基于UPLC-Q-TOF-MS/MS技术分析菠萝蜜多糖小鼠肠道代谢产物及代谢途径
2022-04-27马之原陈玉子谭乐和张彦军吴刚朱科学
马之原 陈玉子 谭乐和 张彦军 吴刚 朱科学

摘 要:菠蘿蜜在我国海南、广东、广西、云南、福建和台湾等地大量栽种,其果实营养丰富,含有糖类化合物、蛋白质、氨基酸、多酚、脂肪酸、维生素、矿物质等多种营养成分。其中,菠萝蜜多糖是一类由鼠李糖、阿拉伯糖、半乳糖、葡萄糖、木糖和半乳糖醛酸组成、分子量约为1668 kDa的生物大分子;具有促进小鼠脾淋巴细胞增殖、提高免疫细胞抗氧化活性及诱导细胞因子TNF-α、IFN-γ和IL-1β分泌等生物活性;可被人体肠道菌群酵解利用,产生丰富的SCFAs等代谢产物,有益于人体肠道健康。然而,有关菠萝蜜多糖的肠道代谢鲜有报道,本文旨在利用UPLC-Q-TOF-MS/MS技术研究菠萝蜜多糖的小鼠肠道代谢产物及其可能代谢途径。健康小鼠分为4组:50 mg/kg小鼠体重菠萝蜜多糖组(低剂量组)、100 mg/kg小鼠体重菠萝蜜多糖组(中剂量组)和200 mg/kg小鼠体重菠萝蜜多糖组(高剂量组)以及同等体积蒸馏水(空白对照组)。连续处理2周后,利用超高效液相色谱串联四级杆飞行时间质谱(UPLC-Q-TOF-MS/MS)鉴定菠萝蜜多糖在小鼠肠道内的代谢物种类并分析相关代谢通路。通过主成分分析(PCA)、偏最小二乘判别分析法(PLS-DA)及二级质谱技术共鉴定出30种特征代谢标志物,负离子模式下有22种,5种上调,17种下调;正离子模式下有8种,6种上调,2种下调。涉及到的代谢通路有苯丙氨酸、丙氨酸、天冬氨酸、色氨酸、胆固醇、2-氧羧酸和核苷酸代谢,以及三羧酸循环、PPAR信号通路、A类视紫红质样受体、苯甲酸的降解及其与氨基酸的结合、核受体、谷胱甘肽和一碳循环、尿素循环与氨基代谢通路,参与小鼠体内胆固醇、脂肪酸、甘油三酯、ω-3脂肪酸、ω-6脂肪酸和次级代谢产物的合成。研究结果可为揭示菠萝蜜多糖发挥功能活性的物质基础及其作用途径提供理论依据。
关键词:菠萝蜜多糖;代谢组学;通路;超高效液相色谱串联四级杆飞行时间质谱中图分类号:R151 文献标识码:A
Fecal Metabolomics of Polysaccharide from Jackfruit Pulp in Mice Based on UPLC-Q-TOF-MS/MS
MA Zhiyuan1,2,3, CEHN Yuzi1,2,3, TAN Lehe1,3, ZHANG Yanjun1,3, WU Gang1,3, ZHU Kexue1,3*
1. Spice and Beverage Research Institute, Chinese Academy of Tropical Agricultural Sciences, Wanning, Hainan 571533, China; 2. College of Food Science and Technology, Huazhong Agricultural University, Wuhan, Hubei 430070, China; 3. Key Laboratory of Processing Suitability and Quality Control of the Special Tropical Crops of Hainan Province, Wanning, Hainan 571533, China
Abstract: Artocarpus heterophyllusLam. is rich in nutrients, including carbohydrate, protein, amino acid, polyphenol, fatty acid, vitamin and minerals, which can be used as good sources for some important nutrients. Nowadays,A. heterophyllusLam. trees are widely distributed in Hainan, Guangdong, Guangxi, Yunnan, Fujian, and Taiwan provinces. Polysaccharide, as an important biological molecule, participates in cell activities. Recently, a water-soluble polysaccharide named JFP-Ps was isolated fromA. heterophyllusLam. pulp, which consisted of rhamnose, arabinose, galactose, glucose, xylose and galacturonic acid, with an average molecular weight of 1668 kDa. JFP-Ps exerted immunomodulatory effect by inducing lymphocyte proliferation, enhancing antioxidant activity and increasing the secretion of TNF-α, IFN-γ and IL-1β. Moreover, JFP-Ps can be fermented into short-chain fatty acids, including acetate, propionate, butyrate and valerate acid by gut microbiota. However, there was little research about the the metabolism of JFP-Ps during gastrointestinal digestion. Based on our previous research, the present study was aim to investigate the fecal metabolomics of JFP-Ps on fecal metabolites from mice. Healthy Kunming mice were divided into four groups, including 50 mg/kg mouse body weight (low dose group), 100 mg/kg mouse body weight (medium dose group), and 200 mg/kg mouse body weight (high dose group) and the same volume of distilled water (blank control group). After experimental treatment for 2 weeks, fresh fecal samples were collected for metabolomics analysis. A metabolomics method based on Agilent 1290 series UPLC and with 6530B series Q-TOF mass spectrometer (UPLC-Q-TOF-MS/MS) was developed to identify the fecal metabolites. Then related metabolic pathways were analyzed by matching KEGG and Wiki pathways. Our results showed that 30 potential biomarkers were authenticated using principal component analysis (PCA) and partial least squares discriminant analysis (PLS-DA), including 22 types in the negative ion mode and 8 characteristic metabolites in the positive ion mode. The results of metabolomics pathway analysis showed that the metabolites were related to the biological pathways and processes, including metabolism of phenylalanine, alanine, aspartic acid, tryptophan, cholesterol, 2-oxocarboxylic acid and nucleotide. They also have been proven to be related to the tricarboxylic acid cycle activity, PPAR signaling pathway, activation pathways in class A GPCRs, degradation of benzoic acid and derivatization of amino acids, nuclear receptors, glutathione and one-carbon cycle, urea cycle and amino metabolism pathways. The results indicated that JFP-Ps could regulate the metabolism of cholesterol, biosynthesis of fatty acid and triglycerides, omega-3 and omega-6 fatty acids metabolism and the secondary metabolites in mice. The results could provide theoretical basis for elucidating the bioactive substances and its mechanism of JFP-Ps.
Keywords: polysaccharide from jackfruit pulp; metabolomics; pathway; UPLC-Q-TOF-MS/MS
DOI: 10.3969/j.issn.1000-2561.2022.04.005
代谢组学是一种研究生物系统在外界刺激或干扰后产生的全部内源性小分子的定性、定量和动态变化的分析方法[1]。UPLC-Q-TOF-MS/MS是一种常见的代谢组学检测设备,具有高分辨率、高灵敏度、高通量等优点,能够快速鉴定代谢物。植物多糖因其抗肿瘤[2-3]、免疫调节[4]、降血糖[5]、抗炎症、抗病毒[6]、抗氧化[7]等生物活性而受到广泛关注,多糖发挥其生物活性的机制尚未完全清楚。因此,借助UPLC-Q-TOF-MS/MS探究多糖在胃肠消化过程的变化规律,有助于揭示其发挥生理活性的物质基础。
肠道粪便中含有的糖、有机酸和氨基酸等小分子代谢物能够反映肠道菌群和胃肠道对营养物质的摄取、消化和吸收的效果[8]。对葡萄酒的粪便代谢组学研究发现,适量饮用葡萄酒使苯甲酸和4-羟基戊酸等总酚代谢物含量显著升高[9];研究表明,连续食用富含β-葡聚糖的整粒谷物2個月,粪便中短链脂肪酸、支链脂肪酸、芳香醇、吲哚类、醛和酮含量增加[10-11]。
菠萝蜜多糖(polysaccharide from jackfruit pulp, JFP-Ps)主要由鼠李糖、阿拉伯糖、半乳糖、葡萄糖、木糖和半乳糖醛酸组成,平均分子量为1668 kDa,具有较强的体外抗氧化及免疫调节等
活性[12-13];其在体外酵解过程中,可被人体肠道微生物利用产生短链脂肪酸等代谢产物,有益于肠道健康[14];经体外胃肠消化的JFP-Ps产物具有较强的×OH、O2–×和DPPH×清除能力[15],JFP-Ps消化液中还原糖含量增加、分子量降低、结构和构象发生明显的变化[16]。JFP-Ps的免疫增强活性与其促进小鼠脾淋巴细胞增殖、提高抗氧化活性及诱导细胞因子TNF-α、IFN-γ和IL-1β分泌有关[13];TAN等[17]研究发现,菠萝蜜粗多糖可能通过提高小鼠胸腺质量指数和吞噬率来增强小鼠的免疫活性。为进一步探究JFP-Ps在体内的代谢规律、挖掘JFP-Ps发挥生物活性的物质基础和参与的代谢途径,本研究通过灌胃健康小鼠不同剂量的JFP-Ps,利用超高效液相色谱串联四级杆飞行时间质谱(UPLC-Q-TOF-MS/MS)并结合高通量分析JFP-Ps在健康小鼠粪便中的差异代谢物及其相关代谢通路,为JFP-Ps的深入研究及开发利用提供理论依据。
1 材料与方法
1.1材料
清洁级雄性昆明小鼠20只,体重(20.0±2.0)g,购自湖南斯莱克景达实验动物有限公司,动物生产许可证号:SCXK(湘)2016-0002。
菠萝蜜多糖(polysaccharide from jackfruit pulp, JFP-Ps)由中国热带农业科学研究院香料饮料研究所提取、纯化、制备。乙腈、甲酸(色谱纯)(德国Merck公司)。
Z36 HK型超速冷冻离心机(德国Hermle公司);UPLC-Q-TOF-MS/MS(安捷伦科技有限公司);Master-s-plus UVF全自动超纯水设备(上海和泰仪器有限公司)。
1.2 方法
1.2.1 动物的饲养和分组 昆明小鼠饲养温度(25±2)℃,相对湿度60%±10%。所有小鼠给予充足的基本膳食和饮水,适应环境一周后,随机分为4组,每组5只:菠萝蜜多糖溶液灌胃剂量分别为50 mg/kg小鼠体重(低剂量组)、100 mg/kg小鼠体重(中剂量组)和200 mg/kg小鼠体重(高剂量组),并设空白对照组(灌胃与多糖组同等体积的蒸馏水)。每天9:00按时灌胃对应浓度的菠萝蜜多糖溶液,空白对照组小鼠灌胃相同体积的蒸馏水。每天对小鼠进行健康检查,连续喂养2周后,收集粪便,–80℃冻存备用。
1.2.2 样品前处理 称取200 mg粪便于1.5 mL EP管中,加入800 μL乙腈水溶液[乙腈/水,4/1(V/V)]匀浆,12 000 r/min、4℃离心10 min,取上清并过0.22 μm微孔有机滤膜至棕色进样瓶中,供UPLC-Q-TOF-MS/MS测定。
1.2.3 分析方法 经处理的粪便样品,采用UPLC仪结合C18色谱柱进行梯度洗脱分离,Q-TOF-MS/MS进行样品检测。柱子型号:Eclipse Plus C18柱(3.5 μm, 2.1 mm×150 mm,安捷伦科技有限公司);流动相:A:0.1%甲酸水,B:乙
腈;洗脱条件:0~5 min 5%~20% B,5~10 min 20%~35% B,10~15 min 35%~98% B,15~16 min 98%~100% B,16~17 min 100% B;柱温:35℃;流速:0.3 mL/min;进样量:5 μL;检测模式:正(负)离子模式;干燥气温度:350℃;干燥气流速:9 L/min;喷雾器压力:40 psig;碰撞电压:150 V;锥孔体电压:60 V;毛细管电压:3500(?3500)V;一级质谱扫描范围:50~1200m/z。
1.3 代谢图谱分析
质谱图采用Agilent Mass Hunter Qualitative Analysis软件按分子特征查找化合物,并生成.cef文件;将每个样品生成的.cef文件按照实验分组导入到Agilent Mass Hunter Mass Profiler Professional软件,利用偏最小二乘判别分析法(partial least squares discriminant analysis, PLS-DA)对各组小鼠粪便的代谢物进行分析;采用非配对t检验进行组间统计学分析;ID browser调用数据库检索,根据CAS、ChEBI、HMP或KEGG号从分子式水平确认化合物;将分析得到的代谢化合物进行目标MS/MS筛选和靶标分析,根据MFG(MS/MS)和MSC(molecular structure correlation)分析,最终从分子结构水平确证特征代谢物。
2 结果与分析
2.1 JFP-Ps在小鼠肠道粪便中代谢物的统计分析
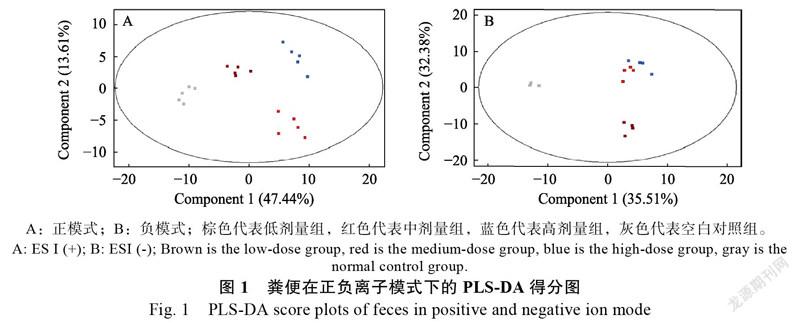
如图1所示,正模式检测条件,高、中、低剂量组和空白对照组完全分离,其中高剂量组小鼠与空白对照组小鼠之间的代谢物差异最大。高、中、低剂量组和空白对照组在負模式检测条件下同样完全分离,但高剂量组与中剂量组之间的代谢差异不大。
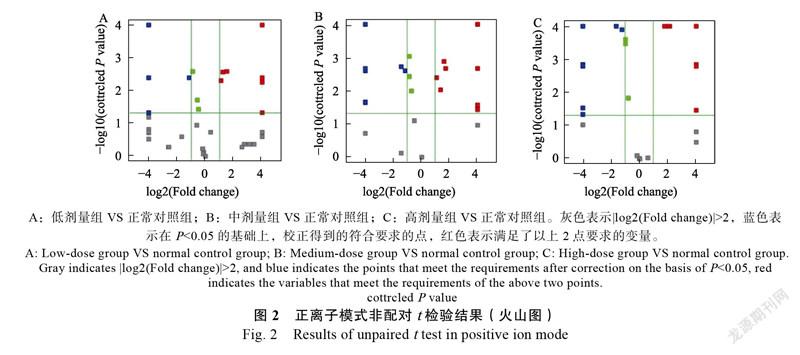
采用独立样本t检验分析,结果表明JFP-Ps高、中、低剂量组与空白对照组间的差异,将具有统计学意义(P<0.05)和Fold change>2的代谢物定义为差异代谢物。在正模式下(表1,图2),与正常对照组相比,低剂量组、中剂量组和高剂量组分别有28种、51种、58种差异化合物;在负离子模式下(表1,图3),与空白对照组相比,低剂量组、中剂量组和高剂量组分别有88种、57种、66种差异化合物。
2.2差异代谢物的鉴定
通过聚类热图分析(图4,图5)并采用二级质谱进行特征目标化合物鉴定。分析发现,正离子模式下特征代谢物主要有8种,其中高、中、低剂量组分别与空白组相比,2-氨基-十六烷酸、β环糊精、蜕皮甾酮、苯甲酰辅酶A等6种物质的相对含量显著上升,天冬氨酸、1-羟基维生素D3纤维二糖苷的相对含量都显著降低。负离子模式下特征代谢标志物主要有22种,其中高、中、低剂量组分别与空白组相比,4-酮-肉豆蔻酸、多巴胺、亮氨酰-亮氨酸、洛伐他汀酸等5种物质的相对含量显著上升,7-羟基哌泊噻嗪葡萄糖醛酸苷、N-棕榈酰甲硫氨酸等17种物质的相对含量显著降低。
2.3代谢通路分析
如表2所示,利用WikiPathway和KEGG数
据库对特征代谢化合物进行代谢通路分析,发现差异代谢物亮氨酰-亮氨酸、洛伐他汀酸、4-酮-肉豆蔻酸、天冬氨酸等13种物质参与调控机体内的氨基酸代谢通路;2,3-二氧古洛糖酸等7种物质参与调控胆固醇代谢与合成、脂肪酸合成、甘油三酯合成、ω-3和ω-6脂肪酸的合成通路;17-十八烷酸等7种物质与三羧酸循环,其中2-甲基丙基硫代葡萄糖苷参与2-氧羧酸循环,是2-氧羧酸循环里硫苷合成模块终产物之一。除此之外,JFP-Ps还能调节苯甲酸的降解、苯甲酸的氨基酸结合、谷胱甘肽和一碳代谢、核苷酸代谢、核受体、尿素循环和氨基代谢、GPCRs、A类视紫红质通路。
3 讨论
研究发现,部分氨基酸及其代谢过程中产生的化合物通过调控细胞因子的生成和分泌进而影响免疫反应[18],其中巨噬细胞内精氨酸经NOS-2途径合成NO并分泌IL-1β、TNF-α和IL-12等炎症细胞因子,并且IL-4、IL-6、TNF-α等能上调氨酸酶活性,促进精氨酸代谢[19]。本研究结果显示JFP-Ps参与调节氨基酸代谢通路,结果与朱科学等[13]研究一致,JFP-Ps可通过氨基酸代谢调节细胞TNF-α、IFN-γ和IL-1β的分泌,提高免疫活性。
PPAR 3种亚型都参与脂质代谢,其中PPARα在脂肪酸代谢中发挥降低血脂的作用,PPARγ参与脂肪细胞分化并调节糖脂代谢,与肥胖的发生及发展密切相关。本研究结果显示JFP-Ps在小鼠体内的代谢物(1S,2S)-3-氧代-2-戊基-环戊烷乙酸参与了PPAR信号通路,推测其可能通过激活PPAR信号通路的方式调节脂类代谢[20-21]。此外,差异代谢物洛伐他汀酸是活性代谢物,能够降低总胆固醇及低密度胆固醇的量,对于高血脂症治疗效果明显[22]。结果表明JFP-Ps的摄入有利于降低小鼠体内甘油三酯的含量。
多巴胺主要是在肠系膜等器官产生的,多巴胺对中枢系统、肾脏和胃肠道有着调节作用[23-25]。多巴胺还可以参与组胺H1受体拮抗剂通路,当H1受体与组胺反应时,H1受体被认为是产生过敏症状的靶点,组胺H1受体拮抗剂通过竞争与H1受体结合,从而抑制了组胺与靶点的相互作用,使其无法表达生理活性,表现出抗过敏的作用[26]。多糖不能被人体直接降解吸收,可作为肠道微生物的主要营养来源被肠道微生物酵解,酵解产物可调节肠道微生物结构[27-28]。肠道微生物的次级代谢产物可通过血液循环影响机体的免疫系统[29];差异代谢物β-环糊精参与环氧合酶抑制剂通路,环氧合酶抑制剂可以抑制肿瘤的发病率,诱导多种肿瘤细胞凋亡[30]。本研究结果表明摄入JFP-Ps可能有利于调节肠道菌群结构、其代谢物可通过缓解细胞损伤发挥其免疫活性[31-32]。
综上所述,JFP-Ps可以调节如多巴胺、苯甲酰辅酶A等30种内源性物质在小鼠体内的代谢,参与小鼠体内的苯丙氨酸、丙氨酸和天冬氨酸、色氨酸、胆固醇、2-氧羧酸、核苷酸代谢以及三羧酸循环、PPAR信号通路、A类视紫红质、苯甲酸的降解及其与氨基酸的结合、核受体、谷胱甘肽和一碳循环、尿素循环与氨基代谢通路。参与小鼠体内胆固醇、脂肪酸、甘油三酯、ω-3和ω-6脂肪酸和次级代谢产物的合成。JFP-Ps调节代谢通路的方式及代谢产物发挥生理活性的机制仍有待进一步研究。
参考文献
- FENG Z, DING C Q, LI W H, WANG D C, CUI D. Applications of metabolomics in the research of soybean plant under abiotic stress[J]. Food Chemistry, 2020, 310: 125914.
- LIN L Y, CHENG K L, XIE Z Q, CHEN C Y, CHEN L, HUANG Y D, LIANG Z. Purification and characterization a polysaccharide from Hedyotis diffusa and its apoptosis inducing activity toward human lung cancer cell line A549[J]. International Journal of Biological Macromolecules, 2019, 122: 64-71.
- FAN S R, ZHANG J F, NIE W J, ZHOU W Y, JIN L Q, CHEN X M, LU J X. Antitumor effects of polysaccharide from Sargassum fusiforme against human hepatocellular carcinoma HepG2 cells[J]. Food and Chemical Toxicology, 2017, 102: 53-62.
- WANG Y F, TIAN Y Q, SHAO J J, SHU X, JIA J X, REN X J, GUAN Y. Macrophage immunomodulatory activity of the polysaccharide isolated from Collybia radicata mushroom[J]. International Journal of Biological Macromolecules, 2018, 108: 300-306.
- WANG P C, ZHAO S, Yang B Y, WANG Q H, KUANG H X. Anti-diabetic polysaccharides from natural sources: A review[J]. Carbohydrate Polymers, 2016, 148: 86-97.
- MING K, CHEN Y, YAO F K, SHI J T, YANG J J, DU H X, WANG X Y, WANG Y X, LIU J G. Phosphorylated Codonopsis pilosula polysaccharide could inhibit the virulence of duck hepatitis A virus compared with Codonopsis pilosula polysaccharide[J]. International Journal of Biological Macromolecules, 2017, 94(Part A): 28-35.
- WANG W, ZHANG F M, LI Q, CHEN H, ZHANG W J, YU P, ZHAO T, MAO G H, FENG W W, YANG L Q, WU X Y. Structure characterization of one polysaccharide from Lepidium meyenii Walp, and its antioxidant activity and protective effect against H2O2-induced injury RAW264.7 cells[J]. International Journal of Biological Macromolecules, 2018, 118(Part A): 816-833.
- KARU N, DENG L, SLAE M, GUO A C, SAJED T, HUYNH H, WINE E, WISHART D S. A review on human fecal metabolomics: Methods, applications and the human fecal metabolome database[J]. Analytica Chimica Acta, 2018, 1030: 1-24.
- MUNOZ-GONZALEZ I, JIMENEZ-GIRON A, MARTIN- AL?VAREZ P J, BARTOLOME B, MORENO-ARRIBAS M V. Profiling of microbial-derived phenolic metabolites in human feces after moderate red wine intake[J]. Journal of Agricultural and Food Chemistry, 2013, 61(39): 9470-9479.
- ANGELIS M D, MONTEMURNO E, VANNINI L, COSOLA C, CAVALLO N, GOZZI G, MARANZANO V, CAGNO R D, GOBBETTI M, GESUALDO L. Effect of whole-grain barley on the human fecal microbiota and metabolome[J]. Applied and Environmental Microbiology, 2015, 81: 7945-7956.
- TRIMIGNO A, KHAKIMOV B, MEJIA J L C, MIKKELSEN M S, KRISTENSEN M, JESPERSEN B M, ENGELSEN S B. Identification of weak and gender specific effects in a short 3 weeks intervention study using barley and oat mixed linkage β-glucan dietary supplements: a human fecal metabolome study by GC-MS [J]. Metabolomics, 2017, 13: 108.
- ZHU K X, ZHANG Y J, NIE S P, XU F, HE S Z, GONG D M, WU G, TAN L H. Physicochemical properties and in vitro antioxidant activities of polysaccharide from Artocarpus heterophyllus Lam. pulp[J]. Carbohydrate Polymers, 2017, 155: 354-361.
- 朱科学, 王颖倩, 张彦军, 贺书珍, 徐 飞, 吴 刚, 谭乐和. 菠萝蜜多糖对脾淋巴细胞抗氧化作用及免疫功能的影响[J]. 食品科学, 2017, 38(23): 207-212.ZHU K X, WANG Y Q, ZHANG Y J, HE S Z, XU F, WU G, TAN L H. Antioxidant and immunoenhancing activity of polysaccharide from Artocarpus heterophyllus Lam. on spleen lymphocytes[J]. Food Science, 2017, 38(23): 207-212. (in Chinese)
- 姚思雯, 何佳丽, 朱科学, 谭乐和, 张彦军, 吴 刚. 菠萝蜜多糖体外酵解特征研究[J]. 现代食品科技, 2019, 35(3): 87-94.YAO S W, HE J L, ZHU K X, TAN L H, ZHANG Y J, WU G. In vitro fermentation characteristics of polysaccharides from Artocarpus heterophyllus Lam. pulp[J]. Modern Food Science and Technology, 2019, 35(3): 87-94. (in Chinese)
- 姚思雯, 朱科学, 何佳丽, 吴 刚, 谭乐和. 菠萝蜜多糖体外消化过程中抗氧化活性变化规律[J]. 热带农业科学, 2019, 39(2): 66-73, 99.YAO S W, ZHU K X, HE J L, WU G, TAN L H. Study on the change in antioxidant activity of polysaccharides from Artocarpus heterophyllus Lam. pulp during in vitro digestion[J]. Chinses Journal of Tropical Agriculture, 2019, 39(2): 66-73. (in Chinese)
- ZHU K X, YAO S W, ZHANG Y J, LIU Q B, XU F, WU G, DONG W J, TAN L H. Effects of in vitro saliva, gastric and intestinal digestion on the chemical properties, antioxidant activity of polysaccharide from Artocarpus heterophyllus Lam. (Jackfruit) pulp[J]. Food Hydrocolloids, 2019, 87: 952-959.
- TAN Y F, LI H L, LAI W Y, ZHANG J Q. Crude dietary polysaccharide fraction isolated from jackfruit enhances immune system activity in mice[J]. Journal of Medicinal Food, 2013, 16(7): 663-668.
- 胡秀红, 任文波, 黄 晶. 细胞因子与氨基酸代谢关系的研究进展[J/OL]. 中国免疫学杂志, [2021-11-24]. https://kns.cnki.net/kcms/detail/22.1126.R.20210128.1806.002.html.HU X H, REN W B, HUANG J. Research progress on the relationship between cytokines and amino acid metabolism[J/OL]. Chinese Journal of Immunology, [2021-11-24]. https://kns.cnki.net/kcms/detail/22.1126.R.20210128.1806.002.html. (in Chinese)
- TAKEDA Y, COSTA S, DELAMARRE E, RONCAL C, OLIVEIRA R L, SQUADRITO M L, FINISGUERRA V, DESCHOEMAEKER S, BRUYERE F, WENES M, HAMM A, SERNEELS J, MAGAT J, BHATTACHARYYA T, ANISIMOV A, JORDAN B F, ALITALO K, MAXWELL P, GALLEZ B, ZHUANG Z W, SAITO Y, SIMONS M, PALMA M D, MAZZONE M. Macrophage skewing by Phd2 haplodeficiency prevents ischaemia by inducing arteriogenesis[J]. Nature, 2011, 479: 122-126.
- 王 青. 枸杞多糖通过PPARγ调节脂肪细胞功能的机制研究[D]. 银川: 宁夏医科大学, 2016.WANG Q. Mechanism of LBP on regulating adipocyte function by PPARγ[D]. Yinchuan: Ningxia Medical University, 2016. (in Chinese)
- WANG M, WANG B, WANG S S, LU H, WU H, DING M Y, YING L L, MAO Y J, LI Y. Effect of quercetin on lipids metabolism through modulating the gut microbial and AMPK/PPAR signaling pathway in broilers[J]. Frontiers in Cell and Developmental Biology, 2021, 9: 616219.
- SERAJUDDIN A T M, RANADIVE S A, MAHONEY E M. Relative lipophilicities, solubilities, and structure-pharmaco?lo?gical considerations of 3-hydroxy-3-methylglutar?yl-coen?z?yme A (HMG-CoA) reductase inhibitors pravastatin, lovastatin, mevastatin, and simvastatin[J]. Journal of Pharmaceutical Sciences, 1991, 80(9): 830-834.
- ABRANTES DIAS A S, AMARAL PINTO J C, MAGALHAES M, MENDES V M, MANADAS B. Analytical methods to monitor dopamine metabolism in plasma: Moving forward with improved diagnosis and treatment of neurological disorders[J]. Journal of Pharmaceutical and Biomedical Analysis, 2020, 187: 113323.
- MEISER J, WEINDL D, HILLER K. Complexity of dopamine metabolism[J]. Cell Communication and Signaling, 2013, 11:34.
- ELDRUP E. Significance and origin of DOPA, DOPAC, and dopamine-sulphate in plasma, tissues and cerebrospinal fluid[J]. Danish Medical Bulletin, 2004, 51: 34-62.
- 苗 菁. 组胺H1受体与拮抗剂的相互作用[J]. 广州化工, 2012, 40(16): 72-73, 124.MIAO J. The interactions between histamine H1 receptor and histamine H1 receptor antagonists[J]. Guangzhou Chemical Industry, 2012, 40(16): 72-73, 124. (in Chinese)
- 刘荣瑜, 王 昊, 张子依, 宋冬雪, 陈锦瑞, 汲晨锋. 多糖与肠道菌群相互作用的研究进展[J/OL]. 食品科学, [2022-01-24]. http://kns.cnki.net/kcms/detail/11.2206.TS.2?0?210205.1625.043.html.LIU R Y, WANG H, ZHANG Z Y, SONG D X, CHEN J R, JI C F. Progress on interaction of polysaccharides with intestinal flora[J/OL]. Food Science, [2022-01-24]. http://kns. cnki.net/kcms/detail/11.2206.TS.20210205.1625.043.html. (in Chinese)
- DAVANI-DAVARI D, NEGAHDARIPOUR M, KARIMZADEH I, SEIFAN M, MOHKAM M, MASOUMI S J, BERENJIAN A, GHASEMI Y. Prebiotics: Definition, types, sources, mechanisms, and clinical applications[J]. Foods, 2019, 8(3): 92.
- DODD D, SPITZER M H, VAN TREUREN W, MERRILL B D, HRYCKOWIAN A J, HIGGINBOTTOM S K, LE A, COWAN T M, NOLAN G P, FISCHBACH M A, SONNENBURG J L. A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites[J]. Nature, 2017, 551: 648-652.
- YU J, TANG B D, LEUNG W K, TO K F, BAI A H C, ZENG Z R, MA P K, GO M Y Y , HU P J, SUNG J J Y. Different cell kinetic changes in rat stomach cancer after treatment with celecoxib or indomethacin: implications on chemoprevention[J]. World Journal of Gastroenterology, 2005, 11(1): 41-45.
- 陳 秋, 夏永鹏, 邱宗荫. 蜕皮甾酮对胰岛素抵抗细胞模型胰岛素敏感性和糖代谢的影响[J]. 中国药理学通报, 2006, 22(4): 460-464.CHEN Q, XIA Y P, QIU Z Y. Effects of ecdysterone on insulin sensitivity and glucosem etabolism in insulin-resistant cell model[J]. Chinese Pharmacological Bulletin, 2006, 22(4): 460-464. (in Chinese)
- ZHANG X H, XU X X, XU T, QIN S. β-ecdysterone suppresses interleukin-1β-induced apoptosis and inflammation in rat chondrocytes via inhibition of NF-κB signaling pathway[J]. Drug Development Research, 2014, 75: 195-201.
