancer[56,57].
ACC
ACC catalyzes the ATP-dependent carboxylation of acetyl CoA to generate malonyl-CoA,which is a rate-limiting step inde novoFA synthesis.In pancreatic cancer cells,inhibition of ACC inhibits Wnt and Hedgehog(HH)signal transduction by inhibiting palmitoylation of their ligands,and inhibits the growth of pancreatic tumorsin vivoandin vitro.ACC inhibitors can restore tumor cells to histological epithelial phenotypein vitro[58].Moreover,ACC is highly expressed in induced pluripotent stem cells(iPSCs).Pharmacological inhibition of ACC significantly reduced reprogramming efficiency in iPSCs[59].Research reveals that inhibiting the activation of ACC can effectively restore intracellular lipid levels,reduce EMT,and inhibit the features of CSCs[60].
FASN
FASN,the key enzyme ofde novolipogenesis,is highly expressed in human pluripotent stem cells(hPSCs)compared with that in hPSC-derived cardiomyocytes(hPSC-CMs)[61].In addition,it is highly active in adult neural stem and progenitor cells,which require FASN-dependent lipogenesis for proliferation[62].Data suggest thatde novolipogenesis is higher and FASN expression is upregulated in glioma stem cells(GCSs).Pharmacological inhibition of FASN dramatically decreases the expression of GSC stemness markers,including Sox2,Nestin,CD133,and FABP7,and thus inhibits cell proliferation and invasiveness of GSCs[63].Moreover,downregulation of FASN suppresses CSCs in breast cancer[64]and pancreatic cancer[65].
SREBP1
SREBPs are a class of transcription factors that regulate lipid homeostasis by controlling the expression of a series of key enzymes required for cholesterol and FA synthesis.Three SREBP subtypes have distinctive roles in lipid synthesis:SREBP1a regulates FA and cholesterol synthesis,and cholesterol absorption,SREBP1c regulates FA synthesis,and SREBP2 specifically regulates cholesterol synthesis and uptake.SREBPs are downstream molecules of the PI3K/AKT/mTOR signaling pathway.Regulation of SREBPs through the PI3K/AKT/mTOR pathway can regulate glucose production and FA synthesis,and affect the proliferation and invasion of cancer cells[66,67].Downregulation of SREBP inhibited the growth of non-small-cell lung cancer cells and liver cancer cells[67,68].SREBP1 targets key enzymes of FA synthesis,such as ACLY,ACC,FASN,and stearyl coenzyme A desaturase 1(SCD1),to regulate lipid metabolism[69],and is highly expressed in various cancers[69-71].Compared to differentiating melanosphere-derived cells,the expression of SREBP1 is enhanced in melanosphere-derived CSCs[42].Gemcitabine is a standard treatment for advanced pancreatic cancer patients but can cause chemoresistance during treatment.The chemoresistant cells have features of CSCs.Gemcitabine is widely used in chemotherapy for advanced pancreatic cancer,but chemotherapy in turn promotes the stemness of CSCs.Resveratrol inhibits SREBP1,resulting in the inhibition of lipid synthesis and the stemness induced by gemcitabine,and enhances the sensitivity of gemcitabine[72].
Lipid desaturation in CSCs
MUFAs,such as palmitoleic acid and oleic acid,are key substrates in the formation of complex lipids such as phospholipids,triglycerides,and cholesterol esters,and maintain optimal fluidity of cellular membranes.Moreover,MUFAs have a protective function against the lipotoxicity caused by excess saturated FAs and other cellular stresses[73,74].SCD catalyzes the committed step in the biosynthesis of MUFAs from saturated FAs[75,76].There are two isoforms in humans,SCD1 and SCD5.The expression of SCD5 is high in the brain and pancreas,while SCD1 is the main subtype,and is highly expressed in adipose tissue,the brain,liver,heart,and lung[77].SCD1 is overexpressed in a variety of tumors,including ovarian cancer[78],breast cancer[79],prostate cancer[80],and colon cancer[81].The upregulation of SCD1,which increases lipid desaturation and relieves endoplasmic reticulum stress,promotes ovarian cancer progression and metastasis[82].Inhibition of SCD1 can inhibit the growth of leukemic cells in the central nervous system[83].A growing number of studies on SCD1 have indicated that it plays a key role in tumorigenesis and maintenance of stemness[84-86].SCD1 promotes the activation of NF-κB by increasing the synthesis of polyunsaturated FA(PUFAs)to promote CSC characteristics.In turn,the NF-κB pathway regulates the expression of lipid desaturase by regulating transcription.This supports a positive feedback loop involving the NF-κB pathway and lipid desaturase in ovarian CSCs[46].Furthermore,SCD1 controls the fate of breast CSCs by regulating Wnt/β-catenin signaling[87].
Cholesterol synthesis in CSCs
Cholesterol is an important component of cell membranes and lipid rafts.Highly proliferating cancer cells require increased cholesterol synthesis to meet the need for rapid production of cell membranes.At the same time,metabolically active cancer cells need lipid rafts to form signal complexes for multiple complex signal transduction[88,89].Cholesterol is produced by a variety of biosynthetic processes or obtained from the diet.Cholesterol synthesis occurs in most tissues and cells.The synthetic pathway involves the conversion of acetyl-CoA to cholesterol through a series of enzymatic reactions,including the biosynthesis of mevalonate(MVA)and squalene[90,91].There are three crucial players in the cholesterol synthesis pathway,namely,SREBP2 and the two key rate-limiting enzymes,3-hydroxy-3-methylglutaryl-CoA reductase(HMGCR)and squalene epoxidase(SQLE).Of these,SREBP2 is the master transcriptional regulator of cholesterol biosynthesis.HMGCR and SQLE reduce HMG-CoA to MVA and catalyze the oxidation of squalene to 2,3-epoxy-quinenone,respectively[92].Increased cholesterol synthesis is considered to be a unique hallmark of many cancers[93].Pharmacological inhibition of cholesterol biosynthesis dramatically suppressed crypt growthin vivoandex vivo,which demonstrates that cholesterol itself acts as a mitogen for intestinal stem cells(ISCs).Cholesterol biosynthesis can drive ISC proliferation and tumorigenesis[94].Proteomic analysis of tumor tissues,patientderived xenograft,and mammospheres known to be enriched in CSCs revealed that the expression of proteins involved in the cholesterol synthesis pathway in CSCs increased.Simvastatin or siRNA blocking cholesterol biosynthesis reduced the formation of mammospheres.These results confirm that CSCs are highly dependent on metabolic processes associated with cholesterol biosynthesis,suggesting that the cholesterol biosynthesis pathway is a potential therapeutic target for the elimination of CSCs[95].
SREBP2
SREBP2 specifically regulates cholesterol synthesis and uptake to maintain intracellular cholesterol homeostasis.Evidence indicates that apoA-I binding protein-mediated cholesterol efflux activates endothelial SREBP2 which in turn transactivates Notch and promotes hematopoietic stem and progenitor cell(HSPC)emergence.SREBP2 inhibition impairs hypercholesterolemia-induced HSPC expansion[96].Biofunctional analyses demonstrated that SREBP2 promotes stem cell-like characteristics and metastasis of prostate cancer cells.The overexpression of SREBP2 increases the population of prostate CSCs and promotes the tumorigenicity of prostate cancer cellsin vivo,while gene silencing of SREBP2 inhibits the growth,metastasis,and stemness of prostate cancer cells[97].In colon cancer,inhibition of SREBP2 blocked the proliferation of cancer cells and reduced CSC properties.Knockdown of SREBP inhibits the growth of xenograft tumorin vivo[98].
MVA pathway
The MVA pathway produces isoprenoids,such as cholesterol and vitamin D,which are essential for a variety of cellular functions from cholesterol synthesis to cell survival and growth[91].Many studies have shown that numerous enzymes(HMGCR,FDPS,squalene synthase,and SQLE)required for cholesterol synthesis in the MVA pathway are overexpressed and overactivated in several cancers,including multiple myeloma,as well as breast,gastric,lung,colon,and prostate cancers.Targeting MVA can effectively inhibit the survival and proliferation ability of cancer cells and reduce the tumorigenic potential[99-104].Overactivation of key enzymes in cholesterol synthesis in the MVA pathway is usually associated with a poor prognosis with shorter disease-free survival and reduced overall survival[105-107].Statins inhibit HMGCR,the rate-limiting enzyme of the MVA pathway.Genetic variants associated with low HMG-CoA reductase function significantly reduced the risk of epithelial ovarian cancer[108].Lovastatin inhibited SOX2 promoter transactivation and reduced the efficiency of mammosphere formation and the percentage of ALDH+ cellsin vitro.Gene set enrichment analysis indicated that lovastatin downregulates genes that are involved in stemness and invasiveness of breast CSCs[109].Atorvastatin has a stronger anti-proliferative effect on CSCs by inhibiting the MVA pathway[110].Cholesterol and MVA increase the proliferation of breast CSCs and promote breast cancer progression,invasion,and chemotherapy resistance through activation of the estrogen-related receptor α pathway[111].Long non-coding RNA(lncRNA)/mRNA microarray assays showed that a novel lncRNA(named lnc030)cooperates with poly(rC)binding protein 2(PCBP2)to stabilize SQLE mRNA,resulting in increased cholesterol which activates PI3K/Akt signaling in governing BCSC stemness[112].
In addition,the MVA pathway is the only source of intracellular isopentenyl- diphosphate,which produces farnesyl-diphosphate and geranylgeranyl-diphosphate(GGPP)for the prenylation of proteins.For example,different types of preacylation enable the RasGTPase superfamily,including Ras and Ral/Rho,to be correctly directed to specific subcellular membranes to function.The RasGTPase superfamily affects a variety of cellular processes in cancer progression and participates in EMT,tumor progression,metastasis,and chemotherapy resistance.Inhibition of the MVA pathway can reduce GTPases prenylation and can induce the death of cancer cells,suggesting that these MVA pathway metabolites are essential for cancer cell viability[91,110].In addition,inhibiting the MVA pathway with small-molecule inhibitors such as statins has been shown to cause inhibition of YAP/transcriptional coactivator with PDZ-binding motif(TAZ)activity.Studies have shown that the activation of RhoGTPases requires GGPP,and the Rho-dependent YAP/TAZ regulatory pathway inhibits YAP/TAZ phosphorylation and promotes their nuclear accumulation to play a role[113-115].Decreasing the activation of Rho-GTPases and Hippo-YAP/TAZ represses the expression of genes associated with breast cancer stemness[116].YAP/TAZ nuclear accumulation and transcriptional activity are attenuated by Rho-GTPase/F-actin signaling to increase the sensitivity to chemotherapeutic drugs and suppress breast cancer chemoresistance[117].
MECHANlSM OF LlPlD SYNTHESlS REPROGRAMMlNG lN CSCS
In CSCs,there are a series of pathways involved in lipid metabolism to maintain cell stemness,and sustain their survival,proliferation,and invasion,including Notch,hippocampal cascade,HH,and Wnt signaling(Figure 3).
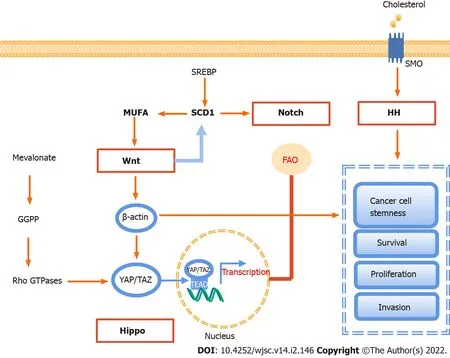
Figure 3 Signaling pathways involved in lipid metabolism in cancer stem cells.There are four major signaling pathways,including Notch,Wnt,Hippo,and Hedgehog signaling,involved in lipid metabolism to maintain cell stemness,and sustain their survival,proliferation,and invasion.GGPP:Geranylgeranyl pyrophosphate;MUFA:Monounsaturated fatty acids;YAP:Yes-associated protein;TAZ:Transcriptional co-activator with PDZ-binding motif;SREBP:Sterol regulatory element-binding protein;SCD1:Stearyl coenzyme A desaturase 1;TEAD:Transcriptional enhanced associate domain;FAO:Fatty acid oxidation;SMO:Smoothened;HH:Hedgehog;SMP:Scalp micropigmentation.
Notch signaling
Notch signaling is a highly conservative signal transduction pathway,which is closely related to various biological behaviors such as tumor metastasis and immune escape[22,118,119].In terms of lipid metabolism,the Notch signaling pathway can regulate the expression of peroxisome proliferatoractivated receptor α and lipid oxidation genes to achieve lipid homeostasis and redox homeostasis[120].In colon cancer,targeting SCD1-dependent lipid desaturation selectively eliminates colon CSCs by inhibiting Notch signaling[49,121].
Wnt signaling pathway
The Wnt signal cascade includes three main pathways:The canonical Wnt pathway,which leads to the accumulation of β-catenin,activates the transactivation complex,and participates in tumorigenesis,the non-canonical planar cellular polarity pathway,and the non-canonical Wnt-calcium pathway[119].At least 19 Wnt family members have been identified in humans,all of which are lipid-modified secretory glycoproteins.They are the ligands of ten Frizzled family receptors[22,122].
Wnt signaling plays a key role in regulating CSCs[13,123,124].The canonical Wnt signaling pathway,activated by ligands such as Wnt2β and Wnt3,promotes the proliferation of CSC by up-regulating βcatenin and terminating target β-catenin and STOP-target proteins,such as FOXM1,MYC,and YAP/TAZ,while the non-canonical Wnt signaling pathway in CSCs is activated by non-canonical Wnt ligands such as Wnt5A and Wnt11,thus activating the PI3K/AKT signal and inducing YAP/TAZdependent transcriptional activation to promote survival and therapeutic resistance of CSCs[125].In contrast,tumor invasion and metastasis are driven by both the canonical and non-canonical Wnt signaling cascades.Canonical Wnt/β-catenin and Wnt/STOP signaling cascades cooperatively upregulate SNAI1 to initiate EMT of CSCs[126].
Wnt signaling has also been associated with lipid synthesis in CSCs.The canonical Wnt/β-catenin pathway regulatesde novolipogenesis and fatty acid monounsaturation[127].SCD could be a key regulator between the Wnt signaling pathway and lipid metabolism.In mouse liver CSCs,the expression of SCD is regulated by the Wnt-β-catenin signaling pathway,while MUFAs produced by SCD provide a positive feedback loop to amplify Wnt signaling by promoting the stability and expression of Lrp5/6 mRNA[128].Another study suggests that MUFAs are crucial in the production and secretion of Wnt ligands[129].Finally,FA metabolism,especially SCD1 activity,in YAP/TAZ signaling depends on the activity of the β-catenin pathway in CSCs[130].
Hippo signaling
The core of the Hippo signaling pathway is the kinase cascade involving mammalian STE20-like(MST)1/2 and LATS1/2.MST1/2 activates LATS1/2 by promoting autosphosphorylation of LATS1/2 or by phosphorylation of MOB1,resulting in degradation of the downstream transcriptional coactivators YAP1 and TAZ,thereby limiting YAP activity[22,131].YAP/TAZ activation leads to the induction of CSC properties,including self-renewal,tumorigenic potential,anoikis resistance,EMT,drug resistance,and metastasis,in a wide range of human cancers[132,133].As mentioned earlier,in lung CSCs,SCD1 regulates lung cancer stemness by stabilizing YAP/TAZ and nuclear localization[130].The positive feedback loops of LATS2 and p53 inhibit cholesterol synthesis,and LATS2 binds to the endoplasmic reticulum tethered precursor(P-SREBP)of SREBP1 and SREBP2,and inhibits the transcription of SREBP mRNA,thus inhibiting the activity of cellular SREBP[134].Recent studies have revealed that the cancer-promoting properties of YAP/TAZ depend on cholesterol biosynthesis activity and MVA-dependent nuclear localization and activity of YAP/TAZ[114].YAP/TAZ-mediated lipid synthesis may be an important factor affecting the metabolic changes of CSCs[135].
HH signaling
The HH signaling pathway,which is responsible for the signal transmission from the cell membrane to the nucleus,is a highly conservative pathway.HH ligands mainly include Sonic hedgehog(SHH),Indian HH,and Desert HH.The HH signal pathway is activated by the binding of HH ligands to the transmembrane proteins Patched(PTCH)1/2,which release the inhibition of smoothened(SMO),leading to the activation of glioma transcription factors,thus inducing target gene transcription[22].HH ligands have been found to be activated in CSCs.High fibrillar collagen content resulting from HH pathway activation promotes breast cancer cell stemness.In cholangiocarcinoma,hypoxia promoted SHH pathway activation.Inhibition of the SHH pathway by cyclopamine significantly attenuated the expression of CSC transcription factors,leading to the abrogation of CD133 expression and EMT[136].
Previous evidence suggested that lipids are key regulators of HH signaling.The cholesterol covalent modification of SMO is regulated by the HH signaling pathway and is very important for the signal transduction and cell biological function of HH.PTCH1 inhibits the cholesterol modification of SMO,while the overexpression of SHH increases the cholesterol modification of SMO[137].In addition,SMO activates adenosine monophosphate kinaseviathe non-canonical pathway,directly or indirectly inhibiting FA and cholesterol synthesis[138].
APPLlCATlON PROSPECTS OF LlPlD SYNTHESlS REPROGRAMMlNG lN THE TREATMENT OF CSCS
CSCs can adapt easily to changes in the nearby environment and are more resistant to conventional therapies than other cancer cells.However,their proliferation and survival are highly dependent on lipid synthesis,which provides a point of penetration for the establishment of efficient targeting strategies to eliminate CSCs.Targeted clearance of CSCs can be achieved by interfering with different aspects of lipid synthesis,such as FA synthesis,lipid desaturation,and cholesterol synthesis(Table 1).
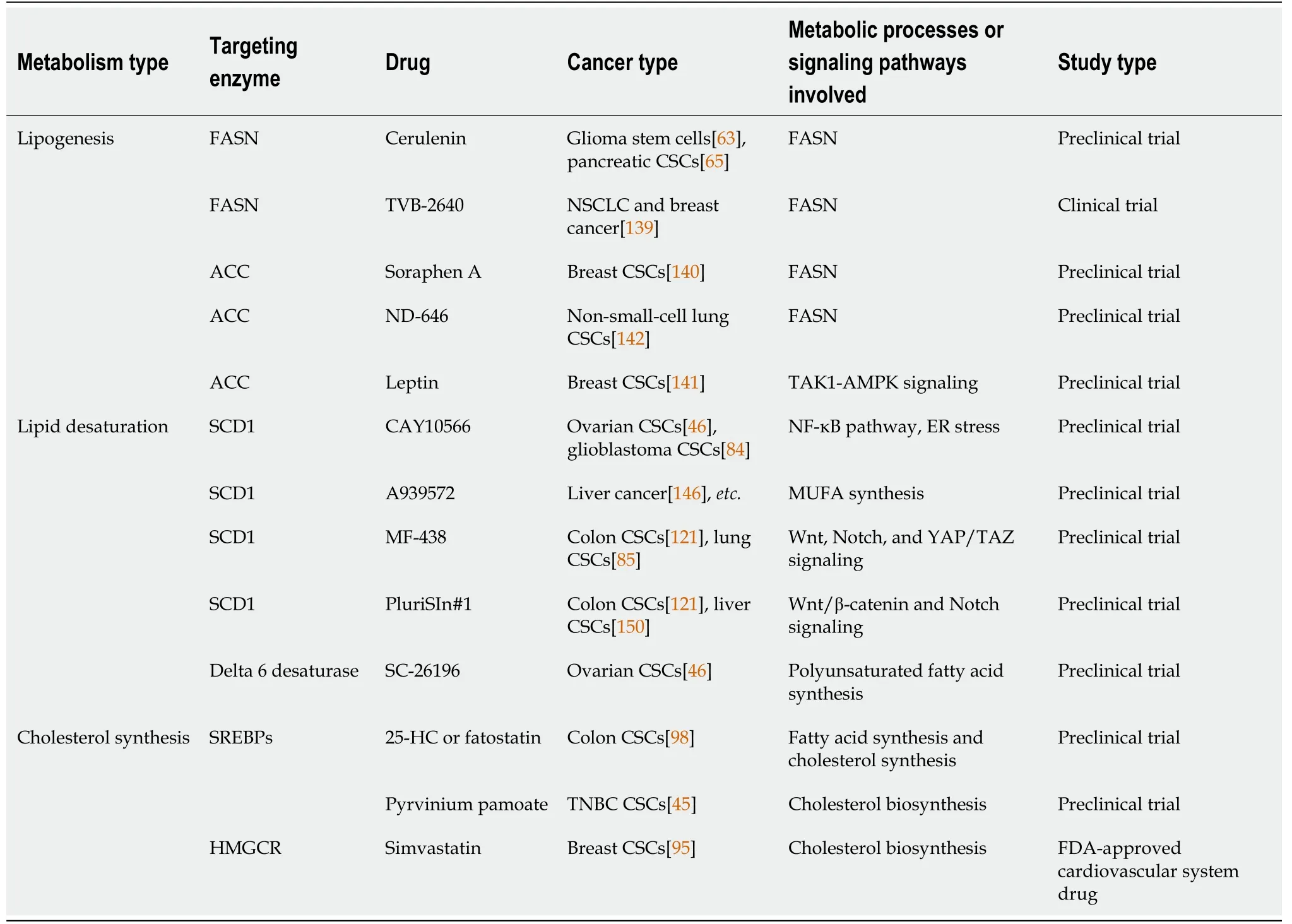
Table 1 lnhibitors related to lipid synthesis enzymes of cancer stem cells
Targeting FA synthesis
FASN is the most targetable among the lipogenesis genes.Some FASN inhibitors have shown anti-CSC and anti-tumor activities.Both inhibitor and RNA silencing of FASN decreased invasiveness,sphere formation,and expression of stemness markers to kill various CSCs[63,65].A new generation of FASN inhibitors is being developed,and data from early clinical trials on TVB-2640,a FASN inhibitor,show a partial tumor response in patients with non-small-cell lung cancer and breast cancer when TVB-2640 was used in combination with paclitaxel[139].Similarly,Soraphen A,an ACC inhibitor,suppressed mammosphere formation.Sorafen A treatment inhibited the self-renewal and growth of CSC-like cells by blocking FA synthesis and eliminated the promoting effect of human epidermal growth factor receptor 2 on CSC proliferation[140].Moreover,inhibition of ACC suppresses tumor growth,metastasis,and recurrence in non-small-cell lung cancer and breast cancer[141,142],indicating that ACC has great significance and potential in inhibiting CSCs and cancer.
However,in addition to being produced through the ACLY pathway,acetyl-CoA can also be produced by glucose or acetate metabolism to enter the process of fatty acid synthesis[143,144].In cancer cells,ACLY silencing increases the expression of ACC2,which maintains lipid synthesis in an acetate-dependent manner[145].Despite the knockdown of ACLY diminishing the number of breast CSCs,the effect of ACLY deficiency remains to be studied in CSCs.
Targeting lipid desaturation
Targeting SCD1,which converts fully saturated fatty acids to MUFAs,can selectively kill CSCs.It is reported that SCD1 inhibitors,such as CAY10566 and A939572,suppress cancer stemness and prevent tumorigenesis,and can counteract cancer cell chemoresistance[46,146].Significantly,MF-438 and PluriSIn #1,as SCD1 inhibitors,selectively eliminate colon CSCs but not the bulk cancer cells[121].Furthermore,inhibition of SCD1 increased the sensitivity of CSCs to cisplatin and reduced drug resistance[85].Therefore,combining SCD1 inhibitors with chemotherapy may be a more effective treatment strategy.Other studies have shown that miR-600 targeting SCD1 regulates Wnt/β-catenin signaling,thereby inhibiting the self-renewal and differentiation of mammary CSCs.Therefore,in addition to SCD1 inhibitors,nanovectorized miR-600 agonists(promiRNAs)may serve as a targeted tumor stem cell therapy[87].Delta 6-desaturase inhibitors block the globular formation and tumorinitiating ability of ovarian CSCs by inhibiting the synthesis of PUFAs[46].
Targeting cholesterol synthesis
Activation of cholesterol synthesis could be relevant to the aggressive and metastatic potential in CSCs.Inhibition of SREBP activation by 25-HC or fatostatin inhibits lipogenesis,including FA and cholesterol,and decreases the expression of genes associated with CSCs[98].PP significantly inhibits lipid anabolism in CSCs.In triple-negative breast cancer,PP exerts cytotoxic effects on TNBCSCs by inhibiting cholesterol synthesis[45].Simvastatin significantly reduced mammosphere formation and growth through inhibition of cholesterol biosynthesis[96].In addition,statins target CSCs by inhibiting the signaling associated with protein farnesylation,and protein geranylgeranylation in the MVA pathway[147,148].Similarly,metformin suppresses CSCs through inhibiting protein prenylation of the MVA pathway in colorectal cancer[149].
CONCLUSlON
In the past few years,many studies have shown that CSCs are responsible for tumor occurrence and development,distant metastasis,and therapy resistance.Metabolic alterations are the main pathways for cancer cells and CSCs to escape from adverse environmental effects.Among the reprogrammed metabolic pathways,alterations in lipid synthesis such asde novolipogenesis,lipid desaturation,and cholesterol synthesis are closely related to CSC generation and stemness maintenance.Furthermore,lipid synthesis is also involved in the activation of several important oncogenic signaling pathways,including Notch,Wnt/β-catenin,Hippo,and HH signaling.Taking the key molecules of lipid synthesis as the target shows promising application potential in the elimination of CSCs.Therefore,we believe that altered lipid synthesis metabolism is a promising target for CSC elimination and tumor therapy.
FOOTNOTES
Author contributions:Wang SY was responsible for conceptualizing this review and writing the original draft;Hu QC was involved in the conceptualization,funding acquisition,and review and editing of the manuscript;Wu T participated in the conceptualization and review of the manuscript;Tao XA,Xia J,and Cheng B contributed to the editing,improving,and finalizing of the manuscript;all authors approved the final version of the manuscript and agreed to be accountable for this work.
Supported bythe National Natural Science Foundation of China,No.82001044 and No.81630025;the China Postdoctoral Science Foundation,No.2020M673019;the Guangdong Basic and Applied Basic Research Foundation,No.2019A1515110071;and the Natural Science Foundation of Guangdong Province,No.2017A030311033.
Conflict-of-interest statement:The research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial(CC BYNC 4.0)license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is noncommercial.See:https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORClD number:Si-Yu Wang 0000-0001-9690-5514;Qin-Chao Hu 0000-0002-4969-0589;Tong Wu 0000-0001-7455-9083;Juan Xia 0000-0002-4966-7119;Xiao-An Tao 0000-0003-1114-9698;Bin Cheng 0000-0001-7288-806X.
S-Editor:Wang JJ
L-Editor:Wang TQ
P-Editor:Wang JJ
杂志排行
World Journal of Stem Cells的其它文章
- Transcription regulators differentiate mesenchymal stem cells into chondroprogenitors,and their in vivo implantation regenerated the intervertebral disc degeneration
- Extracellular vesicles from hypoxia-preconditioned mesenchymal stem cells alleviates myocardial injury by targeting thioredoxininteracting protein-mediated hypoxia-inducible factor-1α pathway
- Anti-fibrotic effect of adipose-derived stem cells on fibrotic scars
- Physical energy-based ultrasound shifts M1 macrophage differentiation towards M2 state
