Chemical design and characterization of cellulosic derivatives containing high-nitrogen functional groups: Towards the next generation of energetic biopolymers
2022-04-19AhmeFouziTrchounDjllTrcheThomsKlpotkeAmirAbelzizMehiDerrjiSlimneBekhouche
Ahme Fouzi Trchoun , Djll Trche , Thoms M. Klp¨otke ,Amir Abelziz , Mehi Derrji , Slimne Bekhouche
a Energetic Materials Laboratory, Teaching and Research Unit of Energetic Processes, Ecole Militaire Polytechnique, BP 17, Bordj El-Bahri,16046, Algiers,Algeria
b Energetic Propulsion Laboratory, Teaching and Research Unit of Energetic Processes, Ecole Militaire Polytechnique, BP 17, Bordj El-Bahri,16046, Algiers,Algeria
c Department of Chemistry, Ludwig Maximilian University Butenandtstrasse 5-13 (D), D-81377, Munich, Germany
d Process Engineering Laboratory, Teaching and Research Unit of Energetic Processes, Ecole Militaire Polytechnique, BP 17, Bordj El-Bahri,16046, Algiers,Algeria
Keywords:Cellulose Surface functionalization Tetrazole Nitrate ester Nitrogen-rich polymer Energetic properties
ABSTRACT In this research, a promising class of insensitive and high-energy dense biopolymers, which contain nitrogen-rich 1H-tetrazol-1-yl acetate and nitrate ester functional groups, was successfully synthesized through tetrazole derivatization and nitration of cellulose and its micro-sized derivative (TNCN and TCMCN). Their molecular structures, physicochemical properties, thermal behaviors, mechanical sensitivities and detonation performances were studied and compared to those of the corresponding nitrocellulose and nitrated micro-sized cellulose (NCN and CMCN). The developed energetic TNCN and TCMCN exhibited insensitive character with excellent features such as density of 1.710 g/cm3 and 1.726 g/cm3, nitrogen content of 20.95% and 22.59%, and detonation velocity of 7552 m/s and 7786 m/s,respectively, and thereby demonstrate their potential applications as new generation of energetic biopolymers to substitute the common NCN. Furthermore, thermal results showed that the designed nitrated and chemical modified cellulosic biopolymers displayed good thermal stability with multistep decomposition mechanism.These results enrich future prospects for the design of promising insensitive and high-energy dense cellulose-rich materials and commence a new chapter in this field.
1. Introduction
Nowadays, the need for high-energy dense materials (HEDMs)based on sustainable and environmentally friendly polymers has created immense interest in both civilian and military purposes to substitute nonrenewable petroleum materials [1-4]. As known,native cellulose (NC) is by far the most plentiful and everlasting polymer existing in the biosphere, which can be acquired from lignocellulosic plants, tunicates, algal biomass and some bacteria[5,6]. This fascinating carbohydrate biopolymer, considered as an inexhaustive wellspring of starting materials, can be easily functionalized and modified with numerous chemical groups,to satisfy the requirements of several industrial uses, and certainly there is still a lot to discover and celebrate in cellulose in the future[7,8].As an important derivative of NC,cellulose microcrystals(CMC)can be produced by disintegration of cellulose fibers to microscale particles using commonly acid hydrolysis to generate highly ordered CMC [9,10]. In addition to the inherent properties of NC, the CMC exhibits some awesome features such as unique microstructure,non-toxicity, high crystallinity index, increased surface reactivity,excellent stiffness and improved thermal stability,attributing to its growing use as prominent biopolymer for advanced cellulosebased materials applications, including nanocomposites, biomedical products, food industry, bioadsorbents and high-energy materials [11-14]. In addition, the presence of several reactive hydroxyl (OH) groups on the cellulosic skeletons allowed it to be functionalized through the incorporation of various chemical functionality to produce new generation of cellulosic materials with attractive properties, which, in turn, improve their effectiveness for a given application [15-17]. On the other hand, the opportunity of introducing energy-rich functional groups (e.g.O-NO, N, N-NO, etc.) in the surface of cellulosic backbone is considered as an hot research subject, which can advance the development of emerging and highly efficient energetic celluloserich materials for advanced applications [4,11,15,18]. In this context, native cellulose nitrate (NCN, nitrocellulose) was the first energetic cellulose derivative to be discovered, and still be employed in a diverse range of military and civilian applications owing to its unique and noticeable characteristics compared to other energetic binders [19-21]. Nonetheless, long-term insight with traditional NCN demonstrates some of its constraints,including the low density,the increased mechanical sensitivity and the inappropriate combustion performance [22,23]. These issues can be efficiently addressed by downscaling modification and surface functionalization of NC precursor to produce promising insensitive and energy-rich cellulosic polymers with excellent features. Recently, several significant achievements in research on advanced cellulose based energetic biopolymers have been realized[11,15,24,25]. Based on the reduced scale process, it was demonstrated that the particle size reduction of NC to its micro-or nanosized derivatives is very helpful for preparing new promising nitrate esters based cellulose materials with better features than the conventional NCN [19,23]. Besides that, the great advancement in the surface functionalization of cellulose and its downscaled derivatives has pushed the scientific community to explore new opportunities to generate highly performance biopolymers with fascinating synergetic effects and excellent physicochemical characteristics [26-29].
Regarding the organic chemistry of energetic materials, tetrazole moieties have received notoriety as outstanding energetic backbones owing to their outstanding features such as environmental safety, tailored performance, high nitrogen content, insensitivity character and good stability [30-32]. Moreover, the high nitrogen content is one of the most important requirements to achieve an increased performance for energetic compounds.In this framework, various research groups such as that of Klap¨otke and co-workers have reported the incorporation of this unique class of nitrogen-rich heterocycles into sample skeleton as most promising trend for the design and construction of promising energetic substances [33,34]. In addition, numerous theoretical and experimental investigations have been conducted over the past few decades to produce highly energetic polymers containing tetrazole moieties for use in advanced applications [35-37]. For instance, a novel 3D energetic coordination polymer has been recently developed by Zhang et al.[38],through tetrazole connections.The authors reported that the synthesized energetic polymer displays a tightly packed 3D framework with high thermal stability,excellent water resistance, and insensitivity to external stimuli. More recently,Tarchoun et al.[2],showed that the surface decoration of anhydroglucose units with 1H-tetrazol-5-yl acetate moieties could offer the opportunities for promising cellulose-rich energetic materials with appropriate qualities. Besides that, it was highlighted by several researches that the combination of tetrazole heterocycles with energy-rich functional groups in one molecule is an efficient approach to enhance the physical stability and energetic properties of energy-rich compounds [39-42]. Therefore, the purpose of the present work is to design a prominent generation of insensitive and high-energy dense cellulose-rich materials through surface functionalization of NC and CMC with high-nitrogen 1Htetrazol-1-yl acetate moieties and explosophoric nitrate ester groups. The physicochemical properties, molecular and crystalline structure, thermal behavior and detonation parameters of the designed energetic biopolymers (2-(1H-tetrazol-1-yl)acetate native cellulose nitrate (TNCN), and 2-(1H-tetrazol-1-yl)acetate cellulose microcrystals nitrate (TCMCN) were deeply investigated,demonstrating their improved features with respect to those of the common nitrocellulose and emergent micro-sized cellulose nitrate.
2. Experimental section
2.1. Materials and reagents
Commercial NC and CMC originated from cotton linter with viscosity-average molecular weights (M) of 4.9 × 10and 2.2 × 10g/mol, respectively, were provided by Merck. N,Ndimethyl acetamide (DMAc), p-toluensulfonyl chloride, lithium chloride (LiCl), triethylamine, and 2-(1H-teraol-1-yl)acetic acid(also named 1H-tetrazole-1-acetic acid) were purchased from Sigma-Aldrich, whereas, acetonitrile, sodium bicarbonate(NaHCO), acetic anhydride (AcO) and fuming nitric acid (HNO)were supplied by VWR-Prolabo.
2.2. Characterization
Electronic Accupyc 1340 II densimeter was used to determine the experimental densities(ρ)of the studied biopolymers and their precursors.Their nitrogen contents(N)were also accessed using a Vario III Elemental analyzer. The X-Ray diffraction (XRD) patterns were collected by a PANalytical X’pert PRO Multi-Purpose diffractometer operating at a generator setting of 45 kV and 40 mA. The crystallinity index (CrI) of samples was calculated using Segal’s equation (Eq. (1)) for starting precursors and Eq. (2) for nitrated derivatives.

Where Istands for the intensity at 200 peak,Iis the intensity of the disordered region between planar reflections 110 and 200.Scr and Samp are the total areas for the ordered and paracrystalline parts, respectively.
A scanning electron microscope (SEM) apparatus, model 157 JEOL JSM-6360 LV, was employed to examine the surface morphology of the different samples. Before analysis, the samples surfaces were coated with carbon by a sputter coater to reduce charging effects.
Fourier transform infrared spectrometry (FTIR) analysis were carried out using a Parkin Elmer spectrometer recorded in ATR mode with a scan number of 64 at a resolution of 4 cm.The solidstateC NMR experiments using the technique of crosspolarization magic angle spinning (CP/MAS) were accomplished employing a Bruker Avance III-500 spectrometer operating at frequency of 76.46 MHz, with an external static magnetic field of 11.74 T.
The thermal stability of the developed energetic biopolymers and their starting materials was investigated by an OZM Research DTA 552-Ex analyzer. For each measurement, a sample mass of 3-4 mg was analyzed under constant nitrogen gas atmosphere(40 ml/min) from 50C to 450C, at a heating rate of 5C/min.
2.3. Energetic performance prediction and sensitivity assessment
The combustion heats of the nitrated biopolymers were determined by an adiabatic calorimetric oxygen bomb (model 6200)from Parr instruments.The obtained results were used to calculate the heats of formation based on the Hess’s law, as described in literature[43].The EXPLO5 V6.04 program was then used to predict the detonation parameters of the designed energetic biopolymers[44]. In addition, sensitivity characteristics against impact and friction were evaluated using a BAM drop hammer and a friction tester following the recommendations of the STANAG 4489 and STANAG 4487, respectively[45].
2.4. Synthetic procedure
The chemical design of 1H-tetrazol-1-yl acetate functionalized cellulosic biopolymers (TNC and TCMC) and their nitrated derivatives was achieved as depicted in Scheme 1.Firstly,a dissolution process of the dried starting biopolymer(NC or CMC)with a ratio of 1:40 (g/ml) solid over liquor was conducted in the well common DMAc/LiCl solvent system as reported in our previous work [28].Afterword, the primary reactive alcohol function on of anhydroglucose units (AGUs) were excited by slowly adding a determined amount of p-toluensulfonyl chloride(5 mol/AGU)dissolved in 50 ml of DMAc,in the presence of triethylamine as base catalyst.This tosylation step was homogeneously performed at low temperature (3-8C) for 24 h. Next, the nucleophilic displacement of primary inserted tosyl moieties with 1H-tetrazol-1-yl acetate ones was carried out through the small portion addition of 2-fold molar excess of 1H-tetrazole-1-acetic acid as compared to AGU. The reaction mixture was then heated at 60C for 24 h, followed by cooling to ambient temperature and precipitation in distilled water.Lastly, the resulted precipitate was thoroughly washed with acetonitrile and distilled water for several times,followed by drying overnight at 70C to obtain the corresponding yellowish 2-(1Htetrazol-1-yl)acetate native cellulose (TNC), and 2-(1H-tetrazol-1-yl)acetate cellulose microcrystals (TCMC).
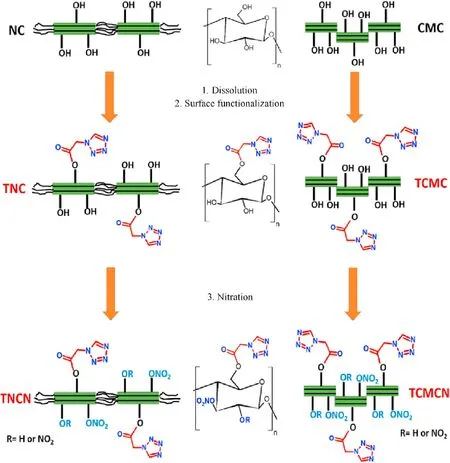
Scheme 1. Synthesis route of surface modified cellulosic biopolymers and their energetic derivatives.
After that, the electrophilic nitration of staring cellulosic biopolymers (NC and CMC) and their 1H-tetrazol-1-yl acetate functionalized derivatives was conducted using a mixture of AcO/fuming HNO(2:1,v/v)with a ratio of 1:45(g/ml)solid over fuming nitric acid.The nitration process was performed at 0C for 10 min,and for another 2 h at ambient temperature. The resulted nitrated compounds were then processed with boiled water and NaHCO(2%,w/v)until neutralization,followed by drying under vacuum to afford the corresponding native cellulose nitrate (NCN), cellulose microcrystals nitrate (CMCN), 2-(1H-tetrazol-1-yl)acetate native cellulose nitrate (TNCN), and 2-(1H-tetrazol-1-yl)acetate cellulose microcrystals nitrate(TCMCN).
3. Results and discussion
3.1. Physicochemical properties
The regioselectivity functionalization of cellulosic precursors was achieved by substituting the activated hydroxyl(OH)functions with tetrazole-acetate moieties through 1H-tetrazole-1-acetic acid esterification process to produce TNC and TCMC with Nof 18.35%and 19.49%,respectively,which are considerably higher than that of the conventional nitrocellulose with maximum nitrogen content of 14.14%. Furthermore, the determined experimental densities reported in Table 1 show that the designed nitrogen-rich cellulosic derivatives present better values than their starting polymers. In addition, as shown in Table 1, the nitrogen content and density of TCMC are obviously higher than those of TNC, which provide evidence for the benefit of using CMC as starting biopolymer rather than NC to increase the surface functionalization degree [11,19].
Another surface modification of the starting precursors and their tetrazole-functionalized derivatives was also accomplished through electrophilic substitution of the free OH groups on the surface of AGUs with explosophoric ONOones as depicted in Scheme 1. The increased density and nitrogen content of the resulted energetic biopolymers with respect to their precursors indicate the successful nitration process. It can be also deduced from Table 1 that the developed TNCN and TCMCN display better Nand ρ than nitrated unmodified samples (NCN and CMCN),following the same tendency of their precursors. These outcomes proved that the combination of nitrogen-rich functional groups in the same AGU could ultimately enhance the physicochemical features of the resulted energetic biopolymers. Moreover, the physicochemical characteristics of the nitrated compounds based on micro-sized cellulose (TCMCN and CMCN) are found to be higher than those based on ordinary cellulose (TNCN and NCN). This expectation is due to the high amount of reactive nucleophilic OH sites and short fiber sizes in the case of CMC and TCMC precursors,which improves the electrophilic insertion of the nitronium ions(NO)into the interior cellulosic fibers and consequently acceleratethe nitration rate[46-48].These findings lead to conclude that the performed chemical modification pathway is effective, and provides a promising generation of energetic cellulosic biopolymers with attractive densities, which are comparable or slightly higher than that of recently developed energetic cellulose-rich materials,such as nitrated azidodeoxy cellulose (1.706 g/cm) [11], nitrated ethylenediamine-functionalized cellulose (1.711 g/cm) [28], and significantly higher than that of glycidyl azide polymer(1.29 g/cm)[49].
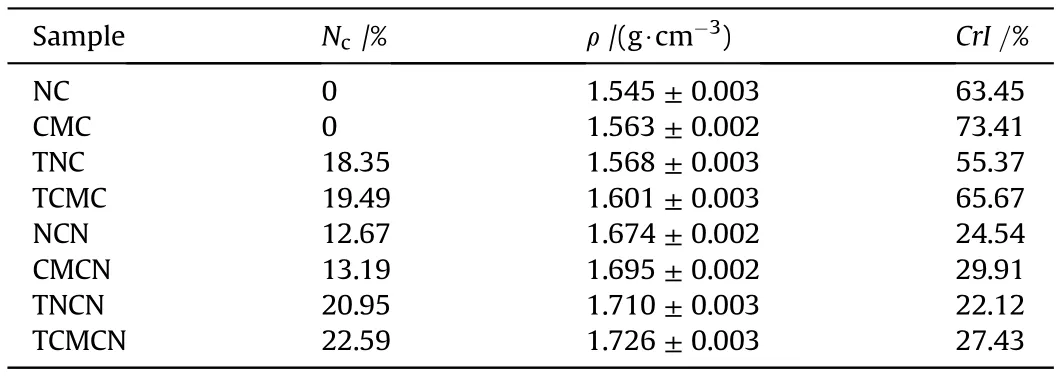
Table 1 Physicochemical characteristics of the unmodified and surface modified cellulosic biopolymers and their energetic derivatives.
3.2. Crystalline structure and surface morphology
Regarding the X-ray diffractograms of nitrated biopolymers,it is revealed from Fig.1(b)that all samples show the typical crystalline structure of nitrocellulose with common diffraction peaks located at 12.5and 15-35, which are corresponded to the crystalline regions and disordered domains of nitrocellulose chains, respectively [19,20]. In order to further elucidate the eventual alteration that can occur in the cellulose crystallization framework after nitration process,the CrI of nitrated compounds were calculated by peak deconvolution method of their diffractograms using Pearson VII function and Eq. (2) [28,54]. The significant decreasing of CrI from unmodified and tetrazole-acetate functionalized cellulosic precursors to their nitrated derivatives is associated with the loss of OH groups and their replacements with explosophoric O-NO,which cause increasing randomness of the amorphous phase and expand the range spacing of linked β-glucopyranose units resulting in the drop in the crystalline order as already reported in the literature [2,46]. It can be also deduced from Table 1 that the crystallinity degree of TNCN (22.12%) and TCMCN (27.43%) are slightly inferior in comparison to those of NCN(24.54%)and CMCN(29.91%), respectively, following the identical evolution of their precursors. It is interesting to mention that the CrI of TCMCN designed in this study from commercially available microcrystalline cellulose is comparable to that of recently synthesized CMC based energetic materials, including nitrated ethylenediaminefunctionalized CMC (27.45%) [28], nitrated azide-functionalized CMC (27.64%) [11], and better than that of nitrated tetrazol-5-ylacetate-functionalized CMC (25.95%) [2].
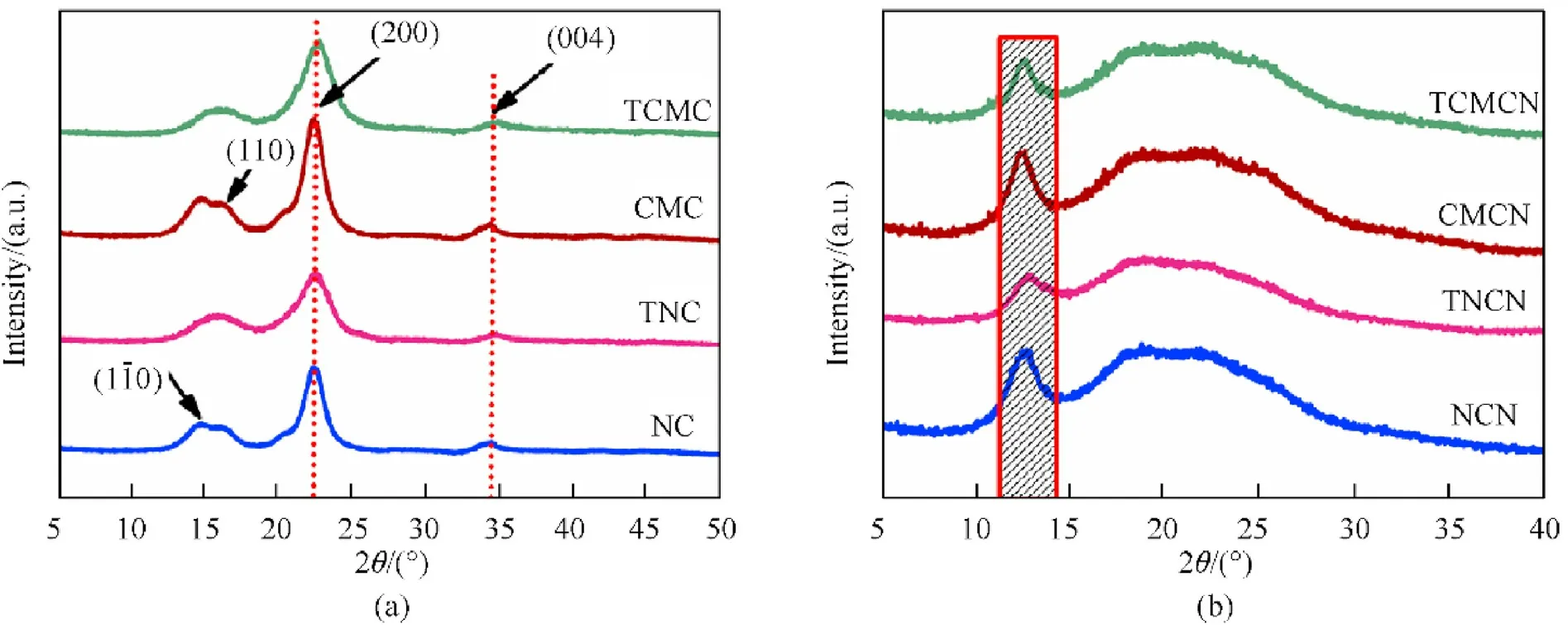
Fig.1. XRD patterns of (a) nitrogen-rich functionalized biopolymers and their cellulosic precursors; (b) nitrated unmodified and tetrazole-acetate functionalized biopolymers.
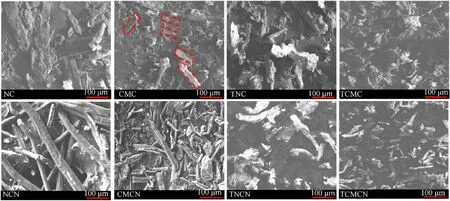
Fig. 2. SEM micrographs of unmodified and tetrazole-acetate functionalized biopolymers before and after nitration.
The surface morphology of the studied biopolymers before and after chemical functionalization are also investigated, and the obtained SEM micrographs are presented in Fig. 2. It can be see that NC presents homogeneous structure of small bundles of fibers and individual long fibers, whereas, TNC exhibits irregular rod-like fibers with rough surface.This microstructure alteration is probably due to the physical swelling and untwisting of the fibers during esterification followed by the formation of linked tetrazole-acetate groups that cover the surface of cellulosic fibers.However,a similar rough rod-shape micronized structure is observed for microcrystalline samples (CMC and TCMC), revealing a homogeneous chemical modification with less significant morphological alteration.Many aggregates are also noted for CMC and TCMC,which are attributed to the van der Waals interactions between hydrogen bonds [55,56]. On the other side, a partial debundling and disintegration of the fibers is noticed after incorporation of electron attractive nitrate esters functions through nitration reaction.Furthermore, it can be revealed from SEM micrographs that all nitrated biopolymers gave nearly analogous surface microstructure as their respective precursors, except for NCN that displays rough surface rather than smooth one for NC,indicating a heterogeneous nitration of NC fibers[46,57].Similar findings about the influence of nitration process on the surface morphology of cellulosic fibers are already mentioned in previous reports[18,19,23].
3.3. Molecular structure characterization
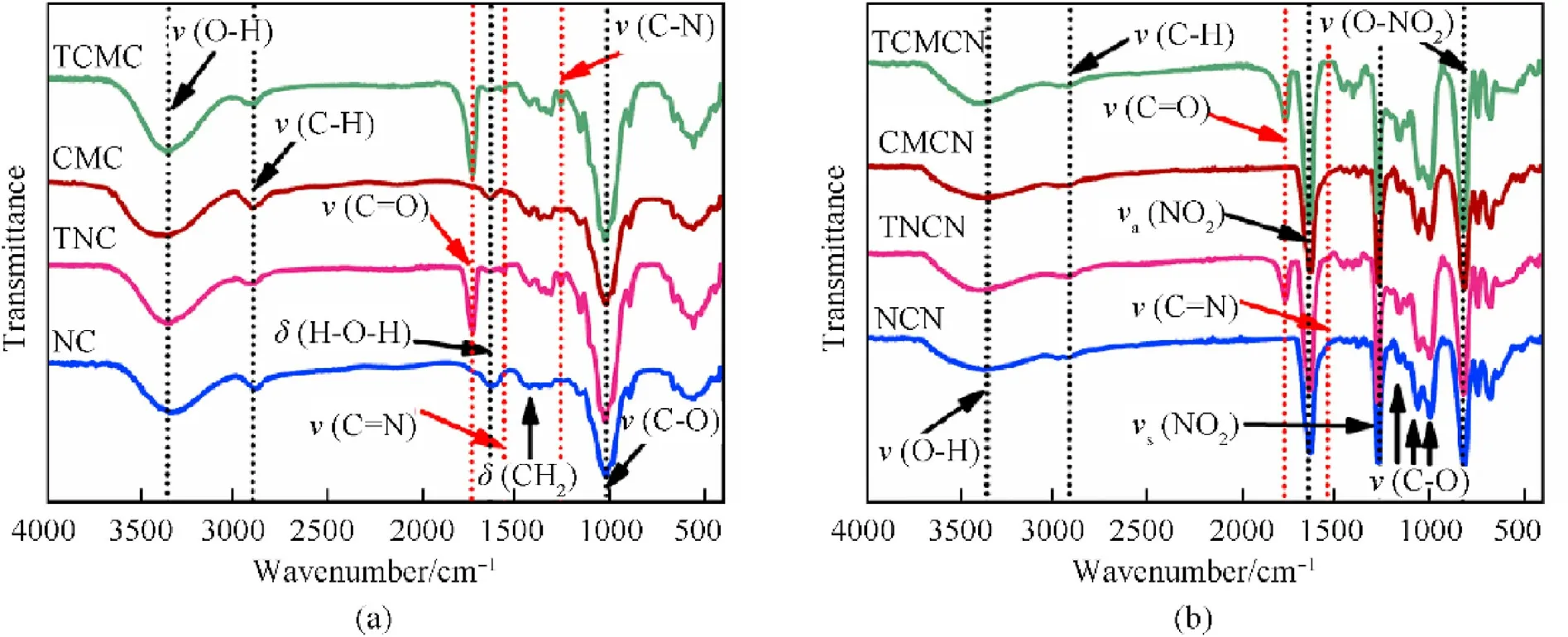
Fig. 3. FTIR spectra of (a) tetrazole-acetate modified biopolymers and their cellulosic precursors; (b) nitrated unmodified and tetrazole-acetate functionalized biopolymers.
The successful formation of the synthesized biopolymers and their starting cellulosic materials were investigated by identifying their molecular structure using FTIR andC solid-state NMR characterizations.The starting biopolymers and chemical modified cellulosic derivatives spectra plotted in Fig.3(a)show the presence of the typical functional groups of cellulose,which are comparable to the spectra of cellulosic materials isolated from other renewable feedstocks [50,56,58]. For instance, the band at 3340 cmis related to the OH stretching,those at 2901,1650 and 1430 cmare assigned to the to the C-H stretching,the O-H bending of absorbed water and the CHbending vibration, respectively, whereas the skeletal vibration of the C-O of pyranose ring and the β-glycosidic linkage vibration are recorded at 1060 and 898 cm, respectively.Furthermore, additional bands are visible in the spectra of functionalized samples belong to the characteristic stretching vibrations of tetrazole ring, detected at 1580 cmfor C=N, and 1260 cmfor C-N [59,60]. The representative band of carbonyl linkage(C=O)between AGU and tetrazole ring is also observed at 1735 cm[2,43]. These findings demonstrate the successful insertion of 2-(1H-tetrazol-1-yl)acetate moieties on the surface of AGUs, without alteration of the main cellulosic skeleton. In addition, the increased intensity of tetrazole-acetate vibrational bands from TNC to TCMC correlates quite well with Nc evolution obtained by elemental analysis.
In the case of the designed energetic biopolymers, it can be observed from Fig. 3(b) that all nitrated samples show the typical FTIR profile of nitrocellulose with representative functional groups located 3340,1650,1280, 830, and 760-680 cm, associated with the free OH stretching,asymmetric and symmetric NOstretching,and stretching and bending vibrations of O-NO, respectively[28,48]. In addition to the common peaks of nitrocellulose, the characteristic vibrational bands of tetrazole-acetate are still present in the spectra of TNCN and TCMCN with a slight shift in numeric value in comparison to their respective precursors, which is probably caused by the steric effect of inserted electron-withdrawing NOfunctions that strongly increase the shieling impact. These results also prove the efficient nitration process and the successful formation of the desired energetic biopolymers. It can be also deduced from Fig. 3(b) that the nitration efficiency of CMC precursors seems to be better than those of NC ones based on the increase of the peak intensities related to the explosophoric NOand O-NOgroups,which is in good agreement with the elevated Nof CMC-based energetic biopolymers found by elemental analysis.
Similarly, solid-state NMR spectroscopy of the nuclideC has been employed to further clarify the chemical structure of the studied cellulose-rich biopolymers. It can be established from Fig. 4(a) that the NMR spectra of the unmodified cellulosic precursors and their surface-functionalized polymers display the common chemical environments of carbon present in the pyranose ring of cellulose backbone that are the C6 at 62-65 ppm, the overlapped C2,3,5 at 71-76 ppm,the C4 at 82-89 ppm,and the C1 at 105 ppm [15,55]. Besides to the above mentioned signals, the functionalized samples spectra show the appearance of new chemical shifts belong to the characteristic carbon resonances of incorporated 2-(1H-tetrazol-1-yl)acetate moiety observed at 51 ppm for the CHlinked to nitrogen atom of tetrazole ring (C8),145 ppm for quaternary tetrazolic carbon(C9)and 170 ppm for the carbonyl of ester linkage (C=O). An obvious decrease in the C6 signal intensity is also recorded after chemical modification,demonstrating the regioselectivity C6 esterification mechanism.These deductions emphasized that the surface functionalization process has been carried out successfully, which agreed well with the FTIR results and nitrogen content assessments.
Regarding theC NMR spectra of nitrated biopolymers presented in Fig.4(b),it should be noted that the NMR experiments of NCN and CMCN were not conducted due to their elevated shock sensitivity (Table 2). As observed, nitrated tetrazole-acetate functionalized samples reveal the well-known resonance signals of carbon present in the backbone of nitrocellulose centered at 72 ppm, 78 ppm and 101 ppm for overlapped C5,6, overlapped C2,3,4 and C1, respectively [15,61]. It is also noticeable from Fig. 4(b) that the carbon chemical shifts of 2-(1H-tetrazol-1-yl)acetate moiety are still present with some up-field shift in numeric value compared to their precursors, demonstrating that the skeleton of the functionalized biopolymers remained unmodified after nitration. Based on theC NMR results and the obtained FTIR finding, we can confirm the successful molecular structure of the synthesized nitrogen-rich energetic biopolymers and their precursors.
3.4. Thermal stability
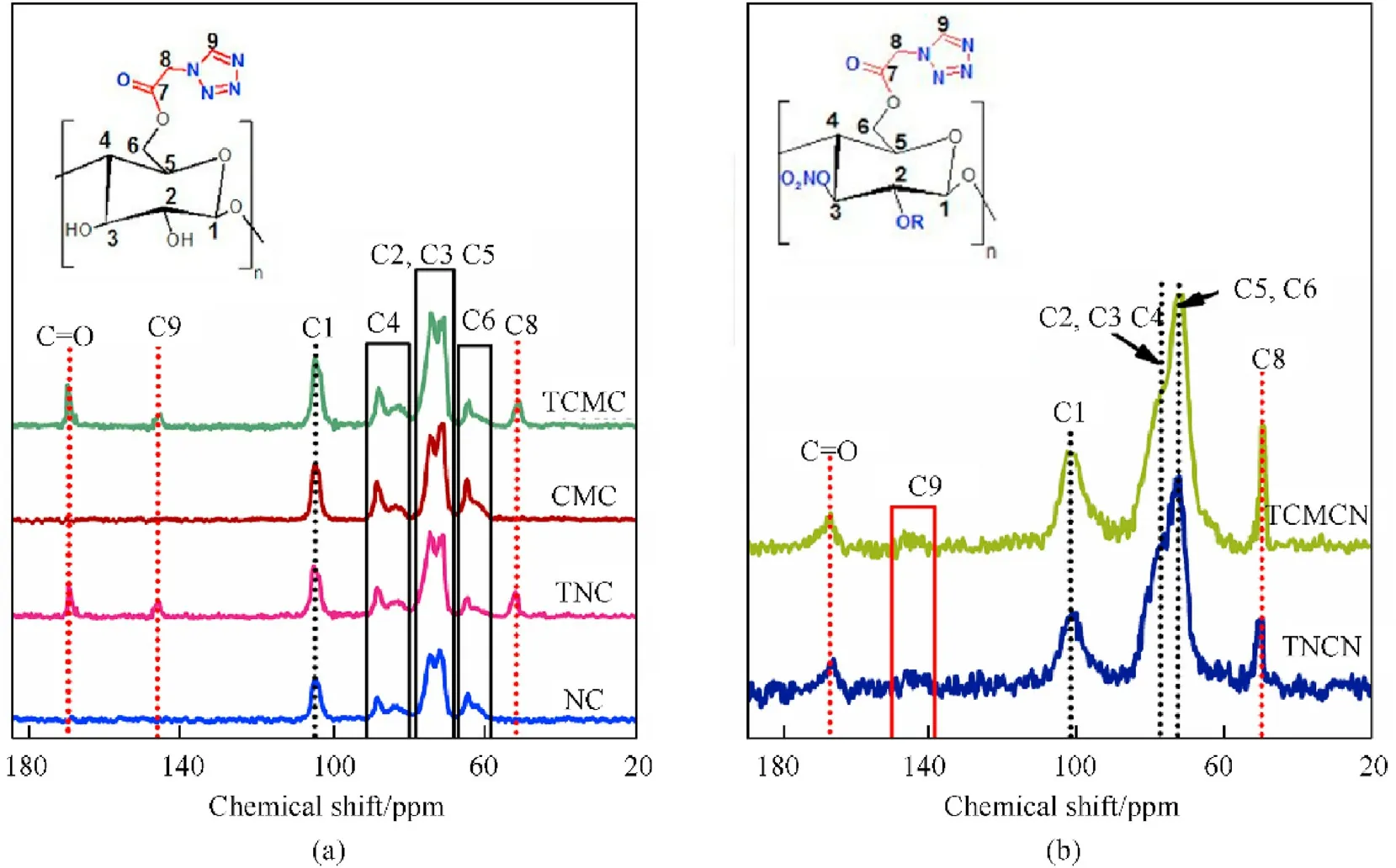
Fig.4. Solid-state 13C NMR spectra of(a)tetrazole-acetate functionalized biopolymers and their cellulosic precursors;(b)nitrated unmodified and tetrazole-acetate functionalized biopolymers.
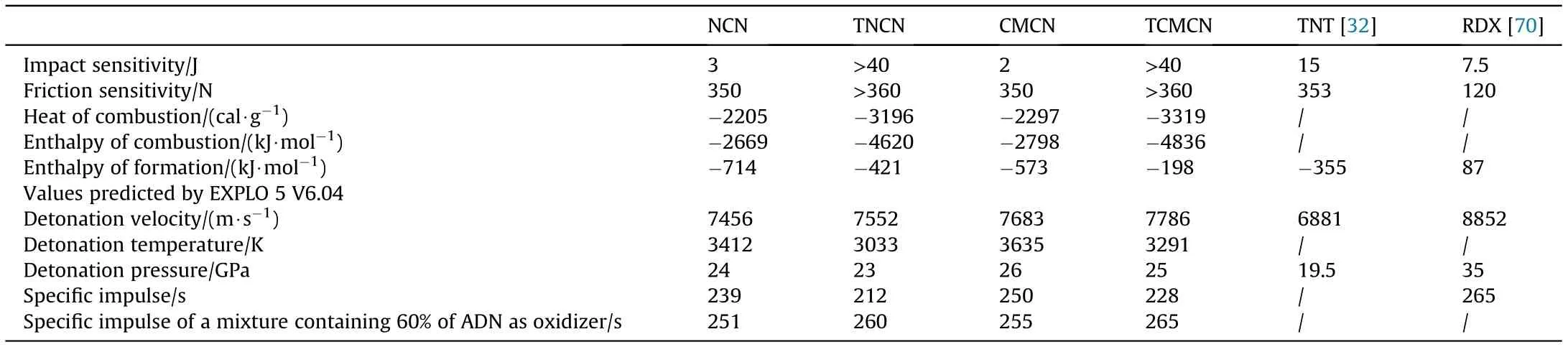
Table 2 Detonation performances of the developed energetic biopolymers.
The thermal study is a key criterion used to investigate the thermal behavior and evaluate the potential of energetic compounds for real-world applications. Hence, DTA experiments were implemented to determine the thermal features of the synthesized nitrogen-rich biopolymers and their precursors. As can be seen in Fig. 5(a), the starting cellulosic materials display a single endothermic decomposition phenomenon detected at peak temperature of 311.7C for NC and 327.9C for CMC, which is attributed to the degradation behavior of cellulosic backbone through different concurrent processes such as decarboxylation, depolymerization,and decomposition of glycosyl units [62,63]. The tetrazole-acetate functionalized biopolymers, however, exhibit two exothermic degradation events occurred within the temperature range of 190-240C and 270-350C, which corresponded to the ring opening of explosophoric tetrazole moieties followed by further destruction of the functionalized polysaccharide structure. The shift of the first exothermic peak to lower temperature from TNC to TCMCN is justified by its high amount of nitrogen-rich functional groups that renders it more susceptible to thermal stimuli [11,64].Besides that, the increased CrI of TCMC compared to TNC is responsible for the delaying of the beginning of its second thermal degradation, since several authors have reported cellulose-rich material with increased crystalline order presented improved thermal stability by the fact that their thermal decomposition starts on the amorphous parts and dissipates to their more ordered domains [15,58,65]. Another interesting observation from Fig. 5(a) is that the surface decorated biopolymers start to decompose at lower temperatures than their precursors, as expected, owing to the presence of explosophoric tetrazole rings that significantly increase their sensitivity against thermal degradation.
Regarding the thermal decomposition behavior of the nitrated biopolymers, it can be seen from Fig. 5(b) that NCN and CMCN present one prominent exothermic decomposition located at 185-210C,which is assigned to the main thermolysis mechanism of the cellulosic chains caused by homolytic breaking of the energyrich CO-NOlinkages [66,67]. Nevertheless, it was found that the decomposition mechanism of the new developed TNCN and TCMCN occurs at three consecutive exothermic events. The first one,noticed at 180-190C,is evidently assigned to the thermolytic decomposition of the weakest explosophoric O-NOgroups, the second process detected at around 205-220Cis associated with the thermal cracking of the tetrazole ring, whereas, the last degradation pathway observed at 290-320C is probably attributed to the thermo-oxidative fragmentation of the polymeric residue.The DTA findings also indicate that the designed nitrogen-rich biopolymers are less thermally stable than the conventional NCN and emergent CMCN, which is expected due to the low CrI, the increased N, and the presence of both thermally unstable nitrate esters and tetrazole rings in the case of TNCN and TCMCN,reducing their heat-resistance.Additionally,it is interesting to highlight that the reported surface decoration of cellulosic biopolymers with explosophoric tetrazole rings and nitrate ester groups conduct to the formation of advanced high-nitrogen dense biopolymers with better thermal stability than recently developed energy-rich macromolecules, such as glycidyl-nitramine-polymer (170C) [68],nano-sized spherical nitrocellulose composite crystal (161.9C)[24],nitrated chitosan(145.2C)[1],and nitrated ethylenediaminefunctionalized microcrystalline cellulose (180.8C) [15].
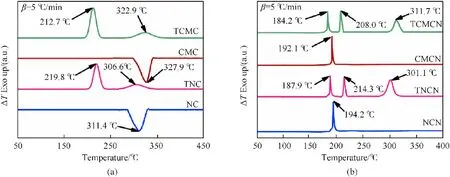
Fig. 5. DTA curves of (a) starting cellulosic materials and their tetrazole-acetate functionalized derivatives; (b) nitrated unmodified and tetrazole-acetate functionalized biopolymers.
3.5. Mechanical sensitivity and energetic properties
In view of the significance of safety performance for energy-rich materials, the sensitivity features of the designed energetic biopolymers toward impact and friction are evaluated,as summarized in Table 2. It is exciting that the new developed nitrogen-rich biopolymers show insensitive mechanical characteristics,indicating a promising safety performance for application. In contrast, the samples subjected only to nitration (NCN and CMCN) are found to be very sensitive toward shock, which is the major limitation for their practical application energetic formulations. The great insensitivity features of TNCN and TCMCN can be related to the presence of nitrogen-rich heterocyclic rings,which exhibit reduced impact sensitivity than nitrate esters [41,69]. Furthermore, the measured constant-volume energy of combustion given in Table 2 indicates that nitrated tetrazole-acetate functionalized biopolymers generate higher amount of combustion heat than directly nitrated cellulosic materials, demonstrating that the surface decoration of cellulose skeleton with high-nitrogen tetrazole rings and energy-rich nitrate esters groups could greatly promote heat release and combustion performance. In addition, the application of the measured heats of combustion and the Hess thermochemical cycle, the enthalpy of formation (ΔH) of the designed energetic biopolymers were calculated and are listed in Table 2. As can be noticed, all nitrated samples display negative ΔH, which reveal that an exothermic process is developed during the formation of the nitrated samples from their basic elements.
Based on the heats of formation and the corresponding densities, the detonation parameters were predicted with the EXPLO5(V 6.04) thermochemical code [44]. They were calculated at the Chapman-Jouguet (C-J) point with the help of a stationary detonation model using a modified Becker-Kistiakowski-Wilson state equation for the system. The C-J point was found by the Hugoniot curve of the system by its first derivative[31,44].Interestingly,the detonation velocity of TNCN and TCMCN exceed those of NCN and CMCN, respectively, owing to their elevated densities and greater amount of explosophoric nitrogen-rich functional groups. However,the nitrated tetrazole-acetate functionalized derivatives display reduced temperature of explosion and similar detonation pressure in comparison to nitrated unmodified ones. With respect to detonation velocity, the values of the new designed TNCN and TCMCN are better than GAP(5047 m/s)[69],polyGLYN(6755 m/s)[71],and GNAP(7165 m/s)[68].Additionally,it is interesting to point out that the detonation performances of the developed energetic biopolymers are even superior to those of TNT[32]and HNS[72],but lower than those of RDX[70],and HMX[73].These outcomes prove that the combination of multiple explosophoric nitrogen-rich groups in the same AGU is a useful trend towards next generation energetic cellulose-rich materials with interesting physical stability and improved energetic performances.
Besides that, the specific impulse (I) of the synthesized energetic biopolymers were also estimated by the EXPLO5 V6.04 program, supposing an isobaric combustion. This crucial parameter is widely determined to investigate the performance of solid-rocket boosters, and it is proportional to the square root of the ratio of the combustion chamber’s temperature and the average molecular mass of products formed during combustion. The theoretical calculations indicate that the new designed TNCN and TCMCN provide lower Ithan the nitrated cellulose and cellulose microcrystals.Nonetheless,it was found that their Icould be notably enhanced by the addition of ammonium dinitramide (ADN) with optimal ratio of 60 wt%, and the Iof the different formulations followed the order:TCMCN/ADN(265 s)>TNCN/ADN(261 s)>CMCN/ADN(255 s) >NCN/ADN (251 s), which are better than that of recently developed nitrocellulose/trinitroethyl-nitrocarbamate formulation(244.46 s) [74]. The optimal ratio allows the oxidation of the ingredients, attaining higher flame temperature. Comparable trend has been highlighted by DeLuca et al., where they reported the employment of ADN in HTPB formulations [75].
4. Conclusions
A promising generation of nitrogen-rich biopolymers were prepared through tetrazole surface functionalization and nitration of ordinary cellulose and its micro-sized derivative. The afforded energetic biopolymers were characterized for their structures,physicochemical features, and energetic properties. The obtained findings confirmed that the new energetic TNCN and TCMCN were successfully designed from their respective precursors with improved densities,increased nitrogen contents and good thermal stability in comparison to the only nitrated ones(NCN and CMCN).Interestingly, the developed nitrated tetrazole-functionalized biopolymers displayed better specific impulse of their energetic formulations with ADN, higher detonation velocities, and lower sensitivities to external stimuli than the state-of-the-art cellulosebased energetic materials including NCN and CMCN. These outstanding results demonstrated that new advances in the synthesis of insensitive and high-energy dense cellulosic biopolymers containing nitrogen-rich functional groups were envisaged to emerge in the foreseeable future, thus providing new avenues toward next generation energetic biopolymers.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
The financial support and the necessary facilities for this study by the Ecole Militaire polytechnique and the Ludwig-Maximilian University of Munich (LMU)are gratefully acknowledged.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.dt.2021.03.009.
杂志排行
Defence Technology的其它文章
- Research on DSO vision positioning technology based on binocular stereo panoramic vision system
- Dynamics of luffing motion of a hydraulically driven shell manipulator with revolute clearance joints
- Blast performance of layered charges enveloped by aluminum powder/rubber composites in confined spaces
- Applicability of unique scarf joint configuration in friction stir welding of AA6061-T6: Analysis of torque, force, microstructure and mechanical properties
- Multi-area detection sensitivity calculation model and detection blind areas influence analysis of photoelectric detection target
- Accurate analysis of limiting human dose of non-lethal laser weapons
