The effect of different Co phase structure (FCC/HCP) on the catalytic action towards the hydrogen storage performance of MgH2
2022-04-27LiutingZhangHaijieYuZhiyuLuChanghaoZhaoJiaguangZhengTaoWeiFuyingWuBeibeiXiao
Liuting Zhang,Haijie Yu,Zhiyu Lu,Changhao Zhao,Jiaguang Zheng,*,Tao Wei,*,Fuying Wu,Beibei Xiao,*
1 School of Energy and Power,Jiangsu University of Science and Technology,Zhenjiang 212003,China
2 Analysis and Testing Center,Jiangsu University of Science and Technology,Zhenjiang 212003,China
Keywords:Hydrogen storage MgH2 FCC/HCP Co particles Catalysis Density functional theory
ABSTRACT High hydrogen desorption temperature and sluggish reaction kinetics are the major limitations for the practical application of MgH2.In this study,Co particles with a face centered cubic (FCC) structure and a hexagonal close packed(HCP)structure were prepared facilely and proved to be good catalysts for magnesium hydride.Co particles with FCC structure presented better catalytic effect on MgH2 than that with HCP structure.Both 7% (mass) Co FCC and HCP particle modified MgH2 decreased the initial dehydrogenation temperature from 301.3 °C to approximately 195.0 °C,but 7% (mass) Co with FCC structure modified MgH2 has a faster desorption rate,and around 6.5% (mass) H2 was desorbed in 10 min at 325 °C.Hydrogen uptake was detected at 70 °C under 3.25 MPa hydrogen pressure and 6.0% (mass) H2 was recharged in 40 min at 150 °C.The hydrogen desorption and absorption activation energy for 7%(mass) FCC Co modified MgH2 was significantly decreased to (76.6±8.3) kJ.mol-1 and (68.3±6.0)kJ.mol-1,respectively.Thermodynamic property was also studied,the plateau pressures of MgH2+7%(mass) FCC Co were determined to be 0.14,0.28,0.53 and 0.98 MPa for 300 °C,325 °C,350 °C and 375°C.The decomposition enthalpy of hydrogen (ΔH) for MgH2+7% (mass) FCC Co was (80.6±0.1)kJ.mol-1,5.8 kJ.mol-1 lower than that of as-prepared MgH2.Moreover,cycling performance for the first 20 cycles revealed that the reaction kinetics and capacity of MgH2-FCC Co composite remained almost unchanged.The result of density functional theory calculation demonstrated that cobalt could extract the Mg-H bond and reduced the decompose energy of magnesium hydride.Our paper can be presented as a reference for searching highly effective catalysts for hydrogen storage and other energy-related research fields.
1.Introduction
With the deterioration of global environment and the shortage of fossil fuel resources,an increasing number of scientists are exploring new environmental-friendly energy alternatives [1–3].Hydrogen energy,which occupies a high calorific value and produces only water after combustion,is hopeful to switch the traditional carbon-based energy economy to a clean and sustainable hydrogen based one [4–6].
It is of great significance to adopt high-capacity and safe storage and transportation techniques of hydrogen for the implementation of hydrogen energy.Solid-state hydrogen storage,especially metal hydrides,is regarded as one of the safest and most efficient hydrogen storage solutions[7].Among the hydrides studied so far,MgH2with advantages of abundant resource,high capacity of 7.6%(mass)and low cost,shows great potential to the realization of hydrogen storage on board [8,9].However,the widespread use of MgH2in practical applications is still hampered by its slow reaction kinetics and stable thermodynamics.In order to solve above technological difficulties,numerous modification methods have been attempted,including alloying [10–12],nanosizing [13–16] and catalysts doping [17–19].
As one of the most feasible modifying strategies,catalyst doping can significantly accelerate the de/rehydrogenation kinetics of MgH2[20–31].In previous researches,transition metal particles have been proved as highly active catalyzing agents for MgH2.For instance,Dmytro et al.proved that the dehydrogenation activation energy for 25% (mass) Ti doped MgH2was decreased from 135 kJ.mol-1to 53.6 kJ.mol-1[21].Pukazhselvan et al.evidenced that the addition of 3% (mass) Fe in MgH2reduced the hydrogen desorption temperature to about 270 °C [32].Hanada et al.found that the MgH2+2% (mass) nano-Ni,prepared by a short mechanical milling at low rpm,could release about 6.5%(mass)from 150°C to 250 °C [33].
As a traditional transition metals,cobalt and cobalt-related compounds are widely adopted to be catalysts for hydrogen storage.Gao et al.reported that nano-sized CoB dispersed on CNTs could decrease the onset dehydrogenation temperature of MgH2to about 214 °C with a hydrogen capacity of 6.62% (mass).Hydrogenation at 300°C revealed that fully dehydrogenated MgH2+10%(mass)CoB/CNTs uptook around 5.7%(mass)H2in 20 s.Moreover,the activation energy of this composite was 89.1 kJ.mol-1,much lower compared to 208.5 kJ.mol-1of pure MgH2[34].Liu et al.evidenced that Mg-Co/Pd@B-CNTs nano-composite rapidly uptook 6.68%(mass)H2in 10 s at 250°C and the activation energy of this composite was reduced to 76.6 kJ.mol-1[35].Xu et al.proved the Co@C modified MgH2system started to desorb hydrogen at around 170 °C and over 6.0% (mass) could be released in the end [36].
It is well known that different morphology and crystal structure can be a vital factor for the catalytic performance of catalysts.He et al.evidenced that different crystal structure in MnO2resulted in different catalytic effects [37].Zhang et al.reported that TiO2nanosheets with {001} surface had a prominent catalytic effect on improvement of the dehydrogenation kinetics of MgH2.The MgH2+TiO2NS sample can release hydrogen before 180 °C and released 6.0%(mass)hydrogen within 3.2 min at 260°C.The Dehydrogenation Eacan be decreased from 186.78 kJ.mol-1of pure MgH2to 67.64 kJ.mol-1of MgH2+TiO2NS [38].
Wang et al.reveal that cobalt presents different phases at different deposition potentials,HCP phase at lower deposition potential,and FCC phase at higher deposition potential[39].In this paper,we successfully prepared two Co particles with different crystal structures (FCC/HCP) and the effect of phase structure on the catalytic action was compared.The remarkably enhanced hydrogen storage performance of FCC/HCP Co modified MgH2was verified through a Sieverts-type apparatus and Differential Scanning Calorimetry(DSC).Moreover,the role of Co played in catalyzing the de/rehydrogenation of MgH2was systematically studied by means of microstructure analysis and theoretical calculations.Based on above investigation results,the catalytic mechanism of Co was also discussed.
2.Experimental
2.1.Sample preparation
Cobalt with HCP structure was synthesized by the reaction of CoCl2and LiH.In brief,2.67 g CoCl2,0.32 g LiH and 120 g balls were milled under an Ar atmosphere for 20 h at a rotary speed of 450 r.min-1(QM-3SP4,Nanjing Chi Shun Technology Development Co.,Ltd).After ball milling,the mixture was washed by centrifugation with tetrahydrofuran and finally vacuum-dried for 12 h at room temperature.
Cobalt with FCC structure was prepared according to a reported paper[27,40].2 g Co powders were mixed with 0.75 ml oleic acid,0.25 ml oleyl amine,15 ml heptane and 80 g balls and milled under an Ar atmosphere for 45 h at a rotary speed of 450 r.min-1.The whole slurry was washed using alcohol in centrifuge tubes for 8 times to remove remaining organic solvents.Ultimately,submicron Co particles were vacuum dried at room temperature for 12 h.
MgH2was prepared in our lab [41].6 g Mg powders were heated under 6.5–7 MPa hydrogen atmosphere at 380 °C for 2 h;after this,the samples were milled under an Ar atmosphere for 5 h at a rotary speed of 450 r.min-1;next the samples after ball milling were continuously heated (380 °C,6.5–7 MPa),followed by 5 ball milling and several tamping;finally,the samples were further hydrogenated for 2 h.To prepare MgH2+Co composites,different amount of as-prepared FCC/HCP Co particles was ball milled (450 r.min-1,0.1 MPa Ar atmosphere) with as-synthesized MgH2for 2 h,denoted as MgH2+x% (mass) FCC/ HCP Co (x=1,3,5,7,9).The ratio of ball to material was 40:1.The information of chemicals used are shown in Table 1.To avoid oxygen and moisture contamination,all the materials were stored and handled in an Argon-filled glovebox (Mikrouna).
Table 1 Information of different samples (company,purity)
2.2.Sample characterization
X-ray diffraction (PANalytical,the Netherlands) and Transmission electron microscopy with X-ray energy spectrometer (Tecnai G2 F20,HORIBAX-MAX) were performed to analyze the microstructure of the samples.Moreover,Differential Scanning Calorimetry (Netzsch STA449F3) measurements were operated at different heat-up rates (5,8,10,12 °C.min-1) from 25 °C to 500°C with flowing argon.The dehydrogenation and rehydrogenation properties of the samples were conducted using a laboratorymade Sieverts-type apparatus.For non-isothermal dehydrogenation process,around 200 mg samples were heated from 25 °C to 400 °C at 2 °C.min-1while non-isothermal hydrogenation experiments were carried out under 3.2 MPa hydrogen pressure,using a heating rate of 2 °C.min-1from 25 °C to 400 °C.In isothermal mode,the samples were quickly heated to designed temperature and maintained constant for the whole process.
2.3.Theoretical methods
All calculations of density functional theory (DFT) were performed using the DMol3code [42,43].The exchange and correlation effects were considered by the generalized gradient approximation with the Perdew-Burke-Ernzerhof functional(GGA+PBE) [44].Relativistic effects were implemented by a DFT semi-core pseudopods (DSPP) core treatment method [45].The double numerical atomic orbital augmented by a polarization function (DNP) was selected as the basis set [46].A smearing of 0.005 Ha (1 Ha=27.21 eV) to the orbital occupation was applied to the orbital occupation.The maximum force level,convergence tolerances of energy level,and displacement level were set as 0.02 Ha.nm-1,1.0 × 10-5Ha and 5.0×10-4nm in the geometric structural optimization,separately.
3.Results and Discussion
3.1.Hydrogen storage properties of Co doped MgH2 system
The microstructure of Co particles were analyzed by XRD measurement.The XRD patterns of Co particles are showed in Fig.1.The black curve has four distinct peaks correspond to the PDF card of Co (PDF#05-0727),implying that the synthesized Co with a hexagonal close packed (HCP) structure.There is only one peakappearing at around 45 degrees for the ball-milled Co,correspond to the PDF card of Co (PDF#15-0806),which means that asprepared Co has a face centered cubic (FCC) structure.

Fig.1.XRD patterns of different Co particles.
To study the catalytic effect of different Co on the hydrogen storage property of MgH2,non-isothermal hydrogen desorption curves of MgH2,and MgH2+7% (mass) HCP Co and MgH2+7%(mass) FCC Co were compared in Fig.2.After the addition of catalyst,the hydrogen desorption performance of MgH2was significantly improved.Although initial dehydrogenation temperatures of the two samples were both reduced from 301.3 °C (pristine MgH2)to 195°C,MgH2+7%(mass)FCC Co released approximately 6.5%(mass)hydrogen with a terminal temperature of 280°C while MgH2+7% (mass) HCP Co released about 6.7% (mass) hydrogen with a higher terminal temperature of 336°C.This result indicated that cobalt with different structures has different influence on the hydrogen desorption performance of MgH2and Co with FCC structure is superior on improving the desorption kinetics of MgH2than that with HCP structure.
To explore the best doping amount of Co particles,Fig.3 compares the non-isothermal hydrogen desorption properties of MgH2+x% (mass) FCC Co (x=1,3,5,7 and 9) composites with additive-free MgH2.It can be noticed that with the increasing doping amount of FCC Co,the initial dehydrogenation temperature of MgH2reduces significantly.The increase in the catalyst concentration also leads to the decrease in the amount of hydrogen released.The MgH2+7%(mass)FCC Co and MgH2+9%(mass)FCC Co composites started to release hydrogen at around 195°C,115°C lower than that of MgH2.By synthetically comparing the release temperatures,dehydrogenation rates and amounts of hydrogen released from Table 2,7%(mass)doping amount occupies the best dehydrogenation behavior.
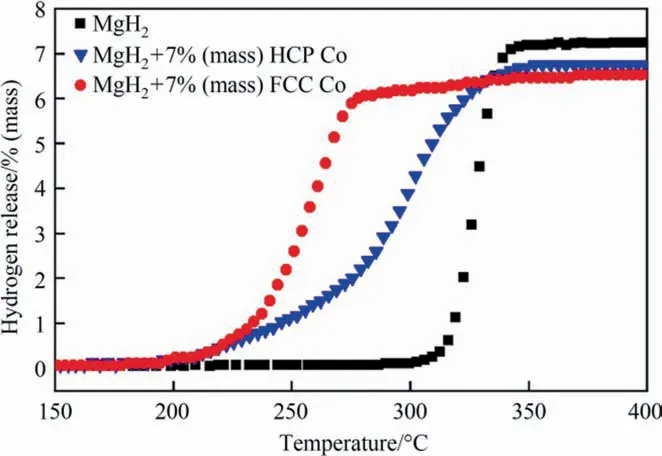
Fig.2.Non-isothermal desorption curves of MgH2,MgH2+7% (mass) HCP Co and MgH2+7% (mass) FCC Co samples.

Fig.3.Non-isothermal desorption curves of MgH2+x%(mass)FCC Co(x=1,3,5,7 and 9) samples.
Fig.4 (a)-(c) show isothermal hydrogen desorption curves of as-prepared MgH2and MgH2+7%(mass)HCP/FCC Co under different temperatures.The dehydrogenation rate is increased with the rising isothermal testing temperature,supporting the concept that the desorption kinetics of MgH2is greatly affected by temperature.When the temperature was set at 325°C,MgH2+7%(mass)FCC Co and MgH2+7% (mass) HPC Co could completely dehydrogenate 6.5% (mass) hydrogen within 10 min and 30 min,respectively,whereas for as-prepared MgH2,more than 1 h was needed to release the same capacity.Even at low temperature of 275 °C,the MgH2+7%(mass)FCC Co composite could fully dehydrogenate in 45 min.It is obvious that the doped composite performed much faster dehydrogenation behavior than the as-synthesized MgH2,and it can be found that the catalytic performance of FCC Co particles is superior to that of HCP Co particles.
To further illustrate this point,the hydrogen desorption activation energy (Ea) was calculated using Kissinger equation [47],which can be written as:

Where β represents the heating rate,Tpdenotes the absolute peak temperature at the max dehydrogenation velocity;A represents a pre-exponential factor and R means the gas constant.DSC curves conducted at different heating rates for the additive-free MgH2,MgH2+7%(mass)HCP Co and MgH2+7%(mass)FCC Co are exhibited in Fig.4(d).Obviously,the peak temperature of the sample decreases after the addition of catalyst.For example,when the heating rate is 12 °C.min-1,the peak temperature is 396.6 °C (MgH2),317.1 °C (MgH2+7% (mass) HCP Co),and 270.6 °C (MgH2+7%(mass)FCC Co),respectively.Fig.4(e)shows the Eacalculated from the fitted lines.Compared with (155.9±14.3) kJ.mol-1of asprepared MgH2,the Eavalue was reduced to (87.2±7.8) kJ.mol-1and (76.6±8.3) kJ.mol-1for MgH2+7% (mass) HCP Co and MgH2+7% (mass) FCC Co,respectively.Due to the excellent catalytic action of FCC Co,about 80 kJ.mol-1reduction in the reaction energy barrier for the hydrogen desorption of MgH2was achieved.Compared with the recently published apparent activation energy of MgH2catalyst system(as shown in Table 3),the activation energy in this paper was significantly reduced.Combining above results,itcan be concluded that phase structure plays an important role in catalytic action of Co and Co particles with FCC structure shows better effect than that with HCP structure,thus,MgH2+7%(mass)FCC was chosen for further study.

Table 2 Information from volumetric release curves of different samples
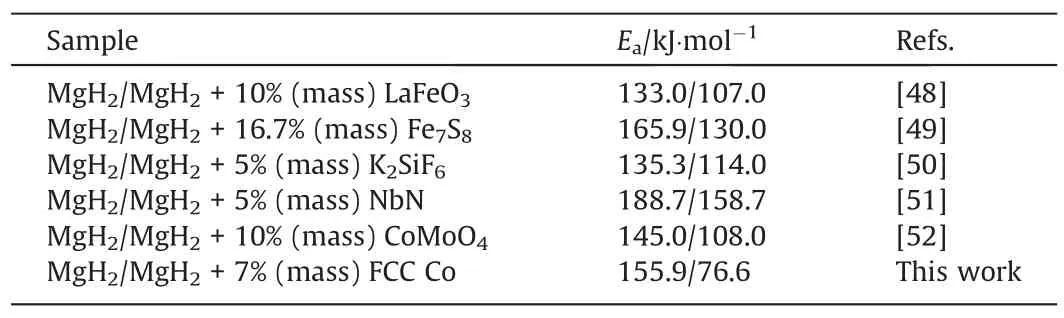
Table 3 The apparent activation energy (Ea) of hydrogen absorption for Mg-based samples

Fig.4.Isothermal hydrogen desorption curves(a,b,c),DSC profiles(d)and Kissinger plots(e)of as-prepared MgH2,MgH2+7%(mass)HCP Co and MgH2+7%(mass)FCC Co composite.
Hydrogen absorption performance is also an important issue to the application of MgH2system.In this study,MgH2with and without HCP/FCC Co dopant were recharged after dehydrogenation under 3.2 MPa hydrogen pressure in both non-isothermal and isothermal modes.Non-isothermal hydrogenation curves for the samples are shown in Fig.5(a).According to the result,MgH2+7% (mass) FCC Co started to absorb hydrogen at approximately 70 °C,which is 110 °C lower than that of fully dehydrogenated additive-free MgH2.Although the hydrogen absorption amount of MgH2+7% (mass) FCC Co is 6.8% (mass),a little lower than that of dehydrogenated MgH2,the hydrogen uptaking rate is greatly accelerated.Fig.5(b),(c)and(d)illustrate the isothermalhydrogenation curves for dehydrogenated MgH2,MgH2+7%(mass)HCP Co and MgH2+7%(mass)FCC Co,respectively.Importantly,the dehydrogenated MgH2+7%(mass)FCC Co could be fully rehydrogenated in 40 min at 150 °C whereas the same rehydrogenation performance for MgH2+7% (mass) HCP Co needed operation temperature around 200 °C and pristine MgH2needed operation temperature higher than 250 °C.When it comes to 175 °C,MgH2+7% (mass) FCC Co could fully absorb hydrogen in 10 min,while MgH2+7% (mass) HCP Co needed more than 1 h to complete absorption at 175°C and MgH2cannot achieve it even at 270°C,further evidencing that the addition of FCC Co can accelerate the rehydrogenation kinetics,reaching a bi-directional catalysis.
To better shed light on the improvement on the rehydrogenation performance,the hydrogenation Eawas calculated by using Johnson-Mehl-Avrami-Kolmogorov (JMAK) method with the following equation [53,54]:(α means the ratio of Mg recharged to MgH2at t moment,n is the Avrami exponent,and k is the kinetic parameter)


Fig.5.Hydrogenation curves in non-isothermal mode(a),isothermal mode(b,c and d)and calculated Ea values(e)for MgH2,MgH2+7%(mass)FCC Co and MgH2+7%(mass)FCC Co samples.
Furthermore,the data of all the parameters could be acquired from fitted plots of the JMAK model.The Arrhenius equation was used to calculate the hydrogenation Eavalue [55]:

As shown in Fig.5(e),the apparent activation energy of hydrogenation for MgH2+7% (mass) FCC Co is (68.3 ± 6.0) kJ.mol-1,8 kJ.mol-1and 13.1 kJ.mol-1lower than that of MgH2+7%(mass)HCP Co ((75.3 ± 2.5) kJ.mol-1) and pure MgH2((81.4 ± 5.6)kJ.mol-1),respectively.Clearly,the addition of Co could reduce the hydrogenation reaction energy barrier of Mg,contributing to the enhanced hydrogen absorption performance of Co modified MgH2system.
Besides the kinetic property of the modified system,the thermodynamic property is also a significant index for practical application of hydrogen storage materials.The equilibrium hydrogen pressure at different temperatures of MgH2with and without FCC Co were measured,shown in Fig.6(a) and (b).The desorption plateau pressures of MgH2were determined to be 0.22,0.41,0.86 and 1.51 MPa for 325 °C,350 °C,375 °C and 400 °C,the plateau pressures of MgH2+7% (mass) FCC Co were determined to be 0.14,0.28,0.53 and 0.98 MPa for 300 °C,325 °C,350 °C and 375°C.The decomposition enthalpy of hydrogen (ΔH) calculated according to the fitting curve is shown in the Fig.6(c).Compared with (86.4 ± 0.4) kJ.mol-1of as-prepared MgH2,the ΔH value for 7% (mass) FCC Co modified MgH2was reduced to (80.6 ± 0.1)kJ.mol-1.It can be seen that Co can not only improve the kinetic property of MgH2but can also tune the thermodynamic property of MgH2.
Apart from the hydrogen absorption and desorption properties,cycling property is also regarded as one of the utmost indicators for the practical application of MgH2.Hence,the cycling property of the MgH2+7% (mass) FCC Co composite was measured for 20 cycles at 300 °C (desorption under 0.02 MPa,absorption under 3 MPa hydrogen pressure),which are presented in Fig.7.It can be clearly observed that no obvious recession in hydrogen absorption and desorption rates could be observed during 20 cycles.Only approximately 0.3% (mass) degradation for MgH2+7% (mass) FCC Co composite could be detected after 20 cycles,with 6.53%(mass)for the first cycle and 6.23% (mass) for the 20th cycle.

Fig.6.The pressure-composites-temperature (PCT) curves of MgH2 (a) and MgH2+7% (mass) FCC Co (b) at different temperatures and corresponding van’t Hoff plot (c).
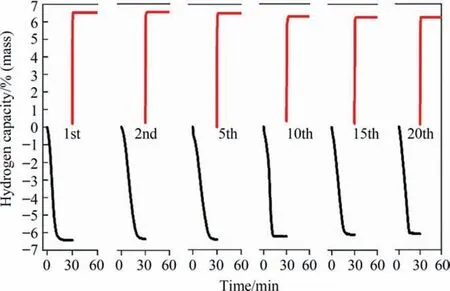
Fig.7.Cycling curves of the MgH2+7% (mass) FCC Co composite in 20 cycles (red for dehydrogenation and black for rehydrogenation).
Through above hydrogen desorption/absorption studies,it is shown that FCC Co particles have a good catalytic efficiency in MgH2.To achieve a better understanding of the evolution of asprepared FCC Co in MgH2system,microstructure measurements(XRD,TEM and EDS)were carried out.XRD profiles in Fig.8 reveals that MgH2/Mg was the main phase in the ball-milled/dehydrogenated sample.For the dehydrogenation sample,a new phase of Mg2Co(PDF#44-1149)appears between 40°and 45°,indicating that Mg reacted with Co in the dehydrogenation process.After rehydrogenation,Mg and Mg2Co were charged to MgH2phase (PDF#12-0697) and Mg2CoH5phase [56].

Fig.8.XRD patterns for MgH2+7% (mass) FCC Co composite in ball-milled,dehydrogenated and rehydrogenated state.
TEM images presented in Fig.9(a)and(b)indicate that the particle size of MgH2+7% (mass) FCC Co composites is around 0.3–1 μm.Fig.9 (c) reveals that bright particles were distributed on the composites,which were confirmed by EDS mapping (Fig.9(d)) to be Co.The TEM analysis indicates that the catalyst was in full contact with MgH2and is well dispersed on the surface of Mg particles,contributing to the re/dehydrogenation reaction on the outer surfaces of Mg/MgH2[57].
3.2.De/hydrogenation mechanism
The particle size of catalyst also affects its catalytic action[40].To further explore the FCC Co,we have provided the TEM images of as-prepared FCC-Co(Fig.10).The diffraction rings in the SAED pattern(Fig.10(b))match well with crystal faces(111),(220),(222)of Co.TEM images (Fig.10(a)) present that the particle size of asprepared Co is 0.5–0.8 μm,which is beneficial for enhancing its catalytic effect.In order to uncover the catalytic mechanism of FCC Co,the DFT calculations were performed to reveal the interaction between MgH2and FCC Co(111)wherein the selected FCC Co(111)is based on the Co strong peak in XRD pattern as revealed in Fig.1.Fig.11(a)represents the MgH2structure transformation during the adsorption process.Clearly,the Mg-H bonds are spontaneously cleaved,indicating FCC Co (111) boosts the formation of the H atoms.That is,FCC Co particles improve the hydrogen sorption,being consistent with the experimental measurements.To shed light on the physical origination,the partial density of states(PDOS)between Mg/H and FCC Co(111)is descripted in Fig.11(b).The Mg p orbitals are mainly distributed above -5 eV meanwhile the H s band is located below-5 eV,demonstrating no direct binding between Mg and H atoms.Furthermore,the intensive orbital coupling between Mg/H and FCC Co (111) enables the stability of H atomic configuration.As a result,the presence of FCC Co plays an important role on enhancing the hydrogen storage performance of the MgH2molecule.

Fig.9.TEM,STEM-HAADF images (a-c) and selected EDS mapping (d,e) for the MgH2+7% (mass) FCC Co composite.

Fig.10.TEM images (a) and SAED image (b) of the FCC Co.
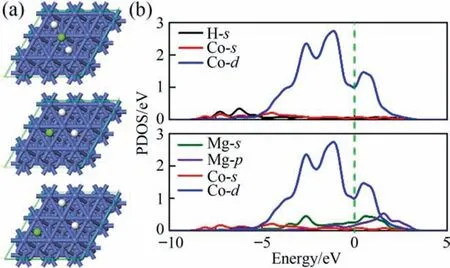
Fig.11.MgH2 absorption on the surface of FCC Co (111).The adsorption configuration (a),the green,white ball represents Mg,and H,respectively,and the corresponding partial density of states (b).
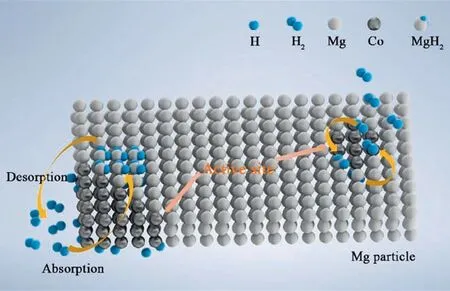
Fig.12.Schematic summary on Co catalyzed Mg particle in hydrogenation process.
In summary,the whole catalytic process was illustrated in Fig.12.In the ball milling process,the prepared Co particles were evenly distributed on the MgH2surface,which created numerous active sites and provided sufficient channels for hydrogen diffusion[58].During dehydrogenation and hydrogenation,the phase change between Mg2Co/Mg2CoH5served as a hydrogen pump to accelerate de/rehydrogenation kinetics.Furthermore,the addition of Co extracted the Mg-H bond of MgH2unit,resulting in the decreased activation energy and operation temperature.Thus,the comprehensive hydrogen storage properties of MgH2+7% (mass)Co composite were dramatically improved.
4.Conclusions
In this paper,two kinds of Co particles with different structure(FCC/HCP)were prepared and experimental results proved that asprepared FCC Co showed superior catalysis on enhancing the comprehensive properties of MgH2than HCP Co.7%(mass)FCC Co particles modified MgH2liberated 6.4%(mass)hydrogen starting from 195 °C.At 325 °C,the MgH2+7% (mass) FCC Co composite can completely dehydrogenate in 10 min with the capacity of 6.5%(mass),much faster than HCP Co particles modified MgH2and pure MgH2.The Eavalue of the MgH2+7%(mass)FCC Co was reduced to(76.6 ± 8.3) kJ.mol-1,about 10.6 kJ.mol-1lower than that of MgH2+7% (mass) HCP Co,and around 50% lower than that of asprepared MgH2((155.9 ± 14.3) kJ.mol-1).Besides,the composite exhibited good cyclic stability and did not decrease significantly in 20 cycles.Microstructure analysis and theoretical calculations revealed uniformly dispersed Co facilitated the break of Mg-H bonds and hydrogen diffusion along the Mg/MgH2interfaces,which was reasonably responsible for the improved hydrogen storage properties of MgH2.In a word,this paper demonstrated the excellent catalysis of Co with for Mg-based hydrogen storage materials and the catalytic performance of Co with FCC structure is better than that of HCP,which may serve as a reference and promote the development of highly effective catalysts in the energy-related fields.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to express gratitude for support from the National Natural Science Foundation of China (Grant Nos.51801078 and 21701083) and the Natural Science Foundation of Jiangsu Province (Grant No.BK20180986 and BK20210884).
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Green hydrogen:A promising way to the carbon-free society
- Electrochemical CO2 mineralization for red mud treatment driven by hydrogen-cycled membrane electrolysis
- Fabrication of azobenzene-functionalized porous polymers for selective CO2 capture
- Significantly enhanced charge transfer efficiency and surface reaction on NiP2/g-C3N4 heterojunction for photocatalytic hydrogen evolution
- CO2 capture by double metal modified CaO-based sorbents from pyrolysis gases
- Methane hydrate crystal growth on shell substrate
