Nitrate reduction by· from UV-activated HCOOH
2022-04-19XuYiqiaoWuLeiZhengTianyi
Xu Yiqiao Wu Lei Zheng Tianyi,2
(1School of Energy and Environment, Southeast University, Nanjing 210096, China)(2Jiangsu Branch of China Municipal Engineering Northwest Design and Research Institute Co., Ltd., Nanjing 210017, China)
Abstract:To address the environmental and health hazards of nitrate in water, a denitrification advanced reduction process(ARP)using only formic acid(HCOOH)activated by ultraviolet(UV)light was proposed.The efficiency, influencing factors, mechanism, and kinetics of the reduction were investigated through component analysis and radical detection.Results show that, after 90 min of UV illumination, the reduction and gas conversion ratios of 50 mg/L reach 99.9% and 99.8%, respectively, under 9 mM of C0(HCOOH), pH = 3.0, and N2 aeration.Meanwhile, 96.7% of HCOOH is consumed and converted into gas.The conversion process includes the transformation to followed by a further reduction to gas and a direct conversion into gas, introducing small amounts of nitrite and ammonia.The carbon dioxide anion radical(· from HCOOH/HCOO- is the principal cause of reduction by UV/HCOOH/N2 ARP.In contrast,· production is caused by the hydroxyl radical(·OH).The reduction efficiency is enhanced by the increase in the light intensity, considerably affected by the initial pH, and less affected by inorganic anions, including Cl-, .The initial HCOOH concentration and light intensity are the main factors that influence the reduction rate.
Key words:nitrate reduction; advanced reduction process; ultraviolet; HCOOH;·
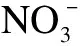
Inspired by the aforementioned systems, this study aims to develop a highly efficient denitrification process utilizing ARPs without producing other pollutants.Herein, a simple system with UV as the activation method and HCOOH alone as the reducing agent was established.
1 Materials and Methods
1.1 Chemicals
HCOOH, sodium nitrate, and phosphate were purchased from Tianjin Kemiou Chemical Reagent Co., Ltd.(Tianjin, China).5,5-Dimethyl-1-pyrrolidine N-oxide(DMPO)was purchased from Aladdin(Shanghai, China).The other chemicals, such as potassium chloride, potassium bicarbonate, and potassium dihydrogen phosphate, were provided by Sinopharm Group Chemical Reagent Co., Ltd.(Shanghai, China).All chemicals were of analytical grade.
1.2 Experimental procedures

1.3 Analytical methods
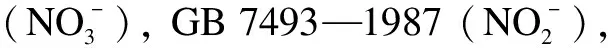
2 Results and Discussion



(a)
2.2 Safety evaluation of UV/HCOOH/N2


(a)
To guarantee the reduction effect,theC0(HCOOH)concentration used in subsequent studies was 9 mmol/L, and the trends of the TOC and HCOOH concentrations were further analyzed(see Fig.2(b)).At the end of UV illumination, more than 96.7% of HCOOH was consumed, and the residual HCOOH concentration was at a negligible level(0.3 mmol/L).
2.3 Reaction mechanism analysis
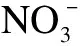

(a)
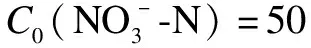

(a)

(a)

(a)
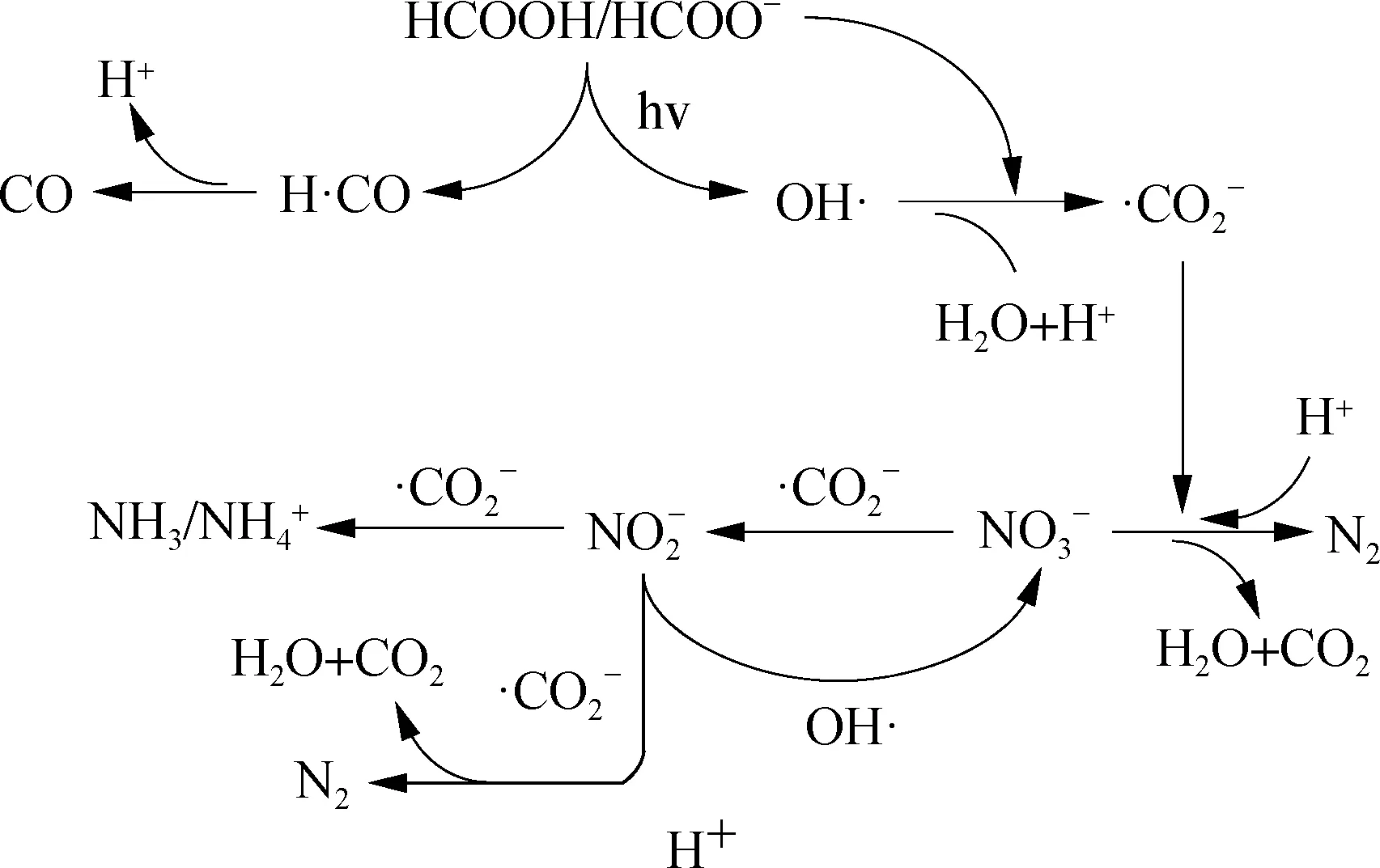
Fig.7 Conceptual reaction mechanism of UV-activated HCOOH denitrification
2.4 Effects of some factors on reduction


(a)
Solution pH variation is shown in Fig.9.When the initial pH was 2.0 and 10.0, the solution pH remained unchanged within 90 min.When the initial pH was 4.0, 6.0, 8.0, and unadjusted(i.e., 3.0), the solution pH increased with time and reached a certain value eventually, indicating that a large amount of HCOOH was consumed in the UV activation process, resulting in the solution pH changing to neutral and weakly alkaline.

Fig.9 pH variation with reaction time
Consequently,the initial pH is one of the main factors that influence the UV/HCOOH/N2denitrification system.The optimal pH is 3 to 4.Meanwhile, a hyperacid environment has an adverse effect on the reduction effect, and a neutral or alkaline environment also reduces the reduction effect but to a slight extent.
The influence of common anions in natural water wasinvestigated under a 125 W high-pressure mercury lamp.The concentration of anions was set at 1, 10, and 100 mmol/L.
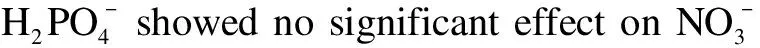

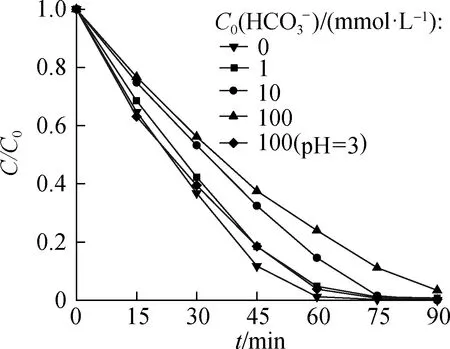
Fig.10 Effects of reduction
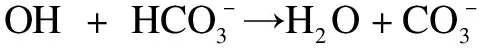
(1)

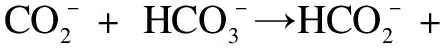
2.5 Kinetic analysis


Tab.1 Kinetic parameters of reduction under different influencing factors
3 Conclusions



杂志排行
Journal of Southeast University(English Edition)的其它文章
- Statistical analysis of nondestructive testing results of cast steel joints in civil engineering structures
- Novel sensitivity analysis method and dynamics optimization for multiple launch rocket systems
- Detection of solar radio burst intensity based on a modified multifactor SVM algorithm
- Metrics analysis of tactile perceptual space based on improved NMDS for leather textures
- Short-term traffic flow prediction with PSR-XGBoost considering chaotic characteristics
- Contract stability and default risk control of pig farming in the company and farmer mode
