Effect of hydrogen-storage pressure on the detonation characteristics of emulsion explosives sensitized by glass microballoons
2022-05-24JipingChenHonghoYixinWngLinglingHungZhowuShen
Ji-ping Chen , Hong-ho M ,b,*, Yi-xin Wng , Ling-ling Hung , Zho-wu Shen
aCAS Key Laboratory of Mechanical Behavior and Design of Materials, Department of Modern Mechanics, University of Science and Technology of China,Hefei, Anhui, PR China
bState Key Laboratory of Fire Science, University of Science and Technology of China, Hefei, Anhui, PR China
Keywords:Emulsion explosives Hydrogen-storage pressure Glass microballoons Underwater explosion
ABSTRACT In this study, hydrogen-storage glass microballoons were introduced into emulsion explosives to improve the detonation performance of the explosives.The effect of hydrogen-storage pressure on the detonation characteristics of emulsion explosives was systematically investigated.Detonation velocity experiments shows that the change of sensitizing gas and the increase of hydrogen pressure have different effects on the detonation velocity.The experimental parameters of underwater explosion increase first and then decreases with the increase of hydrogen pressure.The decrease of these parameters indicates that the strength of glass microballoons is the limiting factor to improve the detonation performance of hydrogen-storage emulsion explosives.Compared with the traditional emulsion explosives,the maximum peak pressure of shock wave of hydrogen-storage emulsion explosives increases by 10.6%at 1.0 m and 10.2%at 1.2 m,the maximum values of shock impulse increase by 5.7%at 1.0 m and 19.4%at 1.2 m.The stored hydrogen has dual effects of sensitizers and energetic additives,which can improve the energy output of emulsion explosives.
1.Introduction
As a special energy source,emulsion explosives are widely used in mining industry, building demolition, explosive welding, which was invented by Bluhm [1,2].They are made by dispersing supersaturated NHNOand NaNOsolutions in hydrocarbon oil containing a surfactant to form a water-in-oil (W/O) structure [3].Wade developed a new type of emulsion explosives in 1978 that used glass microballoons (GMs) for sensitization [4].The compressed microballoons are “hot spots” for triggering the chemical reaction of emulsion matrix [5].Emulsion explosives have the advantages of water resistance, good safety, low production cost,moderate explosion sensitivity and little environmental pollution,but these advantages are also a double-edged sword, which will lead to lower detonation performance compared with military explosives[6,7].
It is of great significance to improve the detonation performance of explosives in both civil and military industries.Wang et al.[8]and Mishra et al.[9] investigated the influence of aluminum content on underwater explosion parameters and detonation velocity,respectively.Explosives such as HMX [10] and RDX [11] were introduced into emulsion explosives to increase detonation performance.Due to high specific energy, environmental friendliness and extensive sources of hydrogen, metal hydride has also been introduced into emulsion explosives for a long time.Cheng et al.[12,13] developed a new type of emulsion explosives containing TiHor MgH,and found that metal hydride had a better effect on improving the detonation performance of emulsion explosives compared with corresponding metal powder.But there are no studies on the introduction of free hydrogen into explosives yet.
The earliest storage of hydrogen in GMs was proposed to solve the problem of hydrogen storage and transportation [14].One can exploit the diffusion of hydrogen through the thin wall of GMs at elevated temperature and pressure, and then trap the gas in GMs upon cooling to room temperature.The storage of hydrogen is effective due to the low diffusivity of hydrogen at room temperature [15,16].Shelby et al.[17] studied the kinetic theory of hydrogen-storage glass microballoons(HGMs)systematically.Kohli et al.[18]designed a hydrogen-storage system and investigated the hydrogen-storage efficiency of GMs with different wall thicknesses and diameters.
In this study,HGMs were introduced into emulsion explosives to improve the detonation performance of the explosives.The effect of hydrogen-storage pressure on the detonation characteristics of emulsion explosives were investigated by underwater explosion system and detonation velocity experiments.
2.Experimental section
2.1.Experimental materials
The emulsion matrix was yellowish and viscous.It was produced by Huainan Shun Tai Chemical Co, Ltd.(Huainan, Anhui,China)and its density was 1.31 g/cm.The component of emulsion matrix is shown in Table 1,where span-80 is an emulsifier used to form the water-in-oil structure.
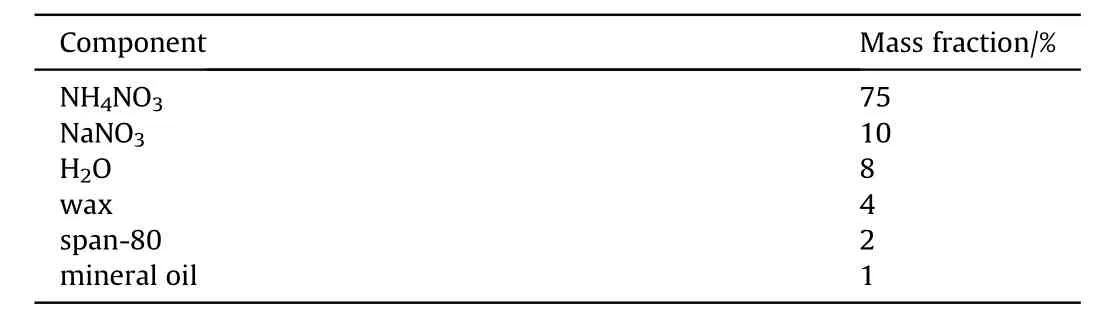
Table 1 The component of emulsion matrix.
The GMs used in this study were purchased from Bengbu Glass Industry Design & Research Institute (Bengbu, Anhui, China).The outer diameter of GMs ranged from 10 μm to 100 μm and the median particle diameter was 50 μm.The apparent density of GMs and the density of spherical shell material were 0.28 g/cmand 2.52 g/cm, respectively.The maximum compressive strength of GMs was 30 MPa.The scanning electron microscope (SEM) was used to observe the microstructure of GMs,as shown in Fig.1.It can be seen that the spherical shell structure remains intact without obvious breakage.
In this study, a high-pressure reaction kettle purchased from Anhui Kemi Machinery Technology Co., Ltd.(Hefei, Anhui, China)was used to store hydrogen for GMs,as seen in Fig.2.It was made of 316 L stainless steel with a design pressure of 20 MPa and a design temperature of 400C.The kettle lid was equipped with a pressure gauge to observe the internal pressure change.The reaction kettle realized temperature programming by a temperature controller and a ring heating furnace.
2.2.Experimental procedure
In the hydrogen-storage experiment, 20 g GM using aluminum foil as a container were put in the reaction kettle and then hydrogen was filled into the reaction kettle through the inlet valve.The hydrogen-storage pressure was set to 2.0, 4.0, 6.0, 8.0 MPa in this study.The maximum hydrogen pressure was set to 8.0 MPa based on the safety considerations of the reaction kettle.Hydrogen permeability of spherical shell material is positively correlated with temperature, an increase in temperature can significantly increase the rate of hydrogen penetration[19].Therefore,the reaction kettle was heated to 300C by a ring heating furnace within 1.5 h and the heating was maintained for 4.0 h to shorten the formation time of HGMs.As the temperature increased, the permeability of the spherical shell material to hydrogen increased and the highpressure hydrogen in the reactor gradually entered into the glass microballoons.After scheduled heating time was reached, the temperature of the kettle gradually lowered to room temperature.The permeability of the spherical shell material to hydrogen reduced and hydrogen was stored in the GMs to form HGMs.As indicated by Cheng et al.[12],the emulsion explosives reached the maximum energy output when the mass ratio of GMs and emulsion matrix was 4:100.HGMs and emulsion matrix were mixed evenly at the mass ratio of 4:100 to prepare hydrogen-storage emulsion explosives (HEE).In addition, the non-hydrogen storage emulsion explosives (NEE) were selected as the control group.The NEE was composed of emulsion matrix and GMs according to 100:4.The density of these five emulsion explosives was 1.17 g/cm.Because the mass of stored hydrogen is tiny, there is no difference in the density of these five emulsion explosives.These five emulsion explosives were conducted detonation velocity and underwater explosion experiments.
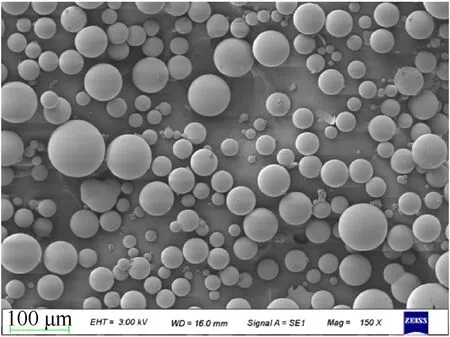
Fig.1.SEM image of glass microballoons.
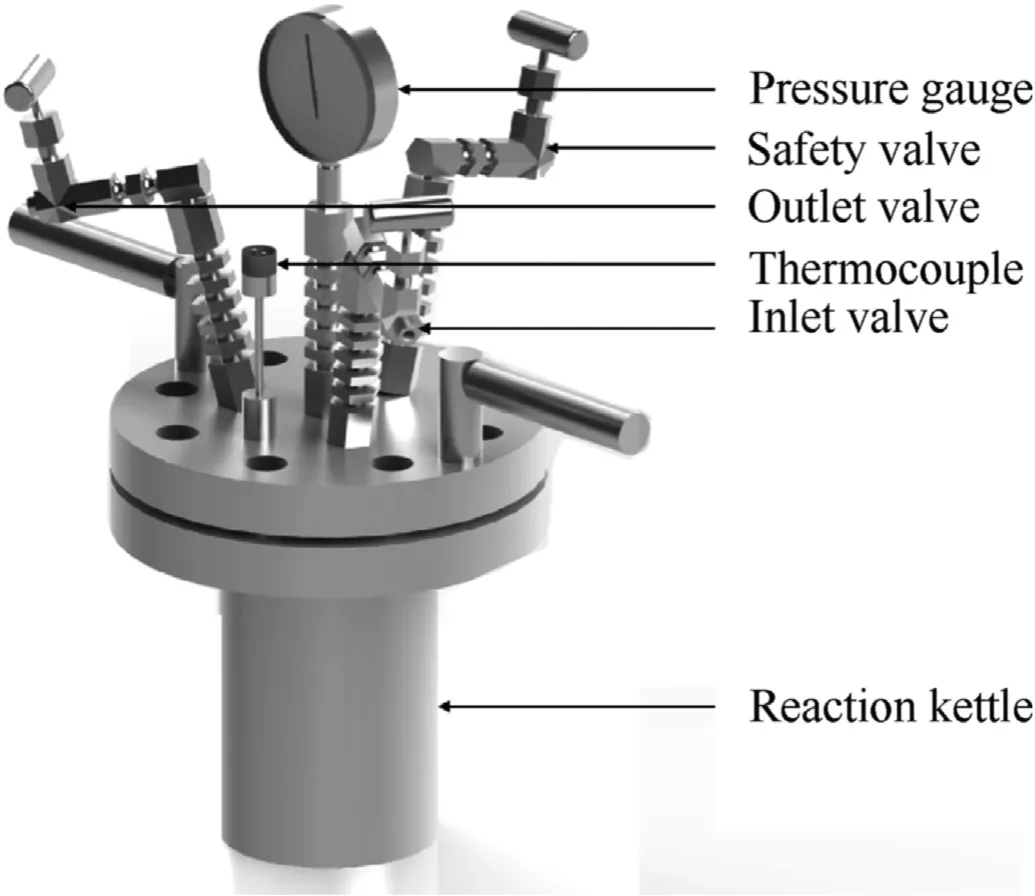
Fig.2.Schematic of high-pressure reaction kettle.
We used discrete points electrical method to measure the detonation velocity of emulsion explosives and the schematic diagram of this measurement system is shown in Fig.3.Four electric probes passed through the detonation velocity tube radially and remained parallel, the distance between them was about 40 mm.The probe was intertwisted from two copper core enameled wires,one end of the probe was disconnected and the other end bent towards the end of the detonation velocity tube was connected to the high-resolution electric timer to record the time gap.Explosives generated a shock wave by a detonator, and then probes were ionized under high temperature, high pressure, luminescence and ionization of shock wave.High-resolution electric timer recorded time gaps of the detonation wave passing through adjacent probes in sequence to obtain the average detonation velocity.
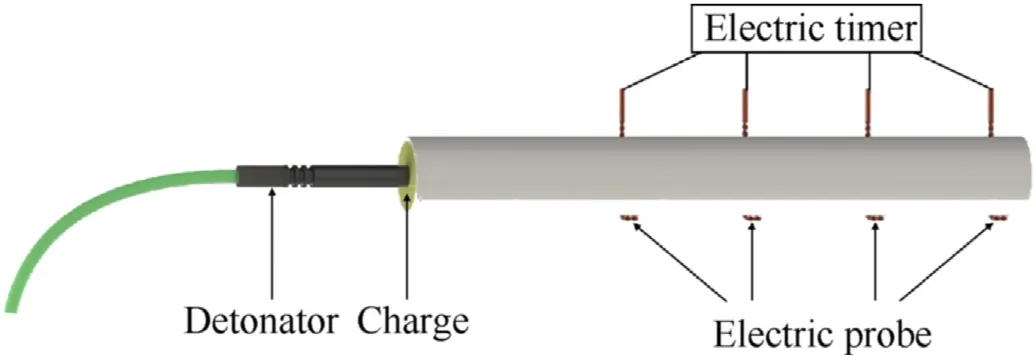
Fig.3.Schematic of detonation velocity experiment.
Underwater explosion experiment system can give the absolute value of work performed by explosives,so it is widely applied in the power test of industrial explosives.As shown in Fig.4, the underwater explosion tank is steel with a diameter of 4.9 m and a depth of 5.0 m.The 30 g spherical charge was placed in the center of the explosion tank after water proofing.Both the charge and two sensors were placed under 3.0 m of the water phase.The distances between two sensors and the charge were 1.0 m and 1.2 m,respectively.The pressure signal generated by the charge was detected by a PCB-W ICP138A25 type pressure sensor and recorded by a Tektronix 4104 digital oscilloscope (Tektronix, Inc.USA).Underwater detonation characteristics of HEE were analyzed by the processing pressure-time history curve.
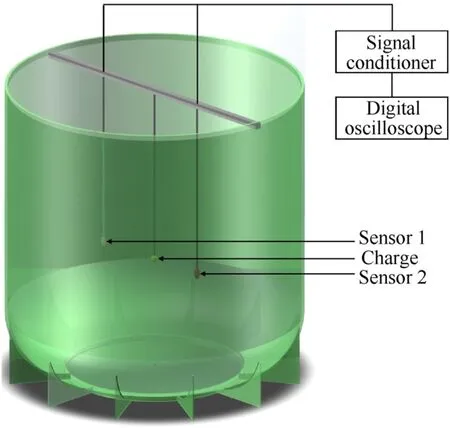
Fig.4.Schematic of underwater explosion system.
3.Results and discussions
3.1.Hydrogen-storage glass microballoons
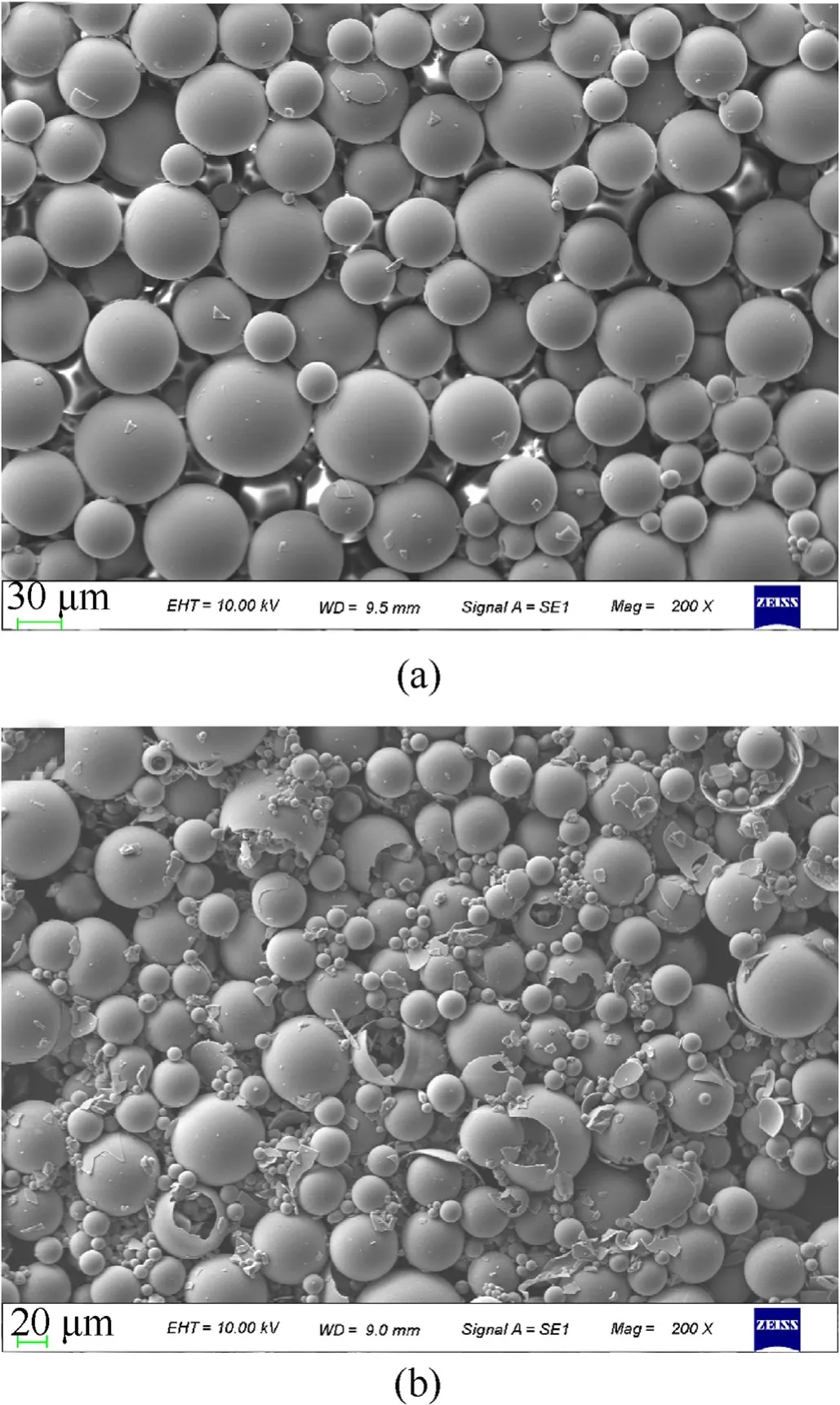
Fig.5.SEM image of HGMs at different hydrogen-storage pressures, (a) 6.0 MPa, (b)8.0 MPa.
During the hydrogen-storage of GMs,the pressure continued to increase in the kettle and the pressure nearly doubled at the end of the temperature rise.After hydrogen penetration was completed,6.0 MPa and 8.0 MPa GM were observed to analyze morphological changes.Fig.5(a) shows that the spherical shell structure remains basically intact at 6.0 MPa and the microstructure of HGMs does not change significantly compared with that of ordinary GMs.However,when the hydrogen pressure was further increased to 8.0 MPa,the crack of GMs could be heard immediately after being taken out from the reaction kettle.It can be seen from Fig.5(b) that the number of broken microballoons increases prominently and there are many completely broken shell fragments at the bottom.Since the maximum compressive strength of GMs could reach 30 MPa,GMs were not basically broken during the process of storing hydrogen.The GMs are less resistant to internal pressure than external pressure[20].Thus,the GMs ruptured under the action of internal high-pressure hydrogen,which was dozens of times more than the ambient pressure.
The hydrogen-storage capacity of HGMs can be calculated by measuring the pressure change of the reaction kettle.The internal nitrogen density, the spherical shell material density and the apparent density of GMs were 1.25 g/L (ρ), 2.52 g/cm(ρ) and 0.28 g/cm(ρ),respectively.Therefore,the volume of internal cavity() and the volume of spherical shell material () satisfy the following relationship:

Each experiment weighted 20 g GM (i.e.71.43 cm).And then according to Eq.(1), the volume of internal cavity was equal to 63.52 cmand the volume of spherical shell material was equal to 7.91 cm.Because the aluminum foil used as a container had only 1-2 g, its volume was negligible.According to the ideal gas state equation=,the hydrogen pressure inside GMs()is obtained by employing the expression as follow[21]:
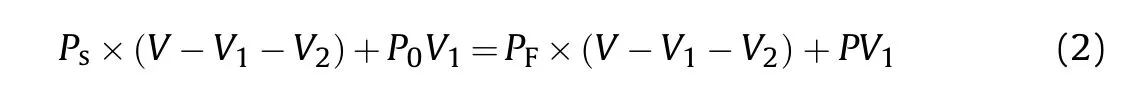
whereis the internal pressure of reaction kettle that starts storing hydrogen (MPa),is the cubage of reaction kettle(300 cm),is the internal pressure of reaction kettle that finishes storing hydrogen (MPa),is the initial pressure inside GMs(0.101 MPa).
The hydrogen-storage capacity of HGMs is calculated by Eqs.(3)and (4):
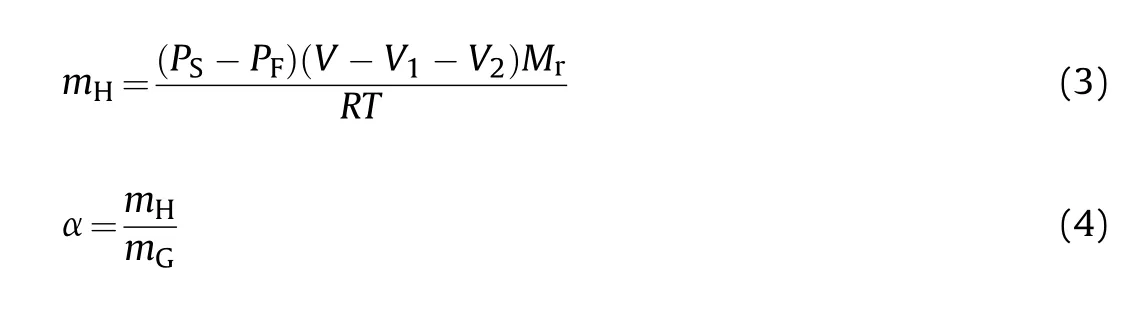
Whereis the mass of hydrogen inside HGMs (g),is the relative molecular mass of hydrogen (g/mol), α is the hydrogenstorage capacity of HGMs (wt%),is the total mass of HGMs (g).
Table 2 shows the calculated results of the pressure inside GMs by recording the gas chamber pressure before and after hydrogen storage.Under hydrogen-storage conditions of 300C and 4.0 h,the hydrogen pressure inside GMs that completed storing hydrogen is positively correlated with the initial hydrogen pressure of the kettle, and the maximum pressure inside GMs is 3.70 MPa in this study.The hydrogen-storage capacity is affected by hydrogenstorage conditions such as storing time and temperature.Therefore,it fails to reach the hydrogen-storage limit,that is,the internal and external pressure of the GMs is consistent.If the hydrogenstorage time and temperature continue to increase, the hydrogenstorage capacity may be further increased.Taking hydrogenstorage pressure at 6.0 MPa as an example, 20 g GM in each test could be used to prepare 524.316 g HEE and the energy release of emulsion explosives would be increased by 0.045 MJ/kg.When the initial hydrogen pressure of reaction kettle is 2.0 MPa,the internal pressure of GMs is higher than the external pressure,which may be caused by the gas leak of the reaction kettle.
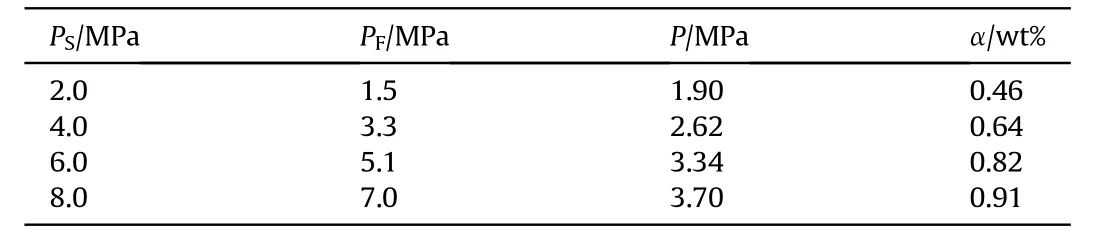
Table 2 Hydrogen-storage capacity of glass microballoons.
3.2.Detonation velocity
The initiation of emulsion explosives is actually the adiabatic compression process of GMs under the action of shock wave or detonation wave [22].Detonation velocity experiment obtains the stable propagation velocity of detonation wave in explosives,which is a crucial parameter of explosion performance.Fig.6 shows the average detonation velocity of different hydrogen-storage pressures.One can see that the change of sensitizing gas and the increase of hydrogen pressure have different effects on the detonation velocity.Stored hydrogen improves the donation velocity to a certain extent at a lower pressure.According to the Chapman-Jouguet (C-J) theory, the relationship between the detonation velocity of emulsion explosives and the chemical reaction energy released from detonation reaction can be given by Ref.[23]:

whereis the detonation velocity of emulsion explosives(m/s),γ is the heat capacity ratio of gas product, andis the chemical reaction energy released during detonation (J/kg).
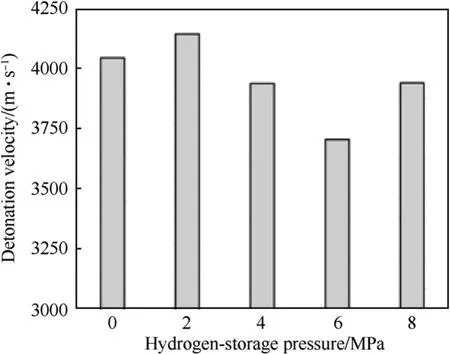
Fig.6.Detonation velocity of five emulsion explosives with different hydrogen-storage pressures.
The detonation velocity of emulsion explosives increases with the increase of chemical reaction energy according to Eq.(3).The energetic hydrogen releases extra energy to ignite more emulsion matrix during the formation of hot spots.The remaining hydrogen participates in the detonation reaction of emulsion explosives and promotes the formation of hot spots.These effects result in the increase of detonation velocity.But this effect gradually weakens with the increase of hydrogen pressure, and even the detonation velocity of HEE at 6.0 MPa is lower than that of the NEE.The increase of hydrogen pressure causes the detonation velocity of HEE to reduce at a higher pressure.Wang et al.[24] found the same experimental results.When GMs stored micromolecular and inert helium, the detonation velocity was significantly reduced.The mechanism of hot spots in emulsion explosives is the adiabatic compression of bubbles.The inside of ordinary glass microballoons is atmospheric pressure nitrogen.The inside of hydrogen-storage glass microballoons is high-pressure hydrogen and the pressure of hydrogen is dozens times of the atmospheric pressure.The higher the hydrogen pressure inside HGMs, the greater the resistance to the formation of hot spots,which is not conducive to the adiabatic compression of bubbles.The formation of hot spots is a prat of the detonation of emulsion explosives,so the decrease of hot spots formation rate leads to the decrease of propagation velocity of detonation wave.The detonation velocity rebounds at 8.0 MPa due to the rupture of GMs.
3.3.Peak pressure of shock wave
All underwater explosion experimental datum can be obtained by processing the pressure-time history curve, as listed in Table 3.The peak pressure of shock wave()reflects the strength of wave front and it can be read from the curve directly.Fig.7 shows that the peak pressure of shock wave increases first and then decreases with the increase of hydrogen-storage pressure and this increasing tread is more remarkable at low hydrogen pressure.The sensors at the two locations reaches the peak at different hydrogen pressures,which may be due to the large interval of hydrogen pressure, the true peak occurs in the range of 4.0-6.0 MPa.However, the peak pressure of shock wave decreases at 8.0 MPa.When the hydrogenstorage pressure was 8.0 MPa, some HGMs were break under the condition of atmospheric pressure and temperature.Part of HGMs were completely broken into fragments and these fragments could not sensitize the emulsion matrix.Part of HGMs only produced cracks but still acted as hot spots.Both of the above two broken method reduced the amount of hydrogen.Therefore, the reduced hot spots and hydrogen causes a reduction in peak pressure of shock wave.Nonetheless, the remaining HGMs still make its underwater detonation performance better than the NEE.The above research indicates that the strength of GMs is the limiting factor to improve the detonation performance of HEE.
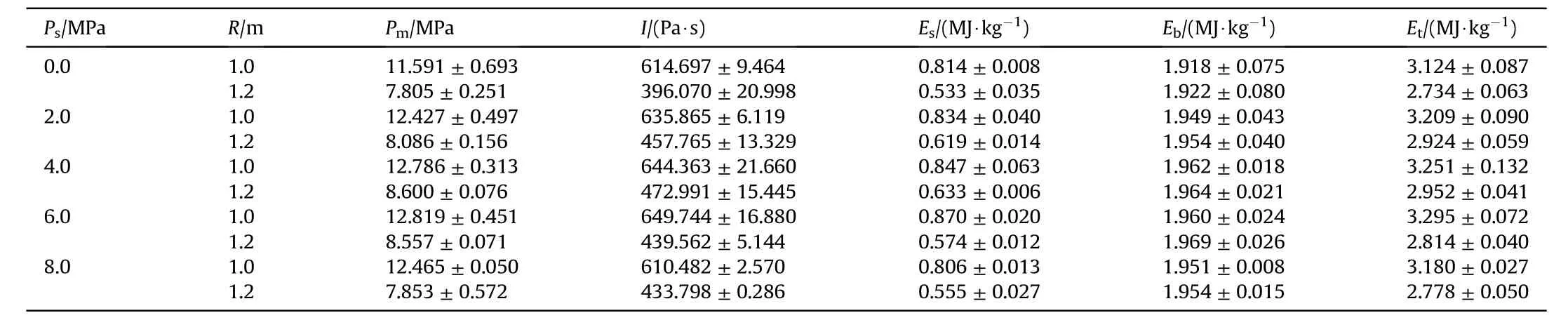
Table 3 Experimental datum of underwater explosion.
Compared with the NEE, the maximum peak pressures of HEE are 12.819 MPa at 1.0 m and 8.600 MPa at 1.2 m,which increase by 10.6% and 10.2%, respectively.The increase amplitude of peak pressure in different locations is basically the same.HGMs changes the internal sensitizing gas from nitrogen to high-energy hydrogen.The combustion of hydrogen releases extra energy to promote the formation of hot spots.The internal hydrogen of HGMs as an energetic group not only sensitizes the emulsion matrix but also participates in the detonation reaction of the emulsion explosives to improve the peak pressure of shock wave.With the increase of hydrogen pressure, the quality of hydrogen also increases and the effect on improving the peak pressure of emulsion explosives is more significant.
3.4.Shock impulse
The work ability of shock wave is related to the integral of pressure with time, namely the impulse ().The impulse is calculated as [25]:

where() is the shock wave pressure (Pa), θ is the shock wave attenuation time constant(s),which is equal to the time interval of the shock wave pressure to decay fromto/e, andis mathematical constant.
Fig.8(a)shows the overall trend of the impulse,it is roughly the same as the peak pressure of shock wave,increasing first and then decreasing.When the distance from the center of the charge to the sensor is 1.0 m and 1.2 m,the maximum values of shock impulse are 649.744 Pa s and 472.991 Pa·s,respectively,which increase by 5.7%and 19.4% compared with the NEE.The increase amplitude of the shock impulse with the HEE at 1.2 m is obviously larger than that at 1.0 m.This may be due to the incomplete reaction of hydrogen during the formation of hot spots.After shock wave front swept through HGMs, the internal hydrogen couldn’t fully react during instantaneous adiabatic compression process, and then the.
Remaining hydrogen continued to generate energy in order to slow down the pressure drop in the course of explosive detonation.Therefore, the shock impulse of the HEE at 1.2 m has a greater increase amplitude compared with that of 1.0 m.
3.5.Specific shock energy
The empirical calculation formula for underwater explosion specific shock energy () can be given by Ref.[26]:

whereis the distance from charge to sensor(m),is the mass of emulsion explosives (kg),ρis the density of water (1000 kg/m),is the sound speed in the water (m/s).
Fig.8(b)shows the calculated results of specific shock energy.At 1.0 m,the maximum value of specific shock energy is 0.870 MJ/kg,which is 6.9%higher than that of NEE.At 1.2 m,the maximum value of specific shock wave energy is 0.633 MJ/kg,which is 18.8%higher than that of NEE.This can be attributed to the fact that hydrogen participates in the detonation reaction of the entire explosives,which provides extra energy to increase the peak pressure of shock wave and to slow down the attenuation of the shock wave.The combined effect of the two increases the specific shock energy.
3.6.Specific bubble energy
The bubble pulsation formed by the interaction between explosive detonation products and hydrostatic pressure is a key point for underwater explosion research.An improved formula for calculating the specific bubble energy () proposed by Bjarnholt[26] can be expressed as
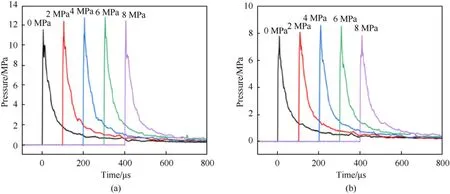
Fig.7.Peak pressure of shock wave of five emulsion explosives at different distances, (a) 1.0 m, (b) 1.2 m.
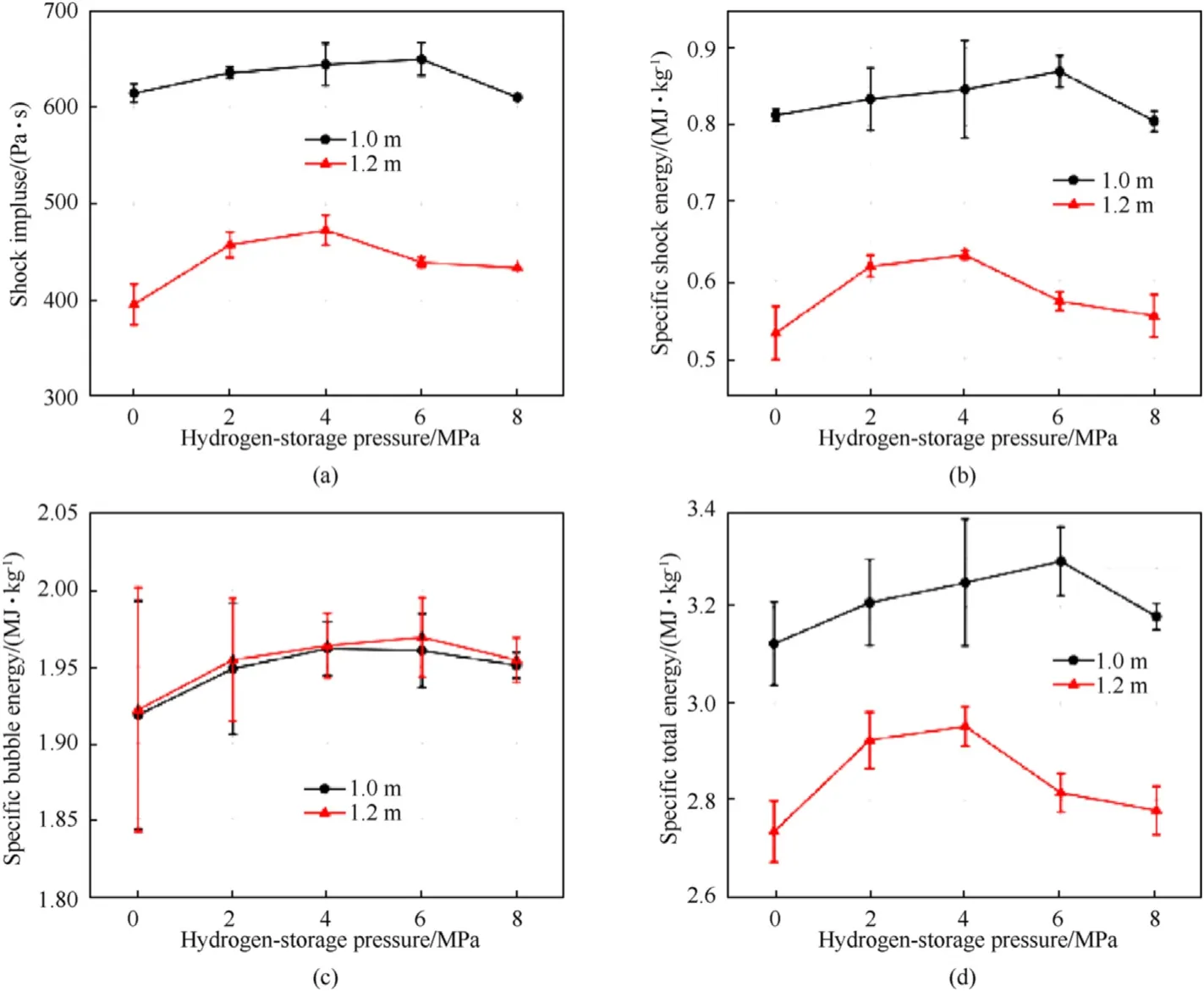
Fig.8.Comparation of experimental parameters of underwater explosion, (a) shock impulse, (b) specific shock energy, (c) specific bubble energy, (d) specific total energy.
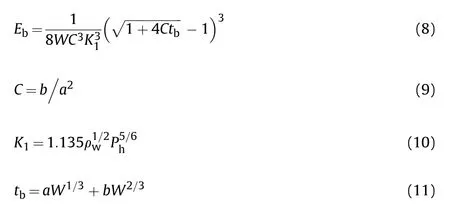
whereis the correction factor of boundary effect calculated by Eq.(9)(s),is an experimental constant determined by Eq.(10),is the first bubble period(s),the constantsandare obtained by making a least squares fit to the (-) data,is the total hydrostatic pressure at the center of the charge including atmospheric pressure(Pa).
Underwater explosion experiments cannot meet the condition of ideal infinite water assumption in the tank, specific bubble energy cannot be calculated from the Willis formula directly [27].A modified equation was adopted to eliminate the effect of boundary condition on the bubble period.The constantsandwere fitted from the previous underwater explosion datum of our laboratory,which were 0.2849 and 0.0746 respectively, and thenwas calculated to be 0.9195 s[28].
From Fig.8(c),the specific bubble energy has a maximum value at the hydrogen-storage pressure of 4.0-6.0 MPa, which increase by 2.3%at 1.0 m and 2.5%at 1.2 m,respectively.This is because the combustion of hydrogen caused the total amount of gas to increase by producing water vapor.The higher the hydrogen pressure, the more water vapor was generated.The specific bubble energy is related to the first bubble period and the total amount of gas generated during the entire detonation reaction is the same,so the specific bubble energy at the two locations are almost no difference.
3.7.Specific total energy
The specific total energy()is determined by the specific shock energy and the specific bubble energy.Thus,it can be calculated by following expressions [26]:
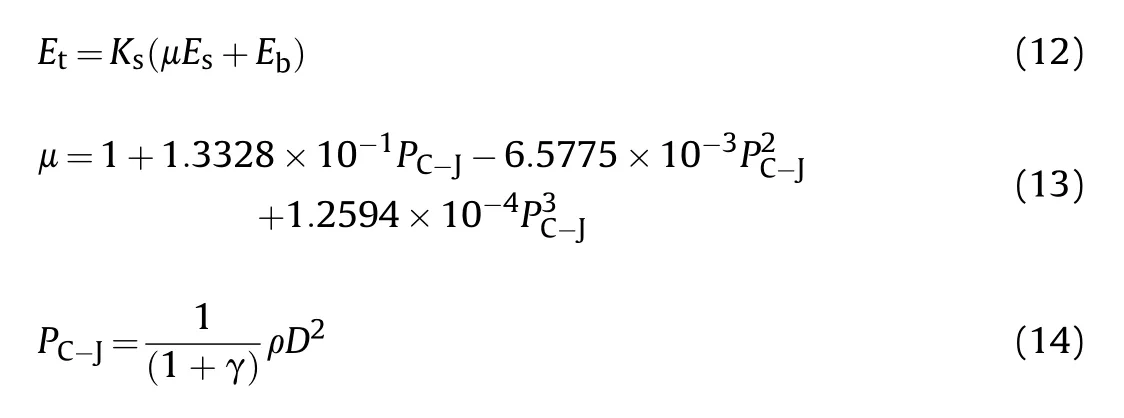
whereis the shape factor of charge, which is 1.00 for the spherical charge used in this experiment, μ is the loss factor of shock wave,is the detonation pressure of emulsion explosives(GPa),γ is the heat capacity ratio,which is equal to 3 for detonation products,ρ is the charge density (kg/m).
Fig.8(d) shows the specific total energy results of different hydrogen-storage pressures.The peak value of specific total energy at 1.0 m and 1.2 m are 3.295 MJ/kg and 2.952 MJ/kg, respectively,which are 5.5% and 8.0% higher than NEE.Since the specific total energy is combination of the specific shock energy and the specific bubble energy,its general trend is consistent with the expectation.The increase of specific total energy is mainly due to the contribution of specific shock energy.According to the previous discussion, if the internal hydrogen completely reacts at 6.0 MPa, the energy of HEE will increase by 0.045 MJ/kg.However, the specific total energy increases by 0.171 MJ/kg at 1.0 m and 0.218 MJ/kg at 1.2 m compared to NEE.Therefore,when the sensitizing gas in the GMs is changed from nitrogen to hydrogen,the hydrogen not only burns to provide energy, but also stimulates the energy output of emulsion explosives.
4.Conclusion
In this study,HGMs were introduced into emulsion explosives to improve the detonation performance of the explosives.The effect of hydrogen-storage pressure on the detonation characteristics of emulsion explosives was investigated.The initial hydrogen-storage pressure of reaction kettle was set to 2.0,4.0,6.0,8.0 MPa,and the improvement of detonation performance was analyzed through underwater explosion and detonation velocity experiments.The following conclusions are summarized:
(1) The detonation velocity increases first and then decreases with an increase in hydrogen-storage pressure.The change of sensitizing gas and the increase of hydrogen pressure have different effects on the detonation velocity.
(2) The parameters of underwater explosion present the same variation trend, increasing first and then decreasing.The maximum value appears between 4.0-6.0 MPa of hydrogenstorage pressure.Compared with the NEE, the maximum peak pressure of shock wave of HEE increases by 10.6% at 1.0 m and 10.2% at 1.2 m, the maximum values of shock impulse increases by 5.7% at 1.0 m and 19.4% at 1.2 m.
(3) The internal hydrogen of HGMs has the dual effects of sensitizers and energetic additives,which provides extra energy to improve the detonation characteristics of emulsion explosives and to slow down the reduction in shock wave pressure.The addition of hydrogen promotes the energy output of the emulsion explosives.
(4) The maximum hydrogen pressure inside GMs can reach 3.70 MPa.The strength of GMs is the limiting factor to improve the detonation performance of HEE.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
This research was financially supported by the National Natural Science Foundation of China under Project NO.51874267 and NO.51674229.The authors would like to thank these foundations for financial support.
杂志排行
Defence Technology的其它文章
- Behind-plate overpressure effect of steel-encased reactive material projectile impacting thin aluminum plate
- Numerical damage evaluation of perforated steel columns subjected to blast loading
- Blast responses of polyurea retrofitted utility tunnel reinforced with basalt fibre reinforced polymer bars
- Experimental study on the accumulative effect of multiple pulses on acceleration sensor
- Higher-order electroelastic modelling of piezoelectric cylindrical nanoshell on elastic matrix
- An improved LuGre model for calculating static steering torque of rubber tracked chassis
