柴胡加龙骨牡蛎汤对慢性应激抑郁大鼠炎症因子及单胺类神经递质受体表达的影响
2022-06-01吴圆圆蔡萧君李浩经蔡英杰李宇王钦
吴圆圆 蔡萧君 李浩经 蔡英杰 李宇 王钦
〔摘要〕 目的 通过研究柴胡加龙骨牡蛎汤对血清炎症因子及海马区单胺类神经递质受体的影响,探讨其治疗抑郁的可能机制。方法 随机从40只大鼠中选取30只建立慢性应激抑郁大鼠模型,造模成功后随机分为模型组、中药组、西药组,每组10只,未进行造模的10只大鼠为空白组。空白组、模型组每天给予蒸馏水6.3 mL/kg,西药组每天给予盐酸氟西汀3.17 mg/kg,中药组每天予柴胡加龙骨牡蛎汤1.638 g/kg,每组均连续灌胃干预8周。干预前后,采用旷场试验观察大鼠活动总路程;采用悬尾实验观察大鼠挣扎静止时间;采用强迫游泳实验观察大鼠游泳不动时间。干预8周后,采用HE染色观察各组大鼠海马组织的病理形态学变化;采用ELISA法检测各组大鼠血清白细胞介素-1β(interleukin-1β, IL-1β)、白细胞介素-2(interleukin-2, IL-2)、白细胞介素-6(interleukin-6, IL-6)、白细胞介素-17(interleukin-17, IL-17)、白细胞介素-18(interleukin-18, IL-18)水平;采用免疫组织化学法观察各组大鼠海马组织中的5-羟色胺2A受体(serotonin 5-hydroxytryptamine 2A receptor, 5-HT2AR)、多巴胺受体D2(dopamine receptor D2, DARD2)表达水平;采用实时荧光PCR检测各组大鼠海马组织中的5-HT2AR、DARD2 mRNA相对表达量。结果 空白组海马区组织结构正常,神经细胞形态正常,排列整齐,细胞质饱满,细胞核清晰;模型组神经细胞数目相对减少,体积变小,细胞核出现皱缩;与模型组比较,柴胡龙骨牡蛎汤组和西药组大鼠神经细胞数目较多、体积较大、细胞核皱缩情况均有不同程度减轻,其中,中药组改善明显。与空白组比较,模型组活动总路程、悬尾挣扎时间均明显缩短(P<0.01),游泳不动时间明显延长(P<0.01),IL-1β、IL-6、IL-18含量明显升高(P<0.01),IL-2含量明显降低(P<0.01),5-HT2AR、DARD2蛋白及mRNA表达水平均明显降低(P<0.01)。与模型组比较,中、西药组活动总路程、悬尾挣扎时间均明显延长(P<0.01),游泳不动时间均明显缩短(P<0.01),IL-1β、IL-6、IL-18含量均明显降低(P<0.05,P<0.01),IL-2均明显升高(P<0.01),5-HT2AR、DARD2蛋白及mRNA表达均明显升高(P<0.05,P<0.01);与模型组比较,中药组IL-17含量明显降低(P<0.05)。中、西药组间活动总路程、游泳不动时间、悬尾挣扎时间比较,IL-1β、IL-2、IL-6、IL-17、IL-18含量比較,5-HT2AR、DARD2蛋白及mRNA表达水平比较,差异均无统计学意义(P>0.05)。干预后,中、西药组活动总路程、悬尾挣扎时间均明显长于干预前(P<0.01),游泳不动时间均明显短于干预前(P<0.01)。结论 柴胡加龙骨牡蛎汤可能通过调控炎症因子及单胺类神经递质功能治疗抑郁。
〔关键词〕 抑郁证;柴胡加龙骨牡蛎汤;炎症因子;单胺类神经递质;郁证
〔中图分类号〕R277.7 〔文献标志码〕A 〔文章编号〕doi:10.3969/j.issn.1674-070X.2022.05.010
Effect of Chaihu plus Longgu Muli Decoction on expression of inflammatory factors and
monoamine neurotransmitter receptors in chronic stress-induced depression rats
WU Yuanyuan, CAI Xiaojun*, LI Haojing, CAI Yingjie, LI Yu, WANG Qin
(Heilongjiang Academy of Traditional Chinese Medicine, Harbin, Heilongjiang 150001, China)
〔Abstract〕 Objective To investigate the effect of Chaihu plus Longgu Muli Decoction on serum inflammatory factors and monoamine neurotransmitter receptors in hippocampus, and to explore the possible mechanism of its treatment for depression. Methods 30 rats were randomly selected from 40 rats to establish chronic stress-induced depression rat model. After successful modeling, they were divided into model group, Chinese medicine group and western medicine group, with 10 rats in each group. The 10 rats without modeling were blank group. Blank group and model group were given 6.3 mL/kg distilled water every day; western medicine group was given 3.17 mg/kg fluoxetine hydrochloride every day; Chinese medicine group was treated with 1.638 g/kg Chaihu plus Longgu Muli Decoction every day. Each group was treated by gavage for 8 weeks. Before and after the intervention, open field test was used to observe the total movement distance of rats; suspension tail test was used to observe the struggle and rest time of rats; the immobile swimming time of rats was observed by forced swimming experiment. Eight weeks after intervention, the histopathological changes of hippocampal tissues were observed by HE staining; serum levels of interleukin-1 (IL-1β), interleukin-2 (IL-2), interleukin-6 (IL-6), interleukin-17 (IL-17) and interleukin-18 (IL-18) were detected by ELISA. The expression levels of serotonin 5-hydroxytryptamine 2A receptor (5-HT2AR) and dopamine receptor D2 (DARD2) in hippocampus were observed by immunohistochemistry; real-time PCR was used to detect the relative expression levels of 5-HT2AR and DARD2 mRNA in hippocampal tissues of rats in each group. Results In the blank group, the tissue structure of hippocampus was normal, the morphology of nerve cells were normal and neatly arranged, and the cytoplasm was full and the nucleus was clear. In the model group, the number and volume of nerve cells decreased, and the nucleus was shrunken. Compared with model group, the number and size of nerve cells in Chinese medicine group and western medicine group were more and larger, and the nuclear shrinkage were reduced to varying degrees, among which the improvement was obvious in Chinese medicine group. Compared with the blank group, the total movement distance and the struggle time of tail suspension in the model group were significantly shortened (P<0.01), the immobile swimming time was significantly prolonged (P<0.01), the content levels of IL-1β, IL-6 and IL-18 were significantly increased (P<0.01), and the content of IL-2 was significantly decreased (P<0.01), the protein and mRNA expression levels of 5-HT2AR and DARD2 were significantly decreased (P<0.01). Compared with model group, the the total movement distance and the struggle time of tail suspension were significantly prolonged (P<0.01), and the immobile swimming time was significantly shortened (P<0.01), the content levels of IL-1β, IL-6 and IL-18 were significantly decreased (P<0.05, P<0.01), IL-2 was significantly increased (P<0.01), 5-HT2AR and DARD2 protein and mRNA expression were significantly increased (P<0.05, P<0.01) in the Chinese medicine group and western medicine group; compared with model group, IL-17 content in Chinese medicine group was significantly decreased (P<0.05). There were no significant differences in total movement distance, immobile swimming time, struggle time of tail suspension, IL-1β, IL-2, IL-6, IL-17, IL-18 content, 5-HT2AR, DARD2 protein and mRNA expression levels between Chinese medicine group and western medicine group (P>0.05). After intervention, the total movement distance, the struggle time of tail suspension were significantly longer than before intervention (P<0.01), and the immobile swimming time were significantly shorter than before intervention (P<0.01) in Chinese medicine group and western medicine group. Conclusion Chaihu plus Longgu Muli Decoction can treat depression by regulating the functions of inflammatory factors and monoamine neurotransmitters.A75A2BD7-E220-481C-A514-6415FC92B4EB
〔Keywords〕 depression; Chaihu plus Longgu Muli Decoction; inflammatory factor; monoamine neurotransmitter; depression syndrome
抑郁症是一种常见的精神疾病,其临床表现为持续性的情绪低落、兴趣减退、负罪感、自我价值感丧失、睡眠障碍、食欲不佳、疲倦及注意力不集中等,抑郁程度严重时患者可出现自杀倾向。抑郁症可通过缩短端粒长度、加速大脑老化和表观遗传老化来促进生物老化,增加患者罹患其他疾病的风险[1]。单胺类神经递质数量减少及其受体功能改变在抑郁症发病机制中的作用已得到广泛认可[2];同时研究表明,抑郁症的发生与免疫激活密切相关,包括炎症标志物、免疫细胞数量和抗体滴度异常等,不同抑郁症状与不同炎症因子表达存在一定的联系[3],中药可通过干预海馬神经递质表达及血清中炎症因子水平对抑郁症进行治疗[4]。抑郁症是一种情志疾病,属于中医学“郁证”“百合病”“脏躁”“癫证”等范畴[5]。《灵枢·本神》言“愁忧者,气闭塞而不行”,郁证起病之初,多因情志内伤而致气机不畅、肝失疏泄,症见多思虑、善太息等。柴胡加龙骨牡蛎汤出自《伤寒论》,具有和解泄热、镇惊安神之功效,临床治疗抑郁症具有良好成效[6]。全方人参、茯苓、大枣,益气养血安神,柴胡、桂枝补土而疏木,大黄、生姜、半夏泻热而降浊,龙骨、牡蛎敛魂补虚而镇逆。本实验研究柴胡加龙骨牡蛎汤对抑郁大鼠炎症因子白细胞介素-1β(interleukin-1β, IL-1β)、白细胞介素-2(interleukin-2, IL-2)、白细胞介素-6(interleukin-6, IL-6)、白细胞介素-17(interleukin-17, IL-17)、白细胞介素-18(interleukin-18, IL-18)及单胺类神经递质受体5-羟色胺2A受体(serotonin 5-hydroxytryptamine 2A receptor, 5-HT2AR)、多巴胺受体D2(dopamine receptor D2, DARD2)表达的影响,探讨其治疗抑郁症的机制。
1 材料
1.1 动物
SPF级SD雄性大鼠40只,体质量(200±20) g,8周龄,购于哈尔滨医科大学实验动物学部,动物许可证号:SCXK(黑)2019-0001。饲养于黑龙江省中医药科学院SPF级实验室,温度(20±1) ℃,相对湿度(50±5)%,每天12 h光照,自由摄食饮水,动物实验伦理审批号:SYXK(黑)2016-008。
1.2 药物与试剂
柴胡加龙骨牡蛎汤:柴胡25 g(批号:18100014),龙骨25 g(批号:19056424),牡蛎25 g(批号:19056614),人参10 g(批号:19071681),桂枝15 g(批号:18100164),茯苓15,g(批号:19071191),清半夏10 g(批号:19052351),黄芩10 g(批号:19020124),大黄6 g(批号:18110294),生姜10 g(批号:18100154),大枣5 g(批号:19046814)均购自江阴天江药业,加蒸馏水置100 ℃恒温水浴锅中加热,充分搅拌至完全溶解,定容至360 mL,生药量为0.26 g/mL,分装后密封,4 ℃冰箱保存备用。盐酸氟西汀分散片(法国礼来有限公司,批号:9492AA)。IL-1β、IL-2、IL-6、IL-17、IL-18试剂盒均购自南京建成生物工程研究所(批号分别为H002、H003、H007、H014-1-1、H015-1-1);DARD2抗体、5-HT2AR抗体均购自北京博奥森生物技术有限公司(批号分别为bs1008R、bs1056R);苏木素、伊红染液均购自南京建成生物工程研究所(批号分别为D005-1-3、D019-1-3);辣根过氧化物酶结合山羊抗兔免疫球蛋白IgG、DAB试剂盒均购自北京中杉金桥生物技术公司(批号分别为210060330、PV-9001)。
1.3 主要仪器
多功能酶标仪(奥地利Tecan公司,型号:M200);荧光显微镜(日本Olympus公司,型号:DP72);实时荧光PCR仪(杭州博日科技股份有限公司,型号:Line Gene 9600)。
2 方法
2.1 慢性应激抑郁大鼠模型建立[7]
采用慢性不可预知温和应激(chronic unpredictable mild stress, CUMS)结合孤养造模,予以悬尾3 min、禁水24 h、禁食24 h、夹尾1 min、昼夜颠倒24 h、电击1 min、4 ℃冰水游泳5 min 7种刺激方法,每天不同时间随机采取不同的方法刺激大鼠,一共刺激28 d。孤养方法为每只大鼠单独1笼以不透光挡板遮挡,建立慢性应激抑郁大鼠模型。
2.2 分组及给药方法
用随机数字表法将大鼠分为4组:空白组、模型组、中药组和西药组,每组10只,给药剂量参照本课题组前期实验[8]。空白组、模型组每天灌胃予蒸馏水6.3 mL/kg;西药组每天灌胃予盐酸氟西汀3.17 mg/kg;中药组每天灌胃予柴胡加龙骨牡蛎汤1.638 g/kg,每组均连续干预8周。
2.3 行为学检测方法[9-10]
2.3.1 旷场试验(open field test, OFT) 干预前后,记录分析各大鼠在箱内的活动总路程,记录时间为5 min,整个过程中保证环境安静,待上1只大鼠测试完成后使用75%乙醇清洁设备。
2.3.2 悬尾实验(tail suspension test, TST) 干预前后,用医用胶布将大鼠尾部距末端约1/3处缠绕固定于悬尾仪上,使大鼠呈倒挂状态,观察并记录3 min内大鼠挣扎静止时间。A75A2BD7-E220-481C-A514-6415FC92B4EB
2.3.3 强迫游泳实验(forced swimming test, FST) 干预前后,在强迫游泳透明玻璃桶中加水,水温(20±1) ℃,加至30 cm处,将大鼠单独且平稳放入水中,使用摄像机及行为学分析软件记录5 min大鼠游泳不动时间,每次更换大鼠时清除“前任者效应”。
2.4 ELISA法测定血清IL-1β、IL-2、IL-6、IL-17A、IL-18
干预8周后,大鼠腹腔注射2%戊巴比妥钠麻醉,腹主动脉采血,采集到的血液于常温下静置2 h后,高速低温冷冻离心机以4 ℃,离心半径10 cm,3000 r/min离心10 min,取上清液,使用相应试剂盒检测上清液中IL-1β、IL-2、IL-6、IL-17A、IL-18含量,严格按照试剂盒说明书进行操作,酶标仪450 nm波长检测样本光密度值,根据标准曲线计算样品浓度。
2.5 HE染色观察海马组织形态
干预8周后,取大鼠海马组织进行石蜡包埋,制成石蜡切片,后脱蜡、水化。苏木精染色,自来水清洗,置于盐酸乙醇分化,并用纯水冲洗。乙醇伊红染色,纯水冲洗。再脱水,分别放入二甲苯Ⅰ、二甲苯Ⅱ中透明。将切片取出,于组织上滴加中性树脂,盖上盖玻片,拍照观察。
2.6 免疫组织化学法检测大鼠海马区DARD2、5-HT2AR蛋白表达情况
干预8周后,取大鼠脑组织放入4%多聚甲醛磷酸盐缓冲液内固定12 h,脱水、包埋、切片,后常规脱蜡至水,PBS缓冲液冲洗,过氧化氢阻断内源性过氧化氢酶,枸橼酸缓冲液进行抗原修复,分别滴加一抗DARD2(1∶100)、5-HT2AR(1∶150),4 ℃孵育过夜,PBS冲洗后分别滴加反应增强液、二抗,DAB染色,封片,于400倍显微镜下观察DARD2、5-HT2AR蛋白表达情况。
2.7 实时荧光PCR检测大鼠海马组织DARD2、5-HT2AR mRNA相对表达水平[11]
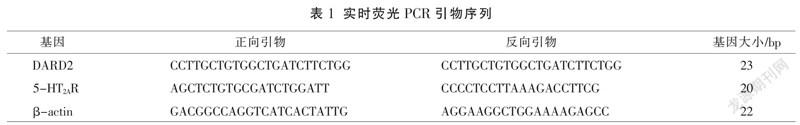
干预8周后,收集各组大鼠海马组织后,加入TRIzol试剂以提取RNA,经多功能酶标仪检测浓度后,选取纯度为1.9~2.0的RNA提取液稀释至100 ng/μL。实验反应体系:1 μL组织RNA,上游、下游引物各1 μL,17 μL反应液(按照PCR试剂盒说明书配制)。扩增条件为:95 ℃ 10 min、95 ℃ 10 s、60 ℃ 30 s、72 ℃ 30 s、40个循环。溶解曲线分析:95 ℃ 10 s,台阶采样、台阶温度0.5 ℃。引物序列详见表1。
2.8 统计学方法
采用SPSS 26.0统计学软件进行数据分析。符合正态分布或近似正态分布的计量资料以“x±s”表示,多组计量资料符合正态性、方差齐性采用单因素方差分析,组间比较采用LSD法;若不满足方差齐性采用Dunnett-T3检验;同组干预前后比较使用独立样本t检验;当各组不满足正态性时,采用秩和检验。以P<0.05表示差异有统计学意义。
3 结果
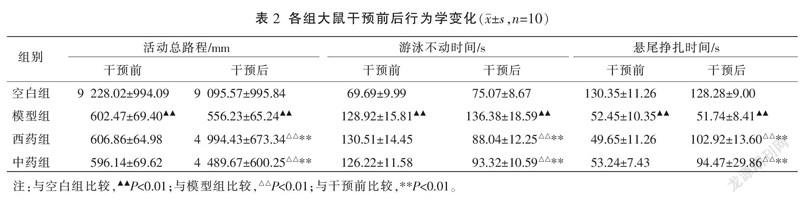
3.1 各组大鼠干预前后行为学比较
与空白组比较,模型组活动总路程、悬尾挣扎时间均明显缩短(P<0.01),游泳不动时间明显延长(P<0.01)。干预前,模型组、西药组、中药组间活动总路程、悬尾挣扎及游泳不动时间比较,差异无统计学意义(P>0.05),具有可比性。干预后,中、西药组活动总路程、悬尾挣扎时间均明显长于干预前(P<0.01),游泳不动时间均明显短于干预前(P<0.01)。干预后,中、西药组活动总路程、悬尾挣扎时间均明显长于模型组(P<0.01),游泳不动时间均明显短于模型组(P<0.01)。干预后,中、西药组活动总路程、游泳不动时间、悬尾挣扎时间比较,差异均无统计学意义(P>0.05)。详见表2。
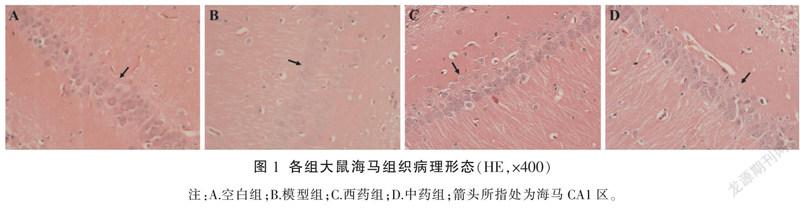
3.2 各组大鼠海马组织病理形态比较
空白组海马区组织显微结构正常,神经细胞形态结构正常,排列整齐,细胞质饱满,细胞核清晰;模型组神经细胞数目相对减少,体积变小,细胞核出现皱缩;与模型组比较,中、西药组大鼠神经细胞数目较多、体积较大、细胞核皱缩情况均有不同程度减轻,其中,中药组改善明显。详见图1。
3.3 各组大鼠细胞血清因子水平比较
与空白组比较,模型组IL-1β、IL-6、IL-18含量明显升高(P<0.01),IL-2含量明显降低(P<0.01)。与模型组比较,中、西药组IL-1β、IL-6、IL-18含量均明显降低(P<0.05,P<0.01),中药组IL-17含量明显降低(P<0.05),中、西药组IL-2均明显升高(P<0.01)。中、西药组IL-1β、IL-2、IL-6、IL-17、IL-18含量比较,差异均无统计学意义(P>0.05)。详见表3。
3.4 大鼠海马组织5-HT2AR、DARD2蛋白表达比较
模型组大鼠海马结构神经元细胞体积变小,排列紊乱,棕黄色染色颗粒少且染色浅,表示5-HT2AR、DARD2阳性表达较少。详见图2。
与空白组比较,模型组5-HT2AR、DARD2蛋白表达水平均明显降低(P<0.01)。与模型组比较,中、西药组5-HT2AR、DARD2蛋白表达水平均明显升高(P<0.05)。中、西藥组5-HT2AR、DARD2蛋白表达水平比较,差异均无统计学意义(P>0.05)。详见图3。
3.5 各组大鼠海马组织5-HT2AR、DARD2 mRNA相对表达水平比较A75A2BD7-E220-481C-A514-6415FC92B4EB
与空白组比较,模型组5-HT2AR、DARD2 mRNA表达均明显降低(P<0.01)。与模型组比较,中、西药组5-HT2AR、DARD2 mRNA表达均明显升高(P<0.05,P<0.01)。中、西药组5-HT2AR、DARD2 mRNA表达比较,差异均无统计学意义(P>0.05)。详见表4。
4 讨论
《医学衷中参西录·补络补管汤》云:“人身阳之精为魂,阴之精为魄。龙骨能安魂,牡蛎能强魄。魂魄安强,精神自足,虚弱自愈也。是龙骨、牡蛎,固为补魂魄精神之妙药也。”龙骨、牡蛎相须为用,补虚弱而安心神、定魂魄。《长沙药解·茯苓》言茯苓“利水燥土,泻饮消痰,善安悸动”,《长沙药解·人参》言人参“入戊土而益胃气,走己土而益脾阳,理中第一,止渴非常”,《長沙药解·大枣》言大枣“补太阴己土之精,化阳明戊土之气,生津润肺而除燥,养血滋肝而息风”,此三药合用,和胃益气、养血安神。柴胡,清胆经之郁火,泻心家之烦热;黄芩清相火而退肝经之郁蒸;大黄泻里热而和胃气,三药合用和解清热安神。桂枝性灵动,疏木达郁以安惊悸,温扶脾阳以行水化痰,诸阴药得之调和阴阳,理中安神。由于中药成分复杂,动物实验可以为柴胡加龙骨牡蛎汤治疗抑郁的机制提供更多可靠信息,同时选择合适的动物模型在整个研究实验过程中尤为重要。
本实验以CUMS结合孤养模式复制抑郁大鼠模型,研究表明由该方法制备的模型可出现机体免疫系统激活、单胺类神经递质减少等抑郁症状,与临床患者发病机制相似[12-13]。OFT、TST、FST可用于评价应激性抑郁模型的表面效度[14],单胺类神经递质、炎症因子等可用于检测模型的结构效度[15]。磁共振成像显示抑郁症患者神经元密度变小、神经元细胞体积缩小、同时海马体积减少[16]。本次实验行为学测试(OFT、TST、FST)显示,柴胡加龙骨牡蛎汤具有明显的抗抑郁作用,这与Wang[17]的研究结果一致,实验证明,柴胡加龙骨牡蛎汤可增加抑郁大鼠海马区神经元数量并改善其形态,从而减轻大鼠抑郁症状。
中药可通过多靶点、多环节治疗抑郁,不同中药其作用机制也不尽相同[18],本实验主要对柴胡加龙骨牡蛎汤治疗抑郁的机制进行初步研究。炎症因子、单胺类神经递质在抑郁发病机制中的作用已被广泛认可,前期研究表明,当患者体内免疫失衡时,如IL-1β、IL-6、IL-17、IL-18升高以及IL-2水平下降,可导致色氨酸代谢异常,使单胺类神经递质功能障碍、生成减少,从而产生抑郁症状[19]。本研究探讨柴胡加龙骨牡蛎汤对大鼠血清IL-1β、IL-2、IL-6、IL-17、IL-18水平及海马组织中5-HT2AR、DARD2表达的影响,以进一步明确柴胡加龙骨牡蛎汤对抑郁的治疗作用。
压力刺激导致NOD样受体(node-like receptor, NLRs)聚集激活,IL-1β、IL-18、IL-6产生增多触发炎症反应,损伤大脑神经元,干预神经重塑,影响神经元的相互作用及认知功能,导致抑郁[18]。而激活DARD2可导致NLRs被抑制,减少IL-1、IL-6的产生[20-21]。IL-17与IL-17R结合可激活核因子-κB蛋白(nuclear factor kappa-B, NF-κB)通路,上调其他炎症因子基因表达,刺激促炎细胞因子IL-1β、IL-6的产生[22]。研究显示,抑郁症患者的血清中IL-17的表达增加,且随着IL-17的增加患者抑郁情绪更加明显[23],由Th17细胞产生分泌的IL-17A具有中枢毒性[24],可通过激活星形细胞和小胶质细胞,增强神经刺激反应(包括由中枢细胞产生IL-17A),或直接通过诱导神经元损伤,增加抑郁行为的易感性[25]。IL-2生成分泌的减少以及IL-2信号通路受阻、抑炎作用的减弱均与抑郁的发生发展相关,抑郁患者体内IL-2水平降低时,抑郁症状加剧、病程进展加快[26]。柴胡加龙骨牡蛎汤可降低抑郁大鼠IL-1β、IL-6、IL-17、IL-18血清水平,升高IL-2血清水平,以减轻大鼠抑郁症状。
DARD2、5-HT2AR在中枢系统中表达极为丰富,所介导的信号在高级脑部功能中存在重要作用,当其异常时可导致精神疾病[27]。5-HT与小胶质细胞的5-HT2AR结合可调节中枢系统炎症刺激导致的趋化反应,可抑制炎症的进一步发生发展[28],改善抑郁。DA信号主要通过D1受体和D2受体进行传递,其中DARD2研究最为广泛,DARD2基因多态性与精神障碍的发生密切相关[29],选择性DARD2激动剂通过上调酪氨酸羟基酶的表达和增加大鼠海马神经元生成缓解抑郁症状[30]。本次实验结果证明,柴胡加龙骨牡蛎汤可增加抑郁大鼠DARD2、5-HT2AR的表达,从而减少大鼠抑郁行为。
实验证明,柴胡加龙骨牡蛎汤可通过调控炎症因子及单胺类神经递质功能有效治疗抑郁,然而柴胡加龙骨牡蛎汤对抑郁大鼠炎症因子及单胺类神经递质的调控是通过哪些酶和代谢途径发挥作用,有待结合生物分子学、网络药理学、代谢组学等研究手段进一步明确。
参考文献
[1] ALEXOPOULOS G S. Mechanisms and treatment of late-life depression[J]. Translational Psychiatry, 2019, 9(1): 188.
[2] MALHI G S, MANN J J. Depression[J]. Lancet, 2018, 392(10161): 2299-2312.
[3] PRIMO DE CARVALHO ALVES L, SICA DA ROCHA N. Different cytokine patterns associate with melancholia severity among inpatients with major depressive disorder[J]. Therapeutic Advances in Psychopharmacology, 2020, 10: 1-12.A75A2BD7-E220-481C-A514-6415FC92B4EB
[4] 王海蘭,周湘乐,谭 婷,等.百合地黄汤对抑郁症大鼠血清IL-10和海马DA的影响[J].湖南中医药大学学报,2018,38(11):1326-1330.
[5] 蒋 健.郁证发微(一):郁证形态论[J].上海中医药杂志,2015,49(8):4-7.
[6] 张 燕,过伟峰,杜琳琳.柴胡加龙骨牡蛎汤治疗情志病[J].中医学报,2021,36(8):1616-1618.
[7] 李 宇,蔡萧君,王 钦,等.巴戟天对慢性应激大鼠肠道微生物代谢多样性的影响[J].中国中医药信息杂志,2022,29(2):55-62.
[8] 颉彦鹏.基于5-HT2AR、CaMKⅡα表达变化研究柴胡加龙骨牡蛎汤抗抑郁机制[D].哈尔滨:黑龙江省中医药科学院,2020.
[9] 王 钦,蔡萧君,李 宇,等.柴胡加龙骨牡蛎汤对慢性应激抑郁大鼠海马BDNF/TrkB/CREB通路的影响[J].中国实验方剂学杂志,2021,27(21):37-46.
[10] 徐雪娇,李天英,马芸瑄,等.抑郁症动物模型概述[J].医学综述,2021,27(7):1345-1349,1357.
[11] 徐明杭,蒙艳丽,王晓溪,等.黄芩苷对肺纤维化小鼠组织中纤维化因子TGF-β1及mmp2,timp2表达的影响[J].中国中药杂志,2020,45(23):5738-5744.
[12] QIAO Y J, ZHAO J B, LI C, et al. Effect of combined chronic predictable and unpredictable stress on depression-like symptoms in mice[J]. Annals of Translational Medicine, 2020, 8(15): 942.
[13] LI K W, YAN L, ZHANG Y P, et al. Seahorse treatment improves depression-like behavior in mice exposed to CUMS through reducing inflammation/oxidants and restoring neurotransmitter and neurotrophin function[J]. Journal of Ethnopharmacology, 2020, 250: 112487.
[14] 赵洪庆,杜 青,柳 卓,等.不同抑郁模型大鼠行为学及神经营养因子表达的对比研究[J].湖南中医药大学学报,2019,39(7):822-826.
[15] 曲书苑,庞宝兴.应激性抑郁动物模型评价方法研究进展[J].实验动物科学,2019,36(6):59-63.
[16] KRAUS C, CASTRéN E, KASPER S, et al. Serotonin and neuroplasticity-Links between molecular, functional and structural pathophysiology in depression[J]. Neuroscience and Biobehavioral Reviews, 2017, 77: 317-326.
[17] WANG X, CHEN J, ZHANG H L, et al. Immediate and persistent antidepressant-like effects of Chaihu-Jia-Longgu-Muli-Tang are associated with instantly up-regulated BDNF in the Hippocampus of mice[J]. Bioscience Reports, 2019, 39(1): BSR20181539.
[18] 李姿蓉,韩远山,王宇红,等.从抑郁共病失眠机制探寻抑郁症新药研发的方向[J].湖南中医药大学学报,2019,39(10):1279-1284.
[19] ACHTYES E, KEATON S A, SMART L, et al. Inflammation and kynurenine pathway dysregulation in post-partum women with severe and suicidal depression[J]. Brain, Behavior, and Immunity, 2020, 83: 239-247.
[20] ZHU J L, HU Z L, HAN X J, et al. Dopamine D2 receptor restricts astrocytic NLRP3 inflammasome activation via enhancing the interaction of β-arrestin2 and NLRP3[J]. Cell Death and Differentiation, 2018, 25(11): 2037-2049.
[21] WANG X Y, VILLAR V A, TIU A, et al. Dopamine D2 receptor upregulates leptin and IL-6 in adipocytes[J]. Journal of Lipid Research, 2018, 59(4): 607-614.A75A2BD7-E220-481C-A514-6415FC92B4EB
[22] ZEPP J, WU L, LI X X. IL-17 receptor signaling and T helper 17-mediated autoimmune demyelinating disease[J]. Trends in Immunology, 2011, 32(5): 232-239.
[23] LI Y C, CHOU Y C, CHEN H C, et al. Interleukin-6 and interleukin-17 are related to depression in patients with rheumatoid arthritis[J]. International Journal of Rheumatic Diseases, 2019, 22(6): 980-985.
[24] VALLES-COLOMER M, FALONY G, DARZI Y, et al. The neuroactive potential of the human gut microbiota in quality of life and depression[J]. Nature Microbiology, 2019, 4(4): 623-632.
[25] BEUREL E, LOWELL J A. Th17 cells in depression[J]. Brain, Behavior, and Immunity, 2018, 69: 28-34.
[26] BUSPAVANICH P, ADLI M, HIMMERICH H, et al. Faster speed of onset of the depressive episode is associated with lower cytokine serum levels (IL-2, IL-4, IL-6, IL-10, TNF-α and IFN-γ) in patients with major depression[J]. Journal of Psychiatric Research, 2021, 141: 287-292.
[27] 閆 盼,施剑飞,李 静,等.DRD2和5-HTR2A基因多态性及其交互作用对奥氮平治疗精神分裂症疗效的影响[J].中国临床药理学与治疗学,2020,25(10):1131-1138.
[28] XUE Y, WEI S Q, WANG P X, et al. Down-regulation of spinal 5-HT2A and 5-HT2Creceptors contributes to somatic hyperalgesia induced by orofacial inflammation combined with stress[J]. Neuroscience, 2020, 440: 196-209.
[29] 董 震,刘 欣,张丽丽,等.首发精神分裂症与DRD2基因多态性和认知功能的关系研究[J].中国全科医学,2021,24(32):4116-4120,4125.
[30] FATIMA M, AHMAD M H, SRIVASTAV S, et al. A selective D2 dopamine receptor agonist alleviates depression through up-regulation of tyrosine hydroxylase and increased neurogenesis in Hippocampus of the prenatally stressed rats[J]. Neurochemistry International, 2020, 136: 104730.
(本文编辑 黎志清)A75A2BD7-E220-481C-A514-6415FC92B4EB
