Fabrication of azobenzene-functionalized porous polymers for selective CO2 capture
2022-04-27DingMingXueWenJuanZhangXiaoQinLiuShiChaoQiLinBingSun
Ding-Ming Xue,Wen-Juan Zhang,Xiao-Qin Liu,Shi-Chao Qi,Lin-Bing Sun
State Key Laboratory of Materials-Oriented Chemical Engineering,Jiangsu National Synergetic Innovation Center for Advanced Materials (SICAM),College of Chemical Engineering,Nanjing Tech University,Nanjing 211816,China
Keywords:Adsorption CO2 capture Selectivity Azobenzene Photoresponse
ABSTRACT Many solid adsorbents have been prepared for the CO2 capture.In particular,the photoresponsive adsorbents have attracted extensive interests because of their tunable pore structure and variable responsive behaviors provoked by the external light.However,it is challenging to fabricate the photoresponsive adsorbents featured the big CO2 capacity and high CO2 selectivity.Herein,copolymerized between 4-phenylazobenzoyl chloride,2,4,6-trichloro-1,3,5-triazine and melamine,a series of azobenzenefunctionalized porous polymers (PTM-AZOs) are successfully synthesized.The PTM-AZOs are verified in possession of proper pore structures,large surface area and photoconductive properties through a series of characterization.The PTM-AZO-2 with the trans-isomerization exhibits the best CO2 adsorption amount of 2.7 mmol.g-1 (273 K and 0.1 MPa),while the CO2/N2 selectivity can reach 2459 and 607 on the trans-and cis-isomerization,respectively.The regulatable pore structures controlled by the photoresponsive azobenzene groups affect the CO2 capture performance of the PTM-AZOs.
1.Introduction
As a greenhouse gas,CO2has caused the global climate warming problem which results in seriously abominable environment,weather disasters,and negative impacts on plant [1–3].Carbon capture and storage (CCS) is regarded as a promising technology,which may avert the increasing CO2emissions to the atmosphere.Solid adsorbents are regarded as a promising alternative for the efficient removal of CO2based on its advantages including easy preparation,low price,and environmental friendliness.Till now,many kinds of porous materials have been investigated or prepared by researchers as absorbents for the CO2capture,including activated carbons [4,5],metallic oxide [6,7],porous organic polymers[8–10],mesoporous silicas [11,12],metal–organic frameworks[13–15],and natural zeolites [16,17].
In general,in a typical adsorption process for the solid materials,the CO2are usually diffused into the channels and fettered at the hierarchical pores or active sites based on the physical or chemical interactions,which is difficult to be desorbed without extra temperature and pressure.In this case,it is hard to realize the balance between the high CO2adsorption capacity and selectivity.For example,the OMC-20-80-24-700 showed a good CO2capture capacity of 3.7 mmol.g-1and the low CO2/N2selectivity of only 21,because the ordered mesopores of 3.4 nm was beneficial to the CO2and N2molecular diffusion without further hindrance[18].Someone manufactured the porous polymer feathered with small surface area and appropriate pore sizes of 0.6–2.5 nm,of which CO2/N2selectivity reached 125,while only gave a very low CO2capture capacity of 0.68 mmol.g-1[19].Recently,the functional materials with photoresponsive groups,such as dithienylethene,spiropyrane,coumarin,and azobenzene,have been reported owing to the advantages of real-time response,precise positioning,and remote controllability[20–23].As the most popular photoresponsive stimuli,the azobenzene groups can change the configuration after illuminated by different ultraviolet(UV)light or visible (Vis) light in many literatures [24,25].Introducing the azobenzene groups in the porous polymers for the new photoresponsive adsorbents is the hopeful solution for varying the CO2capture by the photo-isomerization of azobenzene,and further obtaining both the high CO2capacity and selectivity.
Herein,we designed and synthesized the azobenzenefunctionalized porous polymers (PTM-AZOs) as the adsorbents,which regulated the CO2adsorption/desorption performance through the structural change of the azobenzene groups responding to external light.In accordance with the conventional postsynthetic strategy,the samples PTM-AZOs applied for CO2capture in the present study are prepared through the azobenzene functionalization of a porous polymer,of which synthesis is based on a nucleophilic substitution reaction between 2,4,6-trichloro-1,3,5-triazine and melamine without any catalyst (Scheme 1).The PTM-AZOs exhibit accessible surface areas,large pore volumes,and appreciate pore sizes by varying the amount of introduced azobenzene-containing groups.The PTM-AZOs show the excellent CO2capture performance,ranging from 1.6 to 2.7 mmol.g-1at 273 K and 0.1 MPa,upon the trans isomerization.It is noticeable that the PTM-AZO-2 exhibits the best CO2adsorption amount of 2.7 mmol.g-1(trans-PTM-AZO-2) and 2.2 mmol.g-1(cis-PTMAZO-2),which is better than some other adsorbents reported in the literature such as NAB-4 (2.14 mmol.g-1) [26],Azo-IRMOF-10(0.71 mmol.g-1) [27],and ECTU-15 (0.28 mmol.g-1) [28].The PTM-AZO-2 also shows brilliant CO2/N2selectivity (273 K and 0.01 MPa) of 2459 and 607 on the trans and cis isomerizations.Additionally,in the four times cyclic irradiation with UV and Vis lights,the PTM-AZO-2 exhibits full regeneration performance without obvious decrease.
2.Materials and Methods
2.1.Materials synthesis
Melamine (99%),2,4,6-trichloro-1,3,5-triazine (98%),4-phenylazobenzoyl chloride (>98%),anhydrous KOH (97%),dichloroethane (DCE,99.8%) and dimethyl sulfoxide (DMSO,99.9%) were purchased from Shanghai Aladdin Biochemical Technology Co.,Ltd.and all the reagents were used without any further purification.
As shown in Scheme 1,the detailed synthesis route of the porous polymer and PTM-AZOs were drawn as the previous work[29].Two monomers of the 1 mmol melamine(M1) and 2.5 mmol KOH were dissolved in the DMSO (25 ml),after the vigorous stirring,a stable and clear solution could be obtained.Then,cautiously add,dropwise,a solution of 2,4,6-trichloro-1,3,5-triazine(M2,1 mmol)in the DMSO(25 ml).Under the protection of the nitrogen gas,the mixture in an eggshell bottle was heated at 160 °C for 24 h with continuous stirring.After cooling to near 20 °C,the dark sediment was washed continuously by methanol and deionized water for three times.With the further vacuum drying for overnight,the obtained residual was the porous polymer.

Scheme 1.Synthetic route of porous polymer and PTM-AZOs.
With continuous stirring,the porous polymer(326.4 mg)and 4-phenylazobenzoyl chloride (M3,30.5 mg) were added in the DCE(25 ml),followed by heating at the temperature of 65 °C for 48 h and further vacuum drying.The obtained composite material was denoted as PTM-AZO-1.Through the similar synthesizing process,the PTM-AZOs samples with different amount of M3 were prepared successfully and denoted as PTM-AZO-1,PTM-AZO-2,PTMAZO-3 and PTM-AZO-4,where 1,2,3 and 4 represent the mass of M3 30.5 mg,61.0 mg,91.5 mg and 122.0 mg in the composites,respectively.
2.2.Characterization
At 40 kV and 40 mA,the X-Ray powder diffraction (XRPD) patterns were recorded by the Bruker D8 Advance diffractometer.Fourier transform infrared (FTIR) spectra of the samples were recorded on a Nicolet Nexus 360 spectrometer.The N2adsorption–desorption isotherms were measured at 77 K with Micromeritics ASAP 2020.The samples should be dried at 110 °C under the vacuum condition for 1 h before the isotherms test.The transmission electron microscope (TEM) image of the materials was captured in a JEM-2010F electron microscope operated at 200 kV.UV–Vis spectra were tested on the PerkinElmer Lambda35(the rated power:2.45 W) in the region of 200–800 nm.The PTMAZOs dissolved in the solution were tested,in this order:the initial state,irradiated for 10 min with UV-light (wavelength 365 nm),irradiated with Vis-light (wavelength 420 nm).
2.3.Adsorption tests
Single component gas tests of CO2,CH4and N2(all three are 99.999%) and the regeneration tests over the adsorbents at 273 K were measured by ASAP 2020 analyzer.In case of the regeneration tests,after degas,the samples in the quartz tube were irradiated for 2 h with UV-light and tested.Then,the samples were irradiated with Vis-light for 2 h and tested.The ideal adsorption solution theory(IAST)selectivity is defined as(xi∙yj)/(xj∙yi),in which xiand yi(xjand yj) are the molar fractions of component 1 (component 2) in the adsorbed and bulk phase,respectively.The adsorption selectivity of CO2/N2(15/85,v/v)and CO2/CH4(50/50,v/v)were estimated according to the above equation at 273 K and 0.1 MPa.The dynamic breakthrough curve of the PTM-AZO-2 was measured to analyse the mixture separation performance.A quantity of PTMAZOs were filled into a fixed bed,which was heated for a pretreatment at 373 K for 1 h.Then it was immersed in the ice water,and fed the certain gas flow (CO2/N2,15/85,v/v) of 2 ml.min-1at 273 K and 0.1 MPa.The dynamic breakthrough curve was recorded by the auto gas chromatography.
3.Results and Discussion
3.1.Characterization
The FTIR spectra of the PTM-AZOs are listed in Fig.1(a),which can confirm the successful fabrication of the PTM-AZOs.The stretching vibration of -N=N-of the monomer M3 is easy to identify through the bands which are located at 1575 cm-1.It is obvious that the PTM-AZOs are successfully fabricated based on the existence of the -N=N-stretching vibrations in the PTMAZOs.Fig.1(b) shows the XRPD patterns of the PTM-AZOs which reflecting the structural information.There are no diffraction peaks can be observed,which display the amorphous characteristics of the porous polymer materials [19].
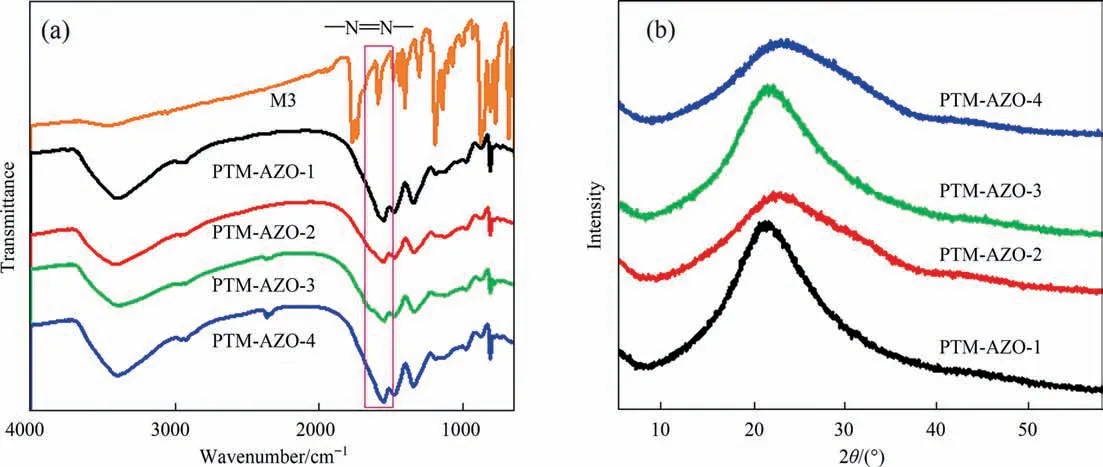
Fig.1.(a) FT-IR spectra and (b) XRPD pattern of the PTM-AZOs.
Fig.2 shows the N2adsorption–desorption isotherms of the PTM-AZOs at 77 K and the related pore size distributions.At the low relative pressure,there are much N2can be adsorbed by the PTM-AZOs,meaning their high porosity.When the relative pressure is larger than 0.1,the PTM-AZOs still show a sharp increase of the N2uptakes and distinct hysteresis loops.All the isotherms of the PTM-AZOs obviously belong to the characteristic of the Ⅳ-type adsorption–desorption isotherm.It is obvious that the PTMAZOs possess plenty of micropores and mesopores,which is based on the results of the previous conclusions.It is convinced that the M3 is successfully drawn into the pore structure of the porous polymer,because of the decreased N2uptakes.
As listed in Table 1,taking the specific surface area and pore volume into account,it is clear that the introduced M3 promotes the formation of the pore structure of the PTM-AZOs.Based on the N2adsorption–desorption isotherms,the calculated specific surface area of the PTM-AZO-1,PTM-AZO-2,PTM-AZO-3,and PTM-AZO-4 decrease from 375 m2.g-1,303 m2.g-1,216 m2.g-1,to 225 m2.g-1,respectively.Meanwhile,the total pore volume of the PTM-AZO-1 (0.38 cm3.g-1) is bigger than that of the PTMAZO-2,-3,and -4 (correspond to 0.31,0.27,and 0.23 cm3.g-1,respectively),which is line with the tendency of the specific surface area.As shown in Fig.2(b),the corresponding pore size distribution of the PTM-AZOs are illustrated,and all the PTM-AZOs have plentiful micropores and mesopores,which are beneficial to the immobilization of the CO2molecules.The PTM-AZO-1 possesses two kind of pores,including the ultra-micropores about 0.5 nm and mesopores between 2.0 nm to 2.6 nm.Meanwhile,the PTMAZO-2 also exhibits a homologous pore size distribution.One pore size is around 0.6 nm which is slightly bigger than that of the PTMAZO-1,the other one is around 1.7–2.3 nm which is less than that of the PTM-AZO-1,resulting from the effective introduction of the M3 into the pore structure of the porous polymer.With introducing the more M3 content,the channels of the porous polymer are blocked obviously.The PTM-AZO-3 and PTM-AZO-4 both show only one pore size of 1.9–2.3 nm and 1.5–2.4 nm,respectively.These results indicate that too much M3 blocks the pores of the porous polymer.The typical TEM images of the PTM-AZO-2 are given in the Fig.3.It is clear that the pore structures formed from the polymerization of the precursor exhibit the worm-shaped pores.

Table 1 Porosity properties of PTM-AZOs
Photoresponsive properties are important for the PTM-AZOs.The PTM-AZO-2 were further studied by using UV/Vis spectrometer as shown in Fig.4.In general,the π →π*transition,representing the trans-azobenzene isomer,can be observed through the wavelength number located at lower than 380 nm [30].Correspondingly,the n → π*transition,representing the cisazobenzene isomer,can be distinguished through the wavelength number located at between 380 nm to 500 nm [25].In the initial state,the curve shows a high peak at the 329 nm and a slight swelling around 440 nm.After the UV-light irradiation,it is clear that the peak intensity at 329 nm falls in a weak and the peak intensity at 440 nm increases inapparently.Then,the curve returns to the initial state with the irradiation of the Vis-light.Therefore,this result indicates that the reversible photoisomerization phenomenon of the azobenzene groups is confirmed.Based on these results,it is apparent that the photoresponsive adsorbents PTMAZOs are successfully designed and prepared through introducing the azobenzene groups into the channels of the porous polymer.

Fig.2.(a)Nitrogen adsorption–desorption isotherms;(b)corresponding pore size distributions of PTM-AZOs.(inset map,pore size distributions between 0.5 nm to 3.0 nm).

Fig.3.The TEM images (a) 20 nm and (b) 5 nm of the PTM-AZO-2.

Fig.4.Alteration in the UV–Vis spectra of PTM-AZO-2 upon UV-light and then vislight irradiation.
3.2.Gas adsorption performance
The single component gas adsorption performance of the adsorbents PTM-AZOs for CO2were tested.As drawn in Fig.5,all the PTM-AZOs deliver a tendency that the adsorbed amount grows continuously and gently for CO2at 273 K and 0.1 MPa.These adsorption curves exhibit the fact that the plenty of the CO2molecules have occupied the accessible structures of the PTM-AZOs.In the case of the PTM-AZO-1 sample,the CO2adsorption capacity is 2.4 mmol.g-1,which is mainly related to the highest surface area(375 m2.g-1) and pore volume (0.38 cm3.g-1).In general,higher specific surface area of the adsorbent often leads to the higher CO2adsorption capacity,owing to the more abundant accessible area [31,32].There is no question that the PTM-AZO-3(1.9 mmol.g-1) and PTM-AZO-4 (1.6 mmol.g-1) exhibit the lower CO2uptake than that of the PTM-AZO-1,resulting from the blocked pore structure including the low surface area (216 m2.g-1and 225 m2.g-1) and small pore volume (0.27 cm3.g-1and 0.23 cm3.g-1).
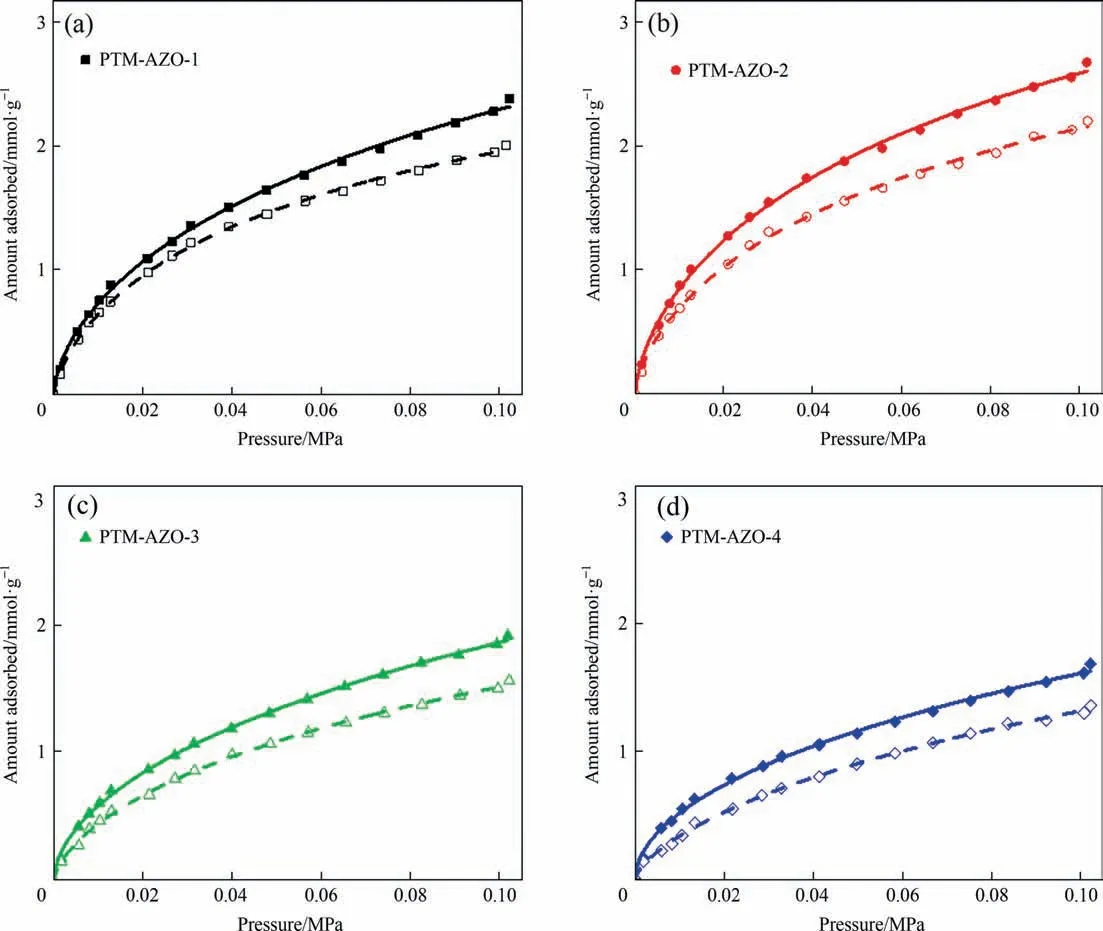
Fig.5.CO2 adsorption isotherms of the sample PTM-AZOs.(solid point and line represent trans-PTM-AZOs,dotted point and line represent cis-PTM-AZOs).
However,the highest CO2adsorptive capacity(2.7 mmol.g-1)is obtained over PTM-AZO-2,which is also better than much other adsorbents reported in the literature,such as ZW MOF(0.63 mmol.g-1) [33],Zn(AzDC)(4,4′-BPE)0.5(1.42 mmol.g-1) [34],F-azo-MIL-53(Al)(0.8 mmol.g-1)[30],Ag/UiO-66–3(1.56 mmol.g-1) [35],and Co2L2(AzoD)2.2DMF (0.81 mmol.g-1)[36],as shown in Table 2.It is very interesting that the PTMAZO-2 shows a lower surface area (303 m2.g-1) and pore volume(0.31 cm3.g-1),in comparison with that of the PTM-AZO-1.This result indicates that the CO2adsorptive capacity is not only rely on the contribution of the surface area.The pore size distribution also efficiently affects the CO2adsorptive capacity of the PTMAZOs.As previous work reported,the pore diameter lees than 0.5 nm prevents the CO2molecules accessing in the narrow space[29].In this case,the micropore sizes about 0.5 nm of the PTMAZO-1 are invalid for the CO2capture,while the micropores size about 0.6 nm of the PTM-AZO-2 are beneficial to the CO2immobilization.Therefore,the combine effect of the specific surface area,pore volume and appropriate pore size of the PTM-AZOs make the CO2adsorptive capacity efficiently improved

Table 2 Comparison of the adsorption performance for different adsorbents
Notably,all the PTM-AZOs show a good photoswitching adsorption behavior upon UV-light as shown in the Fig.5.After UV illumination for 1 h,the CO2uptakes of the PTM-AZOs exhibit a clear decrease (cis-PTM-AZO-1,cis-PTM-AZO-2,cis-PTM-AZO-3 and cis-PTM-AZO-4,correspond to 2.0,2.2,1.5,and 1.4 mmol.g-1,respectively)at 273 K and 0.1 MPa,which indicating that the azobenzene groups have the efficient photoresponsive behavior upon trans/cis isomerizations.Besides,the calculated energy consumption of the trans-PTM-AZO -2 is 0.74 kJ.g-1CO2,which is much less than other adsorbents in the industrial process,such as zeolite 13X APG&activated carbon beads (2.44 kJ.g-1CO2) [39],Ca-X zeolite(2.02 kJ.g-1CO2) [40] and amine-based ion exchange polymer(4.75 kJ.g-1CO2) [41],which indicating that the PTM-AZOs have the advantage of energy savings.
In the case of the highest CO2capture capacity over the PTMAZO-2,the N2and CH4uptakes were also tested at 273 K and 0.1 MPa,and drawn in Fig.6(a).It is clear that the PTM-AZO-2 possesses 0.5 mmol.g-1N2and 0.1 mmol.g-1CH4.After the UV-light,the PTM-AZO-2 upon the cis-isomerization shows a negligible change of the N2and CH4,meaning that the adsorbent has the potential applications for CO2/N2and CO2/CH4.Regeneration property is important to the PTM-AZO-2 and four trans/cis isomerization cycles test are also evaluated without visible loss of CO2adsorption capacity as depicted in Fig.6(b).That means,the PTM-AZO-2 can achieve the full CO2adsorption/desorption performance by the UV/Vis-light without further high temperature or high pressure,indicating the excellent reversibility.This result also shows the consistence between the adsorbents after four cycles with the fresh one in both the appearances and the adsorption performance.The IAST model was employed to estimate the selectivity of CO2/CH4and CO2/N2for the PTM-AZO-2.The CO2/CH4ratio is 50/50 and CO2/N2ratio is 15/85,which are similar with the typical composition of flue gas.As displayed in Fig.6(c),the CO2/CH4selectivity of the PTM-AZO-2 is 18(273 K and 0.01 MPa)upon the trans isomerization,which slightly decreases to 15 upon the cis isomerization.In view of the very few adsorbed amount N2of the PTMAZO-2,the CO2/N2selectivity is as high as 2459 (273 K and 0.01 MPa) upon the trans isomerization in the Fig.6(d).After the UV-light illumination,the CO2/N2selectivity of the PTM-AZO-2 falls into 607 upon the cis isomerization,resulting from the falling of the CO2uptake.The results indicate that the CO2is preferential adsorption for the PTM-AZO-2 in the CO2/CH4and CO2/N2mixed system.

Fig.6.(a) The CO2 N2 and CH4 adsorption isotherms of the PTM-AZO-2 at 273 K and 0.1 MPa.(b) Adsorption cycles of CO2 over trans/cis-PTM-AZO-2.IAST selectivity of (c)CO2/CH4 and (d) CO2/N2 on the PTM-AZO-2.
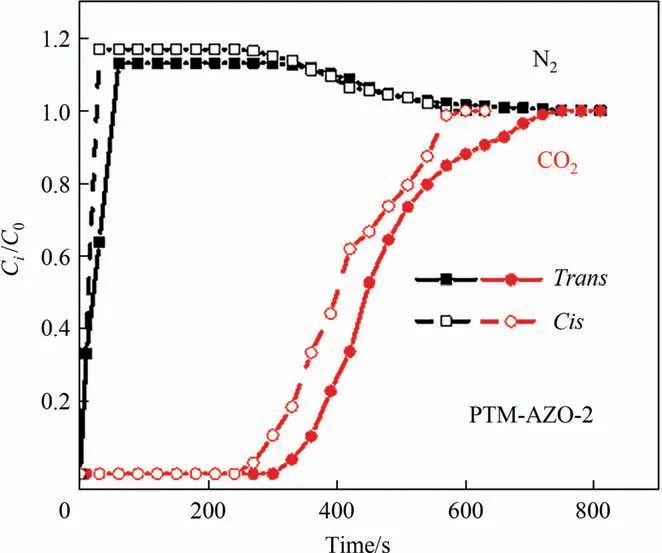
Fig.7.Dynamic breakthrough curves of CO2/N2 over the adsorbents PTM-AZO-2.
Dynamic breakthrough experiments are essential for further evaluating the selective adsorption performance of PTM-AZO-2 in the industrial process.As displayed in Fig.7,for trans/cis-PTMAZO-2,N2is the first component to break through the fixed bed,which may be related with the fact that N2is hardly adsorbed by the PTM-AZO-2.Additionally,the breakthrough time of CO2over trans-PTM-AZO-2 is 300 s,which is longer than that of cis-PTMAZO-2 (240 s).The results indicate that the PTM-AZO-2 possesses the good selective adsorption performance for CO2upon the trans and cis isomerization,which are well consistent with the previous pure CO2uptake.
4.Conclusions
To summarize,a series of azobenzene-functionalized porous polymer PTM-AZOs derived from the polymerization of the melamine,2,4,6-trichloro-1,3,5-triazine and 4-phenylazobenzoyl chloride were synthesized successfully.The different introduction amount of the azobenzene groups play an important role in tailoring the pore structure of the PTM-AZOs upon the trans/cis isomerization.The synergetic cooperation of the high surface area,large pore volume and controllable photoresponsive groups for the PTM-AZO-2 not only achieves the excellent CO2capture capacity of 2.7 mmol.g-1(trans-PTM-AZO-2) and 2.2 mmol.g-1(cis-PTMAZO-2),but also exhibits the extremely high CO2/N2selectivity under different trans/cis isomerization.We believe that our PTMAZOs can be considered to be a promising candidate material for CO2adsorbents.However,if the problem of expanding the difference of adsorption performance under different lights is solved in the future,this strategy may provide researchers with a new way for developing mass-produced photoresponsive absorbers.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We acknowledge the financial support of this work by the National Science Fund for Distinguished Young Scholars(22125804) and the National Natural Science Foundation of China(22178163,22078155,21808105,and 21878149).
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Editorial for special issue on Carbon-neutrality Chemical Engineering
- Design and experiment of high-productivity two-stage vacuum pressure swing adsorption process for carbon capturing from dry flue gas
- 3D multiphase flow simulation of Marangoni convection on reactive absorption of CO2 by monoethanolamine in microchannel
- Top-down strategy for bamboo lignocellulose-derived carbon heterostructure with enhanced electromagnetic wave dissipation
- Structural reconstruction of Sn-based metal–organic frameworks for efficient electrochemical CO2 reduction to formate
- The effect of different Co phase structure (FCC/HCP) on the catalytic action towards the hydrogen storage performance of MgH2
