Significantly enhanced charge transfer efficiency and surface reaction on NiP2/g-C3N4 heterojunction for photocatalytic hydrogen evolution
2022-04-27XiaoqingYanHuaAnZihaoChenGuidongYang
Xiaoqing Yan,Hua An,Zihao Chen,Guidong Yang
XJTU-Oxford International Joint Laboratory for Catalysis,School of Chemical Engineering and Technology,Xi’an Jiaotong University,Xi’an 710049,China
Keywords:Carbon neutrality Charge transfer Internal electric field Photocatalytic H2 generation
ABSTRACTIn this work,a novel NiP2/g-C3N4 heterojunction via homogeneous precipitation method assisted by thermal phosphorization reaction was designed and constructed,and the optimized sample showed the excellent photocatalytic H2 evolution activity under visible-light irradiation,which was nearly 112 times higher than that of pristine g-C3N4 sample.Experimental characterizations and DFT calculations demonstrated that the NiP2 nanoparticles covered on the g-C3N4 surface can form a built-in electric field at the interface to accelerate the transfer of photoexcited electrons from g-C3N4 to NiP2,crucial for hindering the recombination of electron-hole pairs.Moreover,the energy barrier of hydrogen evolution reaction can also vastly reduce when combined NiP2 and g-C3N4 to construct NiP2/g-C3N4 heterojunction.This work represents a method through combing experimental and theoretical tools to thoroughly investigate the mechanism of photocatalytic process.
1.Introduction
Developing clean energy will greatly promote the early realization of carbon neutrality before 2060 in China.The hydrogen,as a promising energy source,has get much attention owing to its very clean.However,current hydrogen mainly produced from the unreproducing resource of fossil fuel,while its production process is high carbon dioxide emissions.Solar driven photocatalytic hydrogen production technology is clean,carbon free and low energy consumption,which has been considered to be the most desirable approaches for H2generation all over the world [1–4].Its basic principle is that under the irradiation of sunlight,the valence band and conduction band of semiconductor photocatalyst could produce holes and electrons with high oxidation and reduction ability respectively,the photogenerated electrons would be reduced water to produce hydrogen,and the photogenerated holes would be oxidized water to produce oxygen [5,6].As a class of important photocatalyst,graphitic carbon nitride (g-C3N4) has attracted many attention due to its prominent chemical,thermal stability and unique electronic properties [7,8],with multiple utilizations in photocatalytic hydrogen production,photocatalytic CO2reduction and photocatalytic ammonia production [9].However,the pure g-C3N4still exhibited poor photocatalytic activity owing to its fast recombination of electron-hole pairs and high overpotential in the process of interface reaction [10].Therefore,in the past decades,great attempts have been made to solve the aforementioned bottleneck problem of g-C3N4[11–13].Among various strategies,the design of special heterojunction structure has been considered to be one of the effective approaches to enhanced the charge carriers separation and improve the photocatalytic hydrogen production activity of g-C3N4.For example,Lin and coworkers[14]have designed a heterojunction structure of g-C3N4and ZnIn2S4to enhance the electron-hole pairs separation.Pan[15]et al.have combined CoP and Pt nanoparticles on g-C3N4nanosheets to construct Pt/g-C3N4/CoP composition,which showed a great photocatalytic activity of water splitting.This is because the introduction of CoP and Pt onto g-C3N4can vastly reduce the overpotential in the process of interface reaction.From these examples,it can be found that the loading metal phosphide on the g-C3N4surface to construct a g-C3N4-based heterojunction exhibited huge advantage for the reducing overpotential in the process of hydrogen production.Recently,nickel phosphides,as a new class of cocatalyst,have garnered considerable attention due to its low cost,high activity,and well stability.The negatively charged P sites with moderate electronegativity could act as Lewis bases to trap the positively charged protons,potentially endowing the nickel phosphides with a Gibbs free energy of adsorbed H (Had) close to zero [16–18].Among these NixPycompounds (Ni3P,Ni5P2,Ni12P5,Ni2P,Ni5P4,and NiP2),NiP2displays better stability toward oxidation,because the corrosion tolerance of them improves with increasing P content[19].For this reason,herein,we report a composite of g-C3N4with NiP2synthesized by homogeneous precipitation method assisted via thermal phosphorization reaction.In this structure,g-C3N4provides the driving force required for the photocatalytic reaction,while NiP2as a co-catalyst provides active sites for hydrogen production,which solves the shortcoming of fast recombination of electron-hole pairs and high overpotential in the process of interface reaction.Experimental studies showed the separation and transfer of charge carriers were obviously improved,and theoretical calculation demonstrated that the built-in electronic field in the interface facilitated the transfer of electrons form g-C3N4to NiP2surface.This work represents a useful method through combing experiment and theoretical calculation to investigate the mechanism of photocatalyst.
2.Materials and Methods
2.1.Synthesis of g-C3N4 with vesicle structure
All chemical reagents were analytical grade and used without further purification.The g-C3N4with vesicle structure was synthesized via two steps.Firstly,4 mmol melamine and 4 mmol cyanuric acid were added into 50 ml methanol,and the homogeneous suspension was vigorous stirring for 48 h at room temperature.Then the product was collected by centrifugation and dried to obtain the white product.Secondly,the product was put into glass bottle with aluminum foil sealing and annealed for 4 h at 550°C with heating rate of 2.3 °C·min-1in a muffle furnace.The faint yellow g-C3N4with vesicle structure was obtained and labeled as CN.
2.2.Preparation of NiP2/g-C3N4 heterojunction
The NiP2/g-C3N4heterojunction was synthesized by homogeneous precipitation method assisted by thermal phosphorization treatment.In a typical process,0.1 g CN,0.03 g NiCl2.6H2O and 0.5 g urea were added into 30 ml deionized water.The mixture was stirring for 4 h at 90°C in oil bath,and the suspension was centrifuged,washed by deionized water and ethanol for one time,respectively.The product was dried in an oven at 60 °C for 12 h.Secondly,0.1 g obtained product and 1g NaH2PO2were respectively placed in the both end of a rectangle porcelain boat.The whole porcelain boat was packaged with aluminum foil and put into a tube furnace,then it was heated from room temperature to 450 °C with a heating rate of 4 °C·min-1and kept at 450 °C for 4 h in N2atmosphere.The final black product was obtained and labeled as PCN (or PCN-2).For comparison,under the same condition,the CN with different mass ratio NiP2were prepared(the mass ratio are 20:1 and 20:5),and those samples were denoted as PCN-1 and PCN-3.
2.3.Characterizations
The microstructure and morphology of all samples were observed by the field emission scanning electron microscope and(FE-SEM;FEI Quanta F250,200 kV) and transmission electron microscopy (TEM;JEOL,JEM-2100).X-ray diffraction (XRD;SHIMADZU,Lab X XRD-6100) and X-ray photoelectron spectrometer(SPECS,Germany) were deployed to investigate the crystal phase,surface chemical composition and binding state of all samples.The optical properties were depicted by UV–vis diffuse reflection spectra using a UV-2600 UV–vis spectrometer (SHIMADZU) with BaSO4as the reference.Using FT-IR spectrophotometer (Nicolet iS50,ThermoFisher) and FLS980 fluorescence spectrophotometer(Edinburgh Instruments)recorded the FT-IR spectra and the photoluminescence (PL) spectra,respectively.The textural properties were obtained by a BET analyzer (BELSORP-Max,Microtrac BEL),and the specific surface area and pore size distribution were calculated by BET and BJH method.
2.4.Photocatalytic H2 evolution
The photocatalytic activity test was carried out in a Pyrex reaction cell using a 300 W Xenon lamp (Nbet,HSX-F/UV300) as the visible-light source.In general,10 mg sample was dispersed in 90 ml aqueous solution mixing with 10 ml triethanolamine as sacrificial electron donor.It is noted that no precious metals (Pt) are added into the reaction cell.Then the system was degassed with N2for 5 min to remove oxygen before reaction.And the amount of H2generation was analyzed by an online gas chromatography(GC) with a TCD detector.The rate of H2generation was obtained by averaging the rate of first 3 h H2production.
2.5.Photoelectrochemical measurement
The transient photocurrent responses were tested by electrochemical station (CHI 660D,Chenhua,China) in a typical threeelectrode system.The FTO glassy (0.64 cm2) modified with photocatalysts used as work electrode,and a platinum wire and Ag/AgCl electrode were employed as the counter and the reference electrode,respectively.For preparing work electrode 10 mg sample,1 ml acetone and 10 μl nafion were ultrasonically mixed to form slurry.Then,400 μl slurry was dropped onto the surface of FTO(2.8 cm2),dried at 120°C for 2 h to obtain work electrode.The electrolytes was KCl solution (1 mol·L-1).
2.6.DFT calculations
All DFT-D2 calculations were based on the Vienna ab initio simulation package (VASP),and the Perdew-Burke-Ernzerhof (PBE)function was adopted for the calculation.The cutoff energy was 520 eV with a plane-wave basis to ensure the accurate result.The Gaussian smearing width was set to 0.2 eV.The K points in Brillouin zone were set to 3 × 3 × 1 Monkhorst Pack grid.The structural optimization were finished until the energy on each atom was less tha 1×10-4eV and the forces on each tom were less than 0.5 eV.nm-1[20].The g-C3N4(0 0 2) facet and NiP2(1 1 1)facet were chosen to construct heterostructure with graphene for obtaining well lattice match.Using 1.0 nm vacuum regions minimizes the interactions between the neighboring systems.The optimized structures,work functions,electron density difference at the interface,free energies and transition states were obtained using PBE functional.The hybrid hse06 functional with the values of α=0.25 and ω=0.2 were used for calculating band gaps and density of states (DOS) of g-C3N4,NiP2and NiP2/g-C3N4.
3.Results and Discussion
The morphological and structural information of CN and PCN were obtained by SEM and TEM,respectively.As displayed in Fig.1(a)–(c),the g-C3N4has a hollow vesicle structure with diameter of about several hundred nanometers,which as a substrate can provide a lot of sites for the loading of NiP2nanoparticles on their hollow vesicle structure surface.So,in Fig.1(d),it can be distinctly found many NiP2nanoparticles with diameter of about 200 nm were uniformly distributed on the surface of CN.To further obtain more precise structural information,the sample was filmed by TEM.As shown in Fig.1(e)and(f),the NiP2nanoparticles cover on the surface of g-C3N4vesicle,forming tight interface connection.Through the HRTEM image (Fig.1(f)),the lattice fringe spacing of 0.273 nm can be indexed to the (2 0 0) facets of cubic NiP2[21],while the lattice fringe of g-C3N4was not observed due to its low crystallinity and covered surface.Additionally,the chemical composition of PCN was further analyzed by the elemental mapping and energy-dispersive X-ray (EDX) spectroscopy measurements.As displayed in Fig.2,the EDX result reveals the co-existence of C,N,P,Ni.The elemental mapping images straightly demonstrate the P and Ni homogeneously disperse on the g-C3N4vesicle surface building a tight interface connection between the NiP2nanoparticles and g-C3N4,which facilitates the transfer of charge carriers though the interface.
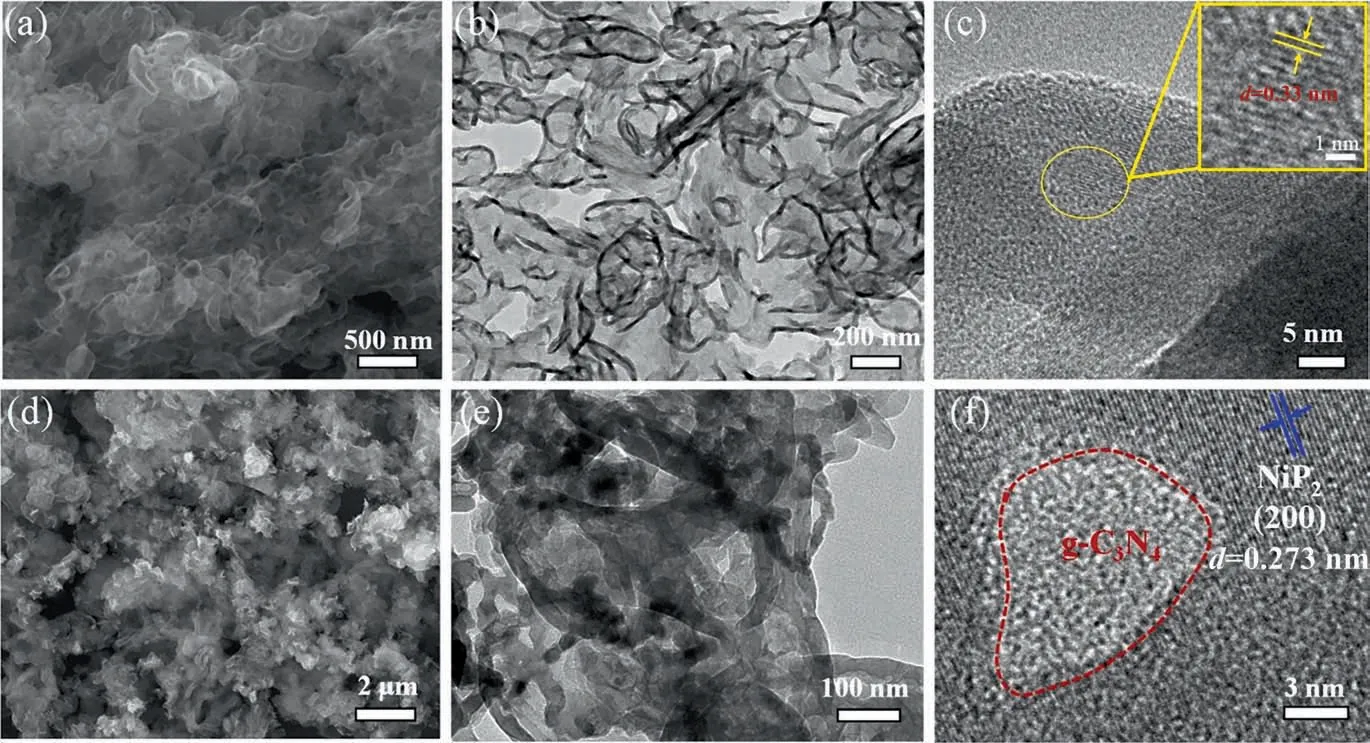
Fig.1.SEM (a),TEM (b) and HRTEM (c) images of CN;SEM (d),TEM (e) and HRTEM (f) images of PCN.

Fig.2.SEM image(a),the corresponding EDX spectrum of PCN(b)and EDX elemental mapping images of C(c),N(d),P(e)and Ni(f)in the selected area of SEM image(a)of PCN nanocomposite.
Fig.3(a) exhibited the X-ray diffraction (XRD) patterns of PCN together with the pure g-C3N4samples.Two characteristic diffraction peaks of pure g-C3N4are observed at 12.66° and 27.58°,and the strong peak and the weak one respectively index to (0 0 2)and(1 0 0)diffraction plane,severally from interplanar and stacking in-plane repeating units of the aromatic system.[22].The diffraction peaks at 13.06° and 27.86° in PCN can be assigned to(0 0 2) and (1 0 0) diffraction plane of g-C3N4,compared with the CN,which both slightly shift to the high degree due to the denser stack and the decrease of gallery distance caused by heat treatment process.Additionally,all characteristic diffraction peaks of cubic NiP2(PDF# 21-0590) are observed in PCN,and the peaks at 28.21°,32.87°,36.84°,40.52°,47.17°,55.86°,61.21° and 63.78°can be well assigned to the (1 1 1),(2 0 0),(2 1 0),(2 1 1),(2 2 0),(3 1 1),(2 3 0)and(3 2 1)diffraction plane of NiP2,suggesting that NiP2was well prepared[23,24].As displayed in FT-IR pattern (Fig.3(b)),the absorption peaks at around 806 cm-1and the region of 1200–1700 cm-1can be attributed to the breathing modes of s-triazine and the stretching vibration of CN heterocycles.The absorption peak at 3165 cm-1is in agreement with the residual N-H components from secondary and primary amines[25,26].No obvious absorption peaks of NiP2is detected in FT-IR pattern of PCN.The X-ray photoelectron spectrum(XPS)analysis was used to analyze the chemical composition and binding states of the PCN sample.The XPS peaks of C1s at 284.5,287.8 and 288.36 eV are assigned to C-C,C=N,and N-C=N bond in Fig.3(c).And as shown in Fig.3(d),the N1s peaks at the bonding energy of 398.5,400.48 and 404.29 eV are indexed to the C-N=C bond and N-(C)3bond in g-C3N4structure and the oxidized state N in the surface,respectively[27,28].The Ni 2p3/2and 2p1/2orbit in NiP2were detected at 835.2 and 870.57 eV,from Ni ions in NiP2.Besides,the XPS peaks of oxidized Ni2+species are also observed at 856.02 and 874.30 eV,and there are two satellite peaks at 861.02 and 879.42 eV.In Fig.3f,the peaks at 128.44 and 129.22 eV can be indexed to P 2p3/2and 2p1/2orbit in NiP2,proving the successful synthesis of NiP2[17].The peak at 127.82 eV represents the P-H bond,which may be originated from the incomplete reaction of PH3.In addition,the peak of oxidized P species also had been detected at 133.89 eV,which commonly observed on the surface of phosphide [18].

Fig.3.(a) XRD of CN,PCN-1,PCN-2 and PCN-3,(b) FT-IR DRS spectra of CN and PCN.XPS patterns of PCN,(c) C 1s,(d) N 1s,(e) Ni 3d,(f) P 2p.
To further observe the structure information,the N2adsorption–desorption isotherms and the pore size distribution curves of CN and PCN were showed in Fig.4.The CN and PCN have similar adsorption–desorption isotherms.According to IUPAC classification,they are the typical IV isotherm with a H3hysteresis loop,indicating that the pore size distribution belongs to the mesoporous region [29].The existence of mesoporous can be further confirmed by the corresponding pore size distribution (Fig.4(b)),which mainly belongs to the mesoporous range at 10–60 nm.As summarized in Table 1,both the average pore diameters of CN and PCN were calculated as 25.75 nm by Barrett-Joyner-Halenda(BJH)method.The Brunauer-Emmett-Teller(BET)specific surface areas of pristine CN and PCN were 18 m2∙g-1and 14 m2∙g-1,and the pore volume for CN and PCN were 0.21 and 0.18 m2∙g-1,respectively.The slight decreases of surface area and pore volume after combination suggest to the surface of g-C3N4was covered by the NiP2nanoparticles.
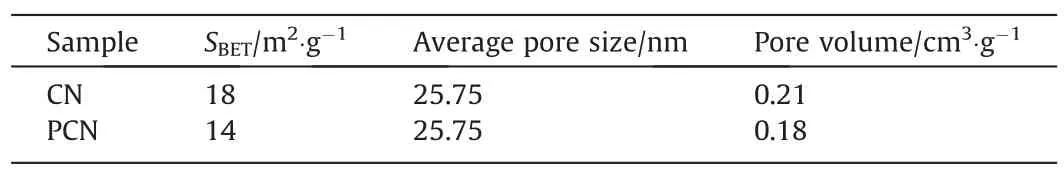
Table 1 Textural properties of CN and PCN
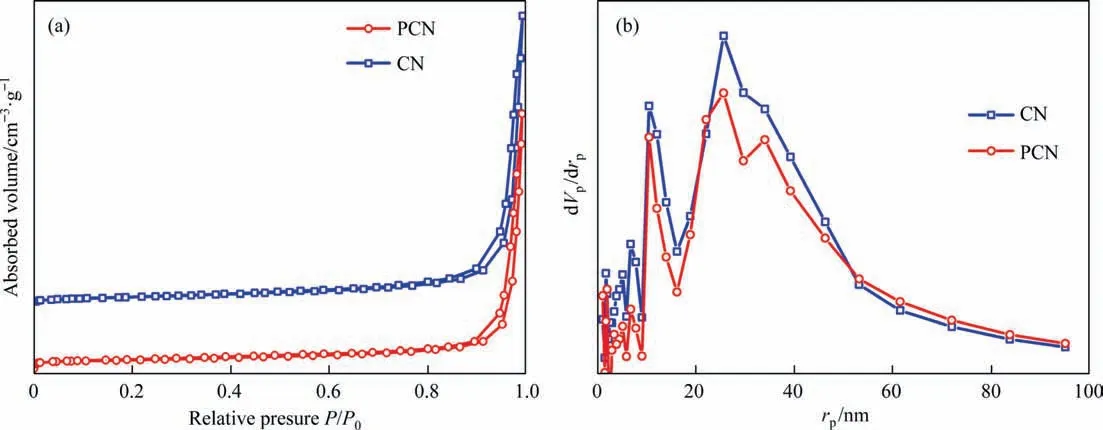
Fig.4.(a) N2 adsorption–desorption isotherms and (b) corresponding Barrett-Joyner-Halenda (BJH) pore-size distribution.
Fig.5(a)shows the transient photocurrent responses of various samples at a bias potential of 0.7 V.It is clear found that pure g-C3N4shows the weakest photocurrent response,illustrating a poor separation and transfer efficiency of photo-induce charge carries.However,when a few Ni2P nanoparticles to decorate the g-C3N4,the samples of NiP2/g-C3N4composited with different mass ratios of NiP2to g-C3N4(especially PCN-2),show a higher photocurrent response.It is indicated that the NiP2as a cocatalyst can vastly accelerate the separation of electron-hole pairs.To further investigate the separation and migration ability of charge carries over the as-obtained different samples,the electrochemical impedance spectroscopy (EIS) were also measured.As manifested in Fig.5(b),the samples of NiP2/g-C3N4,especially PCN-2,have the smaller arc diameter in the EIS Nyquist plot,which means the lower charge transfer resistance compared with that of pure g-C3N4,originating from numerous interfacial channels between g-C3N4and NiP2accelerating charge transfer[30].As shown in Fig.5(c),the separation rate and charge motion dynamics of photo-induced electronhole pairs were also investigated by the photoluminescence (PL).The pure g-C3N4shows the most intense PL signal for its high recombination of electron-hole pairs.With the introduction of NiP2,the PL intensity of PCN sample has an obvious decrease,and it indicates that the charge transfer and separation are effectively boosted.Fig.5(d) exhibited the electrochemical characterization of CN and PCN samples.The found cathode current in the range of -0.4 to -1.0 V vs.reversible hydrogen electrode (RHE)can be attributed to H2evolution occurring at the electrode [15].Compared with the pure g-C3N4,the PCN sample shows a much lower overpotential for hydrogen generation.This is the reason that PCN showed a better hydrogen production performance.

Fig.5.(a)Transient photocurrent responses versus time,(b)Electrochemical impedance spectroscopy(EIS)Nyquist plots,(c)PL spectra and(d)Polarization curves of g-C3N4 and NiP2/g-C3N4 composited with different mass ratios of NiP2 to g-C3N4.
As shown in Fig.6(a) and (b),CN possesses strong ultraviolet absorption property with absorption edge at 450 nm,which can be figure out by the transformed Kubelka-Munk function that the band gap of CN is about 2.54 eV.However,when coupling between g-C3N4and NiP2,the sample of PCN showed an excellent light absorption in both ultraviolet and visible light range.For obtaining more insight electronic structures of g-C3N4,NiP2and NiP2/g-C3N4,the band structure,density of states (DOS) and partial density of state (PDOS) were calculated by using HSE06.As shown in Fig.6(c),the monolayer g-C3N4is an indirect band gap semiconductor in which valence band maximum is located at G point,whereas the conduction band minimum is at M point.The calculated band gap is 2.77 eV,roughly accordant with the test results (2.54 eV).Furthermore,in Fig.6(d),the total density and project density of state indicate that the VBs of g-C3N4are mainly occupied by N 2p orbitals and CBs are dominated by N 2p and C 2p orbitals[31].And as shown in Fig.6(e),the band gap of the bulk NiP2is calculated to be 0 eV,in accordance with its Metal-like properties[32].Thus NiP2can be used as a good acceptor of electrons from g-C3N4.After combination of monolayer g-C3N4and monolayer NiP2,the heterojunction of NiP2/g-C3N4shows a typical direct band structure with a band gap of 0.85 eV.The VBM and CBM are both found at G point,and the CB and VB of PCN are dominated by orbitals of N and P (in Fig.6(f)).

Fig.6.(a)UV–vis diffuse reflectance spectra(DRS)for CN and PCN,(b)plots of(ahv)1/2 versus energy(hv)for CN,calculated band structures(c)g-C3N4 and(e)pure NiP2,TDOS of single-layer (d) g-C3N4 and (f) pure NiP2.
The work function,numerically equal to the difference between vacuum and Fermi levels,is an important parameter in investigating the energy band alignment and charge transfer of semiconductor heterostructure.The work function of monolayer NiP2and g-C3N4could be obtained by computing the electrostatic potential of their slab models.As shown in Fig.7(a) and (b),the work functions of the monolayer NiP2and g-C3N4are 5.17 and 5.45 eV.The work function of NiP2is higher than that of monolayer g-C3N4,which indicates that the electrons would flow from the NiP2to the g-C3N4surface when the monolayer NiP2interacted with the g-C3N4nanosheets to form the heterojunction [33].As the result,the surface of g-C3N4would accumulate negative charges,as the NiP2surface would gather positive charges,further proofed by electron density difference along with Z direction of single-layer NiP2/g-C3N4.As shown in Fig.7(d),the yellow and cyan area represents the electron accumulation and depletion.At the interface of NiP2/g-C3N4heterojunction,the g-C3N4surface is mainly dominated by yellow area,while the surface of NiP2is filled by cyan area[34].The Bader charge analysis for the charge densities of NiP2/g-C3N4heterojunction is adopted to quantify the transfer of charges,and it indicates that 0.07 electrons would transfer from the surface of NiP2to the surface of g-C3N4.The net positive and negative charges induce an internal electric field from NiP2to g-C3N4,which produced another force to the holes and electrons with the opposite direction of the diffusion force.In thermal equilibrium,two forces achieves the balance,and a built-in electric field at the interface is formed,so the photoexcited electrons would transfer from the g-C3N4surface to the NiP2surface under its driving,as shown in Fig.7(e).
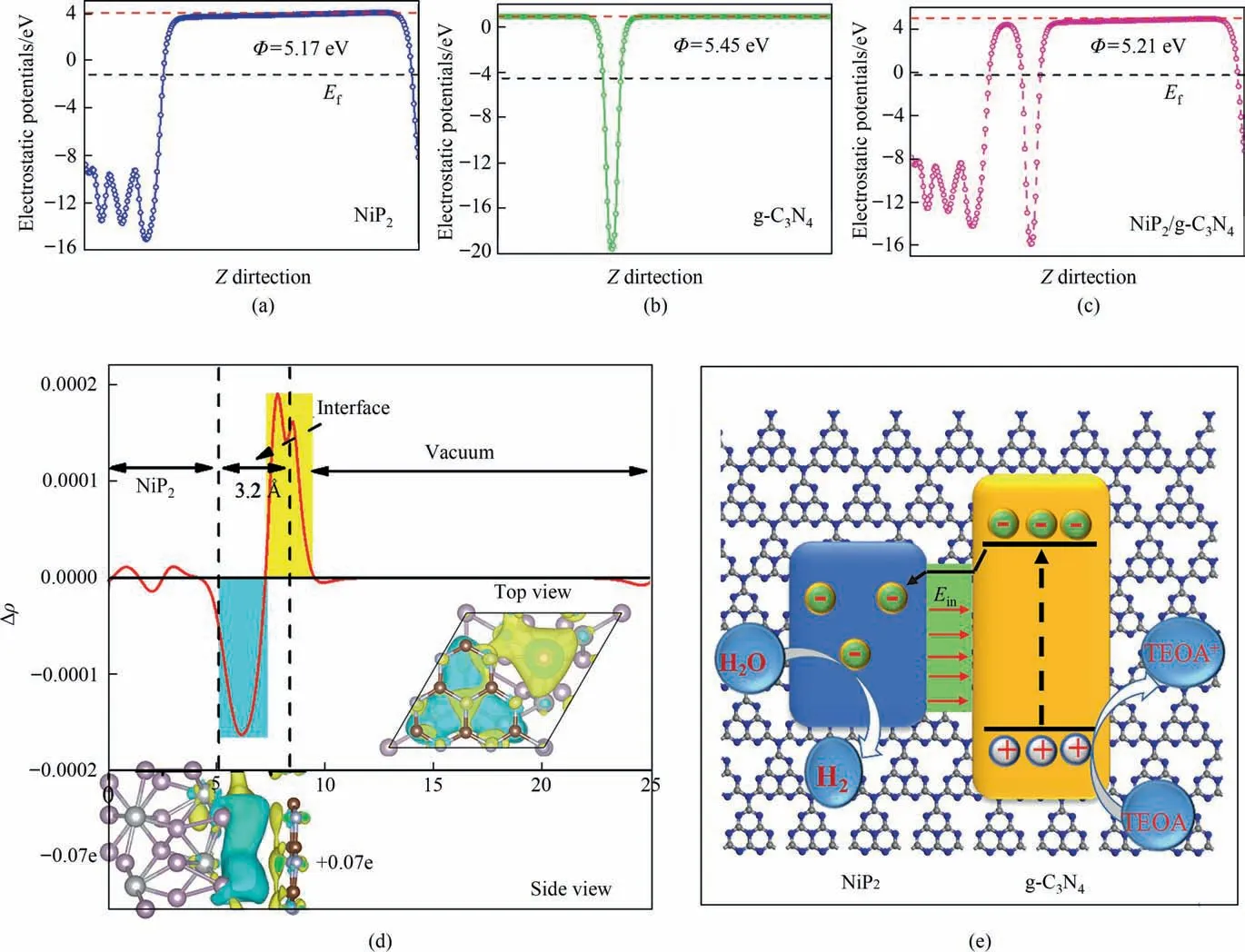
Fig.7.Calculated electrostatic potentials for (a) NiP2,(b) g-C3N4 and (c) NiP2/g-C3N4.(d) Planar-averaged electron density difference along with Z direction of single-layer NiP2/g-C3N4.(e) Scheme illustrating the principle of charges transfer at the interface of NiP2 and g-C3N4.
In order to clarify the hydrogen evolution capability of different crystal planes,the hydrogen adsorption free energy(298 K)of NiP2and g-C3N4typical crystal planes was calculated.The common view is that this value close to zero benefits for hydrogen evolution[35,36].As shown in Fig.8(a),compared with the g-C3N4characteristic crystal plane (0 0 2),the (0 1 1) and (1 1 1) crystal planes of NiP2have better hydrogen evolution ability.Thus,hydrogen desorption and production on these two surfaces are more favorable.Therefore,NiP2can be used as an active site for H2production and accelerate the H2production rate.To investigate the dissociation adsorption of H2O on the catalyst surface,the transition state of water dissociation adsorption on the catalyst surface was calculated by NEB method (Fig.8(b)).The results shows that the dissociation adsorption energy barrier of water on the characteristic crystal surface (0 0 2) of g-C3N4was 3.71 eV.Compared with the NiP2surface,the energy barrier of g-C3N4is relatively low.However,the energy barriers of the dissociation adsorption of H2O on the surfaces of g-C3N4are difficult to overcome at room temperature under visible light.Therefore,it was inferred that the hydrogen is not produced by the direct dissociation and adsorption of water,but by reaction with H+ions in aqueous solution to generate hydrogen gas.
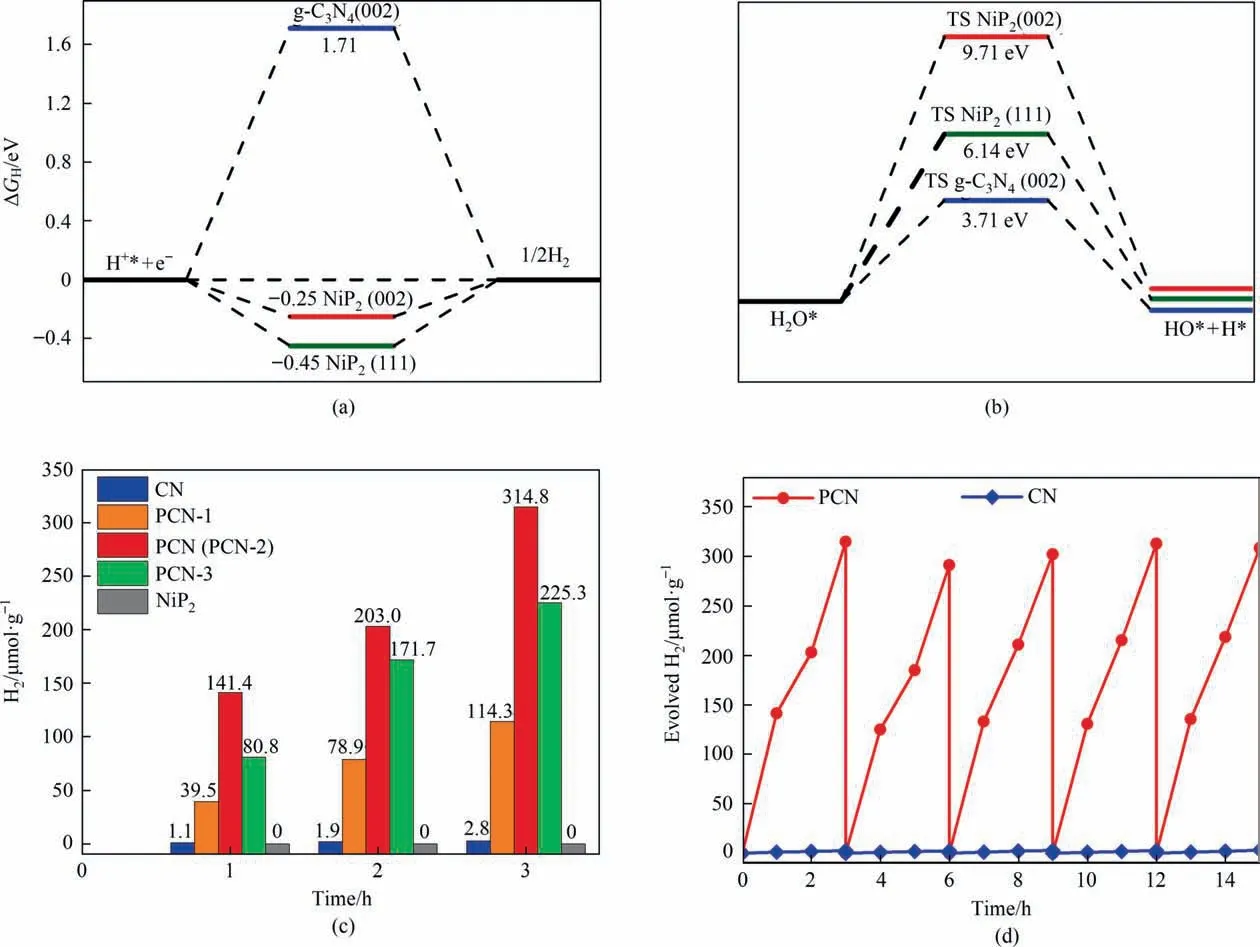
Fig.8.(a) Hydrogen adsorption free energies on different crystal surfaces of g-C3N4 and NiP2,(b) NEB transition state calculations for water splitting on g-C3N4(0 0 2),NiP2(2 0 0) and NiP2(0 1 1),(c) plots of photocatalytic H2 evolution amount vs.irradiation time.(d) recycling H2 evolution curve of CN and PCN for 5 runs.Photocatalytic activity test conditions:visible-light irradiation (λ>420 nm),1 sun,25 °C,photocatalyst 10 mg,deionized water 90 ml,triethanolamine 10 ml and assisted by continuous stirring.
In order to evaluate the activity of the as-prepared samples,the photocatalytic H2evolution from water splitting was tested under visible-light irradiation(1 sun,λ >420 nm,25 °C).As displayed in Fig.8(c),the PCN (PCN-2) sample shows the highest hydrogen generation activity (105 μmol.g-1.h-1),which is 112 times than that of the pure g-C3N4(0.9 μmol.g-1.h-1) and infinitely fold than the NiP2(0.0 μmol.g-1.h-1).Additionally,in compared with PCN(PCN-2) sample,by decreasing or increasing the NiP2,the photocatalytic H2evolution activity are more or less decreased.And it’s worth noting that the hydrogen production rate of the PCN sample had no obvious decline after five consecutive cycles (in Fig.8(d)),which demonstrates that PCN has a good stability during reaction.
According to the above experimental and the calculated results,the charge transfer and photocatalytic generation of H2in PCN composite are proposed.Under visible light illumination,g-C3N4excites large number of electron and hole pairs.But under the driving force of NiP2/g-C3N4built-in electric field,the electrons on the surface of g-C3N4can fast transfer to the NiP2surface.This process can effectively inhibit the hole-electron combination and extend the lifetime of charge carriers.Finally,those electrons on NiP2surface can react with H+ions in aqueous solution to generate hydrogen gas.Additionally,due to the load of the NiP2nanoparticles on the surface of g-C3N4,the overpotential of hydrogen evolution reaction can be effectively reduce,so the hydrogen evolution rate has been effectively promoted [37].
4.Conclusions
In this study,NiP2/g-C3N4heterojunctions were synthesized by homogeneous precipitation assisted by hot phosphating reaction.The samples have an obvious improvement of photocatalytic activity compared with the pure g-C3N4.The results of experiments and DFT calculations show that the g-C3N4with NiP2nanoparticles can construct the interface electron transfer channels and inhibit the combination of electron-hole pairs.And the low energy barriers of hydrogen evolution reaction on NiP2crystal planes can reduce the overpotential and facilitate the surface reaction.This research gives a typical case to understand the function of phosphorous co-catalyst for designing high efficient photocatalyst.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work is supported by the National Natural Science Foundation of China (Grant Nos.U1862105,22108214,22050410267),Natural Science Basic Research Plan in Shaanxi Province of China(Grant Nos.2017JZ001,2018KJXX-008),Fundamental Research Funds for the Central Universities (Grant No.cxtd2017004),China Postdoctoral Science Foundation(Grant No.2021TQ0262),the Promotion Plan for Young People of Shaanxi Association for Science and Technology(20210605)and K.C.Wong Education Foundation,Hong Kong,China.
Author Contributions
Xiaoqing Yan and Hua An contributed equally to this work.Xiaoqing Yan conducted materials synthesis and analysis,Hua An performed materials measurements,data collection and simulations by DFT.Zihao Chen carried out photocatalytic activity testing.Guidong Yang designed the experiments,conceived the project and supervised the research.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Editorial for special issue on Carbon-neutrality Chemical Engineering
- Design and experiment of high-productivity two-stage vacuum pressure swing adsorption process for carbon capturing from dry flue gas
- 3D multiphase flow simulation of Marangoni convection on reactive absorption of CO2 by monoethanolamine in microchannel
- Top-down strategy for bamboo lignocellulose-derived carbon heterostructure with enhanced electromagnetic wave dissipation
- Structural reconstruction of Sn-based metal–organic frameworks for efficient electrochemical CO2 reduction to formate
- The effect of different Co phase structure (FCC/HCP) on the catalytic action towards the hydrogen storage performance of MgH2
