CO2 emission and source partitioning from carbonate and non-carbonate soils during incubation
2022-04-16YiZHAORolandBOLZhaoanSUNYupingZHUGEXiaoxiaSHIWenliangWUandFanqiaoMENG
Yi ZHAORoland BOLZhaoan SUNYuping ZHUGEXiaoxia SHIWenliang WU and Fanqiao MENG∗
1Beijing KeyLaboratoryof Biodiversityand Organic Farming,College of Resources and Environmental Sciences,China Agricultural University,Beijing 100193(China)
2Institute of Bio-and Geosciences,Agrosphere(IBG-3),Forschungszentrum Jülich GmbH,Jülich 52425(Germany)
3KeyLaboratoryof Biologyand Molecular Biologyin Universityof Shandong,College of Biological and Agricultural Engineering,Weifang University,Weifang 261061(China)
4National Engineering Laboratoryfor Efficient Utilization of Soil and Fertilizer Resources,College of Resources and Environment,Shandong Agricultural University,Tai’an 271018(China)
5SinoCarbon Innovation&Investment Jiangsu Co.,Ltd.(SCII),Nanjing 210046(China)
ABSTRACT The accurate quantification and source partitioning of CO2 emitted from carbonate(i.e.,Haplustalf)and non-carbonate(i.e.,Hapludult)soils are critically important for understanding terrestrial carbon(C)cycling.The two main methods to capture CO2 released from soils are the alkali trap method and the direct gas sampling method.A 25-d laboratory incubation experiment was conducted to compare the efficacies of these two methods to analyze CO2 emissions from the non-carbonate and carbonate-rich soils.An isotopic fraction was introduced into the calculations to determine the impacts on partitioning of the sources of CO2 into soil organic carbon(SOC)and soil inorganic carbon(SIC)and into C3 and/or C4 plant-derived SOC.The results indicated that CO2 emissions from the non-carbonate soil measured using the alkali trap and gas sampling methods were not significantly different.For the carbonate-rich soil,the CO2 emission measured using the alkali trap method was significantly higher than that measured using the gas sampling method from the 14th day of incubation onwards.Although SOC and SIC each accounted for about 50%of total soil C in the carbonate-rich soil,SOC decomposition contributed 57%–72%of the total CO2 emitted.For both non-carbonate and carbonate-rich soils,the SOC derived from C4 plants decomposed faster than that originated from C3 plants.We propose that for carbonate soil,CO2 emission may be overestimated using the alkali trap method because of decreasing CO2 pressure within the incubation jar,but underestimated using the direct gas sampling method.The gas sampling interval and ambient air may be important sources of error,and steps should be taken to mitigate errors related to these factors in soil incubation and CO2 quantification studies.
KeyWords: alkali trap,C3/C4 plant,inorganic carbon stock,isotope fractionation,organic carbon stock
INTRODUCTION
Soil is the largest carbon (C) pool on the earth, with an organic carbon(OC)stock of 1 550 Pg and an inorganic carbon(IC)stock of 950 Pg(Lal,2007).In arid and semiarid regions, which occupy a total land area of 4.9×107km2(Lardneret al.,2015),the soil inorganic carbon(SIC)stock is approximately 10 times greater than that of soil organic carbon (SOC) (Schlesinger, 1982).The SIC and SOC pools play a significant role in global C sequestration(Lal,2009).Because SIC significantly contributes to CO2emissions,it should receive greater attention(Tamiret al.,2011;Ramnarineet al.,2012;Chevallieret al.,2016).Zamanian and Kuzyakov(2019)highlighted that,on a global scale, CO2emitted from carbonate soil by nitrogen (N)fertilization(>7.5×1012g C year−1)and from liming of acidic soils(>273×1012g C year−1)accounted for more than 30%of the total global CO2emissions resulting from land-use changes(Zamanianet al.,2018).
The efflux of CO2from soils is recognized as one of the largest C fluxes within the global C cycle, and small changes can greatly affect atmospheric CO2(Schlesinger and Andrews,2000)and consequently,the global climate(Lal,2004;Luoet al.,2010).Since the 1970s,the importance of soil respiration as a source of CO2has been well recognized,and numerous studies have investigated CO2emissions from different ecosystems(Buyanovskyet al.,1986;Conantet al.,2000)and from soils underlying different types of vegetation(Raich and Schlesinger,1992).Other studies have compared methods to quantify soil respiration(Jong and Schappert,1972;Baldocchiet al.,1988).For non-carbonate soils,CO2emissions are mostly generated by soil respiration(i.e.,SOCdecomposition by microbes).However,for carbonate soils,SIC dissolution may also occur during the microbial decomposition of SOC(Stevenson and Verburg, 2006; Bertrandet al., 2007).The CO2produced by SOC decomposition and/or SIC dissolution dissolves in soil water and may react with Ca2+and/or Mg2+to re-precipitate as new carbonates(Nordtet al.,2000).In the field,such re-precipitation may occur in daily cycles if the hydrothermal conditions within the soil fluctuate intensively(Yateset al., 2013; Faet al.,2016).However,under constant hydrothermal conditions in a laboratory setting,such re-precipitation may take several months(Kuzyakovet al.,2006;Ramnarineet al.,2012).
The two most commonly used methods to quantify soil CO2emissions are the alkali trap method and the direct gas sampling method(Rochetteet al.,1997;Bruunet al.,2014;Lardneret al.,2015).Compared with field direct measurement,laboratory incubations have more stable hydrothermal conditions,which reduce interference from environmental factors.Measurements of soil CO2flux in laboratory incubations have been widely used to quantify soil microbial activity(Leitaet al.,1995),SOC decomposition(Kuzyakov and Bol,2005),soil organic matter(SOM)pool dynamics(Haile-Mariamet al., 2008), and the turnover of different SOM fractions (Stevenson and Verburg, 2006; De Troyeret al., 2011).For example, using the alkali trap method,Ramnarineet al.(2012)found that 62%–74%of the CO2emitted during a 14-d incubation of typical Hapludalf soil was derived from SIC.Tamiret al.(2012)conducted a 168-d soil incubation experiment and found that N application to calcareous soil enhanced the dissolution and recrystallization of SIC.The alkali trap method may overestimate or underestimate CO2fluxes because of variations in temperature and moisture and diffusion of external gases,which can result in excessive or insufficient absorption of the released CO2(Rochetteet al.,1997;Ramnarineet al.,2012).Therefore,it is important to compare the efficacies and accuracies between the gas sampling method and the alkali trap method to quantify CO2released from incubated soil.
Isotopic measurement of13C content is an effective tool to identify CO2sources(Stevenson and Verburg,2006;Tamiret al.,2011).Compared with SIC,SOC is less enriched with heavier13C.Isotopic signatures ofδ13C(δ13C represents the ratio of C stable isotope to the standard,indicating the degree of enrichment of13C in the sample)are widely used to distinguish SOC from SIC(Chevallieret al.,2016).Therefore,δ13C value of CO2evolved from SOC(δ13CCO2-SOC)is significantly different from that from SIC(δ13CCO2-SIC)(Salomons and Mook, 1976; Magaritz and Amiel, 1980;Plestenjaket al.,2012).In addition,C3 and C4 plants have differentδ13C values because of physiological differences in the photosynthetic fixation of CO2(Balesdentet al.,1990;Bolet al.,2004;Krullet al.,2007).Based on the differences inδ13C values between C3 and C4 plants, Kuzyakov and Bol(2005)distinguished three sources of CO2emitted from soils.Isotope fractionation may occur during the reactions that emit CO2.The value ofδ13CCO2-SICis 7‰−9‰lower than that of theδ13C value of SIC(δ13CSIC)in field conditions(Szaran,1997;Chevallieret al.,2016).In laboratory incubations, the differences betweenδ13CCO2-SOCand theδ13C value of SOC(δ13CSOC)range from 0 to 1‰(Boströmet al.,2007;Breeckeret al.,2015).Some studies have also highlighted that,compared with field conditions,laboratory conditions lead to much smaller,or even negligible,isotope fractionation(Bertrandet al.,2007;Tamiret al.,2012).For instance,Chevallieret al.(2016)found that the estimated contribution of SIC to CO2emitted from soil exceeded total CO2emissions if isotopic fractionation between SIC and SIC-derived CO2was included in the calculation.
China has a large area of arid and semi-arid soils,which contain approximately 60 Pg SIC,accounting for 5.0%–6.7%of the global SIC pool (Pan, 1999).The Loess Plateau in northwestern China represents a typical carbonate(i.e.,Haplustalf)soil area,in which the SIC content is approximately 50%of total carbon(TC)(Donget al.,2014).In contrast,the northeastern plain in China is dominated by non-carbonate(i.e., Hapludult) soil, in which SOC accounts for>99%of TC(Lanet al.,2016).Both of these regions are important agricultural production areas in China.Analyses of the amount and sources of CO2emissions from these two typical soils not only help us understand the transformation of soil C,but also provide a basis for estimating CO2emissions in two major regions of China.
In this study, we collected Hapludult from the northeastern plain and Haplustalf from the Loess Plateau in northwestern China and conducted a 25-d laboratory incubation experiment.The CO2emitted from the soil was collected and measured using both the alkali trap method and the gas sampling method.The differences between theδ13C value of the CO2emitted(δ13CCO2)andδ13CSOCwere compared to examine the effects of SOC fractionation during the Hapludult incubation process.An isotopic fraction was introduced into the calculation to determine the impacts on partitioning of CO2sources (Chevallieret al., 2016).We aimed to determine the differences in CO2capture between the alkali trap method and the gas sampling method and to explore the contributions of plants with different types of photosynthesis(C3 and C4)and soil C(SIC and SOC)to CO2emissions,as well as the errors associated with the calculations.
MATERIALS AND METHODS
Incubation experiment
Two types of soils,carbonate and non-carbonate soils,were used for this experiment.The carbonate-rich soilwas collected in Shanxi Province in northwestern China(34°17′59.317′′N,108°04′9.384′′E),at the southern edge of the Loess Plateau.This region is in a warm temperate zone with a semi-humid and semi-arid climate and an average annual temperature and precipitation of 13°C and 600–650 mm,respectively.The soil is classified as Eum-Orthic Anthrosol in Chinese Soil Taxonomy, equivalent to Udic Haplustalf in USDA Soil Taxonomy.The soil texture is silt clay loam.The cropping system in the region has two crops per year,i.e., winter wheat (Triticum aestivumL.)and summer maize(Zea maysL.).The non-carbonate soil was collected in Liaoning Province in northeastern China(41°08′24.73′′N,121°19′39.48′′E),where there is a warm temperate sub-humid climate,the average annual temperature is 9°C,and the annual precipitation is 540–640 mm.The soil is Hapludult in USDA Soil Taxonomy.The soil texture is silt loam.The cropping system in the region is one crop per year,i.e., soybean (Glycine maxL.) rotated with maize(Zea maysL.).Historically,maize is the main crop in the region.Before sampling,the last crop planted at both sites was maize.Soil samples(0–20 cm)were collected using a soil auger (diameter of 3.0 cm) from the fields at the two sites.Three samples(each sample was a composite mix of five individual surface soil cores)were obtained from each field,air-dried,passed through a 2-mm mesh sieve,and finally stored at room temperature.
To activate the microbial population and decrease fluctuations in SOC mineralization,we added deionized water to 20-g air-dried soil sample to achieve 70%of the field water capacity and then incubated each sample in a 100-mL beaker(not sealed)at 25°C for 7 d before the start of the incubation experiment(Paulet al.,2006).
For the incubation experiment, each 20-g soil sample was placed in a 240-mL jar.To reduce the interference of carbonate re-precipitation, the incubation period was 25 d,because the dissolution of carbonate re-precipitation normally takes several months under laboratory conditions(Kuzyakovet al.,2006;Ramnarineet al.,2012).Each jar was sealed with a layer of Parafilm and a plastic sealing cap and incubated for 25 d at 25°C.At each sampling,after taking out the NaOH solution (alkali trap method) or collecting CO2with a needled syringe(gas sampling method),the jars were left open for 1 h to allow the internal air pressure to adjust,and the soil water content was re-adjusted to 70%of the field holding capacity by weight using deionized water(Tamiret al.,2011).Afterward,new NaOH solution(alkali trap method)was added to the jars,and the jars were resealed with new Parafilm and plastic sealing caps.
CO2 emissions and δ13C measurements
Alkali trap method.A 10-mL beaker(to hold NaOH solution)was fixed on the wall of the incubation jar using glue, about 2 cm above the soil in the jar.On day 0 of the incubation experiment,10 mL 0.1 mol L−1NaOH was added to the beaker.The NaOH solution was changed on days 2,5,7,9,14,18,and 25 after the start of the incubation(corresponding to sampling times 1, 2, 3, 4, 5, 6, and 7,respectively)to collect the emitted CO2.For the blank(no soil)treatment,sealed jars with only NaOH solution were used.Three replicate jars were prepared for each soil sample and the blank.
The CO2trapped in the NaOH solution was measured using the HCl-SrCl2titration method (Ramnarineet al.,2012).To analyzeδ13C of the emitted CO2,the suspension containing SrCO3was placed in a 250-mL centrifuge bottle,centrifuged at 8 000× gfor 20 min, and then washed three times with deionized water to remove excess SrCl2and NaCl (Ramnarineet al., 2012).The SrCO3samples were dried at 80°C for 8 h and then weighed into tin capsules.Theδ13C values of SrCO3were determined using an Elementar Vario EL cube(Elementar Analysensysteme GmbH,Germany)interfaced to a PDZ Europa 20-20 isotope ratio mass spectrometer (Sercon Ltd., UK) at the Stable Isotope Facility, University of California at Davis, USA.All theδ13C values reported are in the Vienna Pee Dee Belemnite(VPDB)standard.
Gas sampling method.The analysis of CO2captured using the gas sampling method was conducted simultaneously as that using the alkali trap method.Similarly,three replicates of each sample and the blank(no soil)were analyzed.The headspace of each incubation jar was gas-sampled using a 20-mL needled syringe.After removing the plastic sealing cap,the needle was inserted through the Parafilm.The gas sample in the syringe was compressed to 15 mL,after which the overpressure was released,and the volume was reduced to 12 mL.Then,the gas was transferred to a 12-mL vacuumed headspace bottle for analyses of the CO2concentration andδ13C using a gas chromatography-isotope ratio mass spectrometer (Delta Plus, Thermo Fisher Scientific, USA) at the Stable Isotope Facility, University of California at Davis.
δ13C calculation and correction.The amount of C in CO2(CCO2)derived from the incubated soil was calculated using Eq.1.

where for the alkali trap method, Cs(mg kg−1) is the C in CO2emitted from the incubated soil quantified by backtitration with HCl and Cb(mg kg−1)is the C in CO2inside the blank jars.For the gas sampling method,Cs(mg kg−1)is the C in CO2in the headspace within the jar containing soils quantified by gas chromatography and Cb(mg kg−1)is the C in CO2inside the blank jars.
Theδ13CCO2was calculated using Eq.2(Maryet al.,1992;Patakiet al.,2003).

where for the alkali trap method,δ13Csis theδ13C value of C in SrCO3from the incubated soils andδ13Cbis theδ13C value of C in SrCO3from the blank jar, which both were analyzed using a PDZ Europa 20-20 isotope ratio mass spectrometer.For the gas sampling method,δ13Csis theδ13C value of CO2from the incubated soils andδ13Cbis theδ13C value of CO2from the blank jar,analyzed using a Delta Plus isotope ratio mass spectrometer.
TC,SIC,SOC,total nitrogen,δ13C,and pH analyses
Soil TC and total nitrogen(TN)contents were measured using a CN analyzer (Flash EA 2000, Thermo Electron Corporation,Italy)at the Chinese Academy of Agricultural Sciences.The SIC content was measured using the pressurecalcimeter method(Sherrodet al.,2002).The SOC content was calculated by subtracting the SIC content from the TC content.Theδ13C values were analyzed using a Delta Plus isotope ratio mass spectrometer at the Chinese Academy of Agricultural Sciences.Soil pH was measured with a pH meter(soil:water=1:5,weight/volume).
Source partitioning of SOC and emitted CO2
Source partitioning of SOC into C4 and C3 plant origins.The proportion of SOC derived from C4 plants(fC4)in Hapludult(non-carbonate soil)and Haplustalf(carbonaterich soil)was calculated using Eq.3.
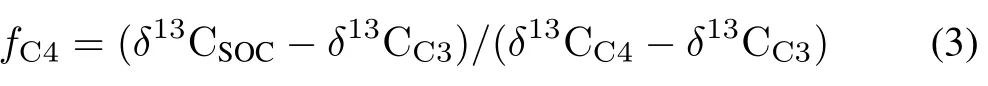
whereδ13CC4is theδ13C value of C4 plants(−12‰)andδ13CC3is theδ13C value of C3 plants(−27‰).The proportion of SOC derived from C3 plants(fC3)is 1−fC4.
Estimation of CO2 released from SOC and SIC.The proportion of CO2evolved from SOC(fSOC)was estimated using the two-end-member mixing model in Eq.4(Balesdentet al.,1987).

For Hapludult,because SOC accounted for>99%of TC,we assumed that all CO2emitted was from SOC;therefore,fSOC=1.The proportion of CO2evolved from SIC(fSIC)is 1−fSOC.
Because all CO2emitted was assumed from SOC for Hapludult,the difference betweenδ13CCO2andδ13CSOCwas due to isotopic fractionation during SOC decomposition into gaseous CO2.For Haplustalf,we assumed that isotopic fractionation during SOC decomposition into gaseous CO2was the same as that for Hapludult;that is,δ13CCO2-SOCwas the sum ofδ13CSOCand the corresponding isotope fractionation.Isotopic fractionation in the process of SIC dissolution into gaseous CO2was set at 7‰;that is,δ13CCO2-SICwas 7‰lower thanδ13CSIC(Chevallieret al.,2016).
Partitioning of CO2 evolved from SOC into C4 and C3 plant-derived SOC.The proportion of CO2evolved from SOC derived from C4 plants(fCO2-C4)was estimated using Eq.5.
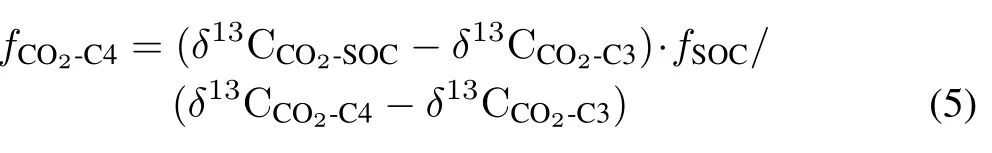
whereδ13CCO2-C3is theδ13C value of CO2evolved from C3 plant-derived SOC andδ13CCO2-C4is theδ13C value of CO2evolved from C4 plant-derived SOC.For Hapludult,theδ13CC3andδ13CC4values were used instead of theδ13CCO2-C3andδ13CCO2-C4values,respectively,during the process of calculation.The proportion of CO2evolved from C3 plant-derived SOC(fCO2-C3)is 1−fCO2-C4.If isotopic fractionation was considered, we assumed that theδ13CCO2-C3andδ13CCO2-C4values were 1‰ higher than theδ13C values of SOC derived from C3 and C4 plants,respectively(Boströmet al.,2007;Breeckeret al.,2015).
During the process of calculation,δ13CSOCwas used instead ofδ13CCO2-SOC,δ13CC4instead ofδ13CCO2-C4,andδ13CC3instead ofδ13CCO2-C3for Haplustalf.If isotopic fractionation was considered,as in Eq.4,δ13CCO2-SOCwas the sum ofδ13CSOCand the corresponding isotope fractionation.Theδ13CCO2-C4andδ13CCO2-C3values were assumed to be 1‰ higher than the values ofδ13CC4andδ13CC3,respectively.
Statistical analysis
Data were analyzed using statistical software SAS(SAS Institute Inc., 2000).Thet-test was used to compare the physical and chemical properties of the two soils.The CO2emission rates andδ13C were tested for normality using the Shapiro-Wilk test.Then,one-way analysis of variance(ANOVA) was used to evaluate the differences between CO2collection methods in the same laboratory incubation experiment.If the effects of the sampling method on the measurement results were significant(P <0.05),thet-test was used to compare the CO2-13C and CO2emissions between the two methods and to compare the estimation results of the two methods for C sources.The Bonferroni method was used to correct for multiple tests, and the significant difference level was set toP <0.05/N,whereNwas the number of CO2sampling times (7 times during the incubation study).Two-factor two-level ANOVA was used to detect soil type×sampling method interactions, and the Bonferroni method was also used forpost-hoctesting,with the significant difference level set toP <0.05/N.
RESULTS AND DISCUSSION
Soil C contents,pH and δ13C values
The C contents,δ13C values, and pH differed significantly between Hapludult(non-carbonate soil)and Haplustalf(carbonate-rich soil) (Table I).The pH of Hapludult (7.2±0.2)was significantly lower than that of Haplustalf(7.8±0.2).The TC content of Hapludult was 10.3±0.1 g kg−1,of which 99%was SOC.In contrast,the TC content of Haplustalf was 17.1±0.1 g kg−1, comprising about 50% SOC and 50% SIC.Theδ13C value of TC (δ13CTC,−20.6‰±0.1‰) in Hapludult was significantly lower(more negative)than that of Haplustalf,reflecting the high SIC content in the TC of Haplustalf.However,the value ofδ13CSOCin Hapludult(−21.2‰±0.1‰)was significantly higher than that of Haplustalf(−22.3‰±0.1‰).The values ofδ13CSICin Hapludult and Haplustalf were−7.6‰and−5.8‰,respectively.

TABLE IProperties of Hapludult(non-carbonate soil)and Haplustalf(carbonate soil)
Quantification of CO2 emissions during incubation
For Hapludult(non-carbonate soil),there were no significant differences in CO2emissions between the two gas collection methods during the entire incubation period(Fig.1a).The cumulative CO2emissions quantified by the gas sampling and alkaline trap methods were similar(147.7±5.3 mg kg−1for the gas sampling method and 146.9±4.8 mg kg−1for the alkaline trap method)(Fig.2a).For Haplustalf(carbonate-rich soil),the CO2emissions measured by the gas sampling and alkali trap methods were similar during the first 9 d of incubation; however, from day 14 onward,the CO2emissions at each sampling event measured by the gas sampling method were significantly lower than those measured using the alkali trap method(Fig.1b).The cumulative CO2emissions during the entire 25 d of incubation measured using the gas sampling and alkali trap methods were significantly different(139.0±1.7vs.165.3±5.7 mg kg−1)(Fig.2b).
For both Hapludult and Haplustalf,the cumulative CO2emissions in the first 9 d in the incubation experiment accounted for approximately 50% of the total CO2emitted during the entire 25-d incubation period(Fig.2).This indicated that SOC decomposed faster in the early phase of soil incubation than in the later phase,consistent with the results of other studies(De Troyeret al.,2011;Tamiret al.,2011).For Haplustalf,the significant differences between the cumulative CO2quantified by the two methods during the later incubation period might be due to SIC dissolution,which is facilitated by the organic acids produced during SOC decomposition(Rovira and Vallejo,2008;Tamiret al.,2011).In the alkali trap method,the CO2emitted was immediately absorbed by NaOH.Thus, the CO2concentration(pCO2)in the sealed jars decreased.This would allow SIC dissolution and CO2generation reaction to reach an equilibrium(Donget al., 2013).In contrast, for the gas sampling method,thepCO2in the sealed jars continuously accumulatedduring incubation.The decrease in O2concentration and the increase inpCO2in the sealed jars would impede SOC decomposition by heterotrophic microorganisms(Ekschmittet al.,2008)and reduce SIC dissolution driven by organic acids derived from SOC decomposition.Moreover,a high concentration of CO2may increase the assimilation of CO2by soil microorganisms(Ekschmittet al.,2008).This may result in the amount of CO2collected being less than the amount of CO2emitted by the soil sample.In addition,in the gas sampling method,the increase inpCO2might reverse the equilibrium of SIC dissolution and reduce CO2generation.Therefore,for carbonate-rich soils such as Haplustalf,CO2emissions may be overestimated using the alkali trap method and underestimated using the gas sampling method.The longer the sampling interval(duration between two gas sampling events),the greater the differences inpCO2within the sealed jar between the two methods.Consistent with this,the difference in CO2emissions measured using the two methods was significant if the sampling interval was longer than 4 d(Fig.1).

Fig.1 CO2 emissions at each sampling event from Hapludult(non-carbonate soil)(a)and Haplustalf(carbonate-rich soil)(b)measured using the gas sampling (GS) and alkali trap (AT) methods.Vertical bars indicate standard deviations of the means (n = 3) (raw data are shown in Table SII, see Supplementary Material for Table SII).The symbol*indicates significant difference between the two gas collection methods at a sampling day(P <0.05/N,where N is the number of CO2 sampling times).

Fig.2 Cumulative CO2 emissions during incubation experiment from Hapludult(non-carbonate soil)(a)and Haplustalf(carbonate-rich soil)(b)measured using the gas sampling(GS)and alkali trap(AT)methods.Vertical bars indicate standard deviations of the means(n=3).The symbol*indicates significant difference between the two gas collection methods at a sampling day(P <0.05/N,where N is the number of CO2 sampling times).
We also analyzed the effect of the interaction between gas collection method and soil type on CO2emissions and found that,in the later period of the incubation experiment(18–25 d),the interactive effect was significant(Table SIII,see Supplementary Material for Table SIII).This highlighted that the measured CO2emissions were also influenced by other factors such as the sampling interval,as well as the gas collection method and soil type.
δ13C values of emitted CO2
After correction by Eq.2,theδ13C values of the CO2emitted (δ13CCO2) from Hapludult (non-carbonate soil)ranged from−19.4‰±0.6‰to−19.1‰±0.6‰(mean=−19.3‰±0.5‰)for the gas sampling method,and from−20.1‰±0.2‰to−19.5‰±0.7‰(mean=−19.8‰±0.7‰)for the alkali trap method(Fig.3a).Overall,the values ofδ13CCO2for Hapludult determined using the gas sampling and alkali trap methods were not significantly different,except at day 18 of incubation.For Hapludult,theδ13CCO2was similar toδ13CSOC(−21.2‰).The differences(1.4‰–1.9‰)may have come from three sources:(1)trace SIC in Hapludult that affected measuredδ13CCO2; (2) C isotope fractionation that occurred during the decomposition of SOC into gaseous CO2,i.e.,0‰–1‰during SOCdecomposition and CO2diffusion from soil(Cheng,1996;Boströmet al.,2007;Breeckeret al.,2015),which was in line with the fractionation coefficient set in Eq.5 (1‰);and (3) operational errors during the experiment, such as measurement and sampling.Because soil contains active and inert OC pools,the preferential decomposition of active OC by microorganisms may be another reason for this difference(Amelunget al.,1999;von Lützowet al.,2007).Therefore,for non-carbonate soil, quantifying genuine isotope fractionation during SOC decomposition into gaseous CO2is necessary for future studies.

Fig.3 δ13C values of CO2 emitted(δ13CCO2),measured using the gas sampling(GS)and alkali trap(AT)methods,during the incubation period for Hapludult(non-carbonate soil)(a)and Haplustalf(carbonate-rich soil)(b).Vertical bars indicate standard deviations of the means(n=3)(raw data are shown in Table SII).The symbol*indicates significant difference between the two gas collection methods at a sampling day(P <0.05/N,where N is the number of CO2 sampling times).
For Hapludult, we found that theδ13CCO2measured by the gas sampling method was slightly higher than that measured by the alkali trap method at each sampling event.There are several possible reasons for this:(1)contamination by trace ambient air in the gas collection needle.As we used a syringe for gas sampling,the trace air stored in the needle may have affected the result.We calculated these possible impacts and found that,in our experiment,theδ13CCO2was decreased(negative)by 0.05‰if the ambient air stored in the needle(Table SIV,see Supplementary Material for Table SIV)was considered;(2)contamination by trace ambient air outside the incubation jar.In the current study,the incubation jars were sealed with a double-layer (i.e., Parafilm and a sealing cap).During the gas sampling process,ambient air may have entered the incubation jar when the outer sealing cap was opened,and the gas sample was collected using the needle inserted through the Parafilm.The ambient air hadδ13CCO2of−8‰,much higher than that of the CO2derived from SOC decomposition(−19.3‰±0.5‰).This implied that for the incubation experiment,contamination by ambient air during the gas collection process should be avoided,for example,by using real-time gas monitoring(Meijideet al.,2010;Ananet al.,2014).
For Haplustalf(carbonate-rich soil),the averageδ13CCO2measured using the gas sampling method was−17.7‰±0.7‰(from−18.0‰±0.3‰to−17.1‰±1.2‰)and that measured using the alkali trap method was−17.4‰±0.5‰(from−18.2‰±1.1‰to−17.0‰±0.5‰) (Fig.3b).There was no statistical difference in theδ13CCO2values between these two gas collection methods.However,compared with Hapludult, Haplustalf showed higher fluctuations inδ13CCO2as measured using both methods during incubation.We concluded that this was due to SIC dissolution during incubation(Tamiret al.,2011).
For both Hapludult and Haplustalf,variations inδ13CCO2may have been due to re-precipitation of CO2into carbonate during incubation.However, because this re-precipitation usually takes several months under laboratory conditions(Kuzyakovet al.,2006;Ramnarineet al.,2012),we assumed its effects onδ13CCO2were negligible in our study.We also examined the effects of gas collection method and soil type onδ13CCO2(Table SV,see Supplementary Material for Table SV)and found that only soil type had a significant effect.
Partitioning sources of CO2
In laboratory incubation experiments,the C isotope fractionation occurring during SOC decomposition and SIC dissolution is much smaller than that in the field because:(1)interference from ambient air is much higher in the field because of wind; (2) the soil used in indoor incubation experiments is much more uniform than that in the field;and (3) the constant temperature in laboratory conditions eliminates the influences of temperature and moisture variations on C isotope fractionation(Chevallieret al.,2016).In addition,CO2capture is more complete with the timely replacement of NaOH(Bertrandet al.,2007),in contrast to a previous study by Fritzet al.(1985).Therefore,in most laboratory incubation studies,C isotope fractionation has not been considered(Bertrandet al.,2007;Tamiret al.,2012).However,to examine the effects of various influential factors,we compared scenarios where C isotope fractionation was considered or not considered in partitioning the sources of CO2emitted.
Using Eqs.4 and 5,we first partitioned the emitted CO2into SOC and SIC and then partitioned the CO2from SOC into C3 and C4 plant-derived SOC.In Hapludult,as the SOC accounted for 99%of the TC(Table I),we assumed that all CO2came from C3 and C4 plant-derived SOC.The average proportion of C4 plant-derived CO2was 52%±3%(51%±4%–53%±6%)for the gas sampling method and 48%±4%(46%±1%–50%±4%)for the alkali trap method(Table II).If C isotope fractionation during SOC decomposition into gaseous CO2(for both C3 and C4 plant-derived SOC)was considered to be 1‰,the average proportion of CO2derived from C4 plant-derived SOC was 45%±3%(44%±4%–46%±6%)for the gas sampling method and 41%±4%(39%±1%–43%±4%)for the alkali trap method(Table II).For both scenarios(considering fractionation or not),there were no significant differences between the gas sampling and alkali trap methods in estimating the proportion of CO2derived from C3 and C4 plant-derived SOC(Table II).Considering that the proportions of SOC derived from C3 and C4 plants in Hapludult soil were 61%(6.2 g kg−1)and 39%(3.9 g kg−1)(Eq.3),respectively,it was concluded that SOC derived from C4 plants decomposed faster than that derived from C3 plants,consistent with the results of other studies(Wynn and Bird,2007;Ponphang-Ngaet al.,2011).
For Haplustalf(carbonate-rich soil),the contribution of SOC to emitted CO2was 72%±4.3%(69%±7%–74%±5%)for the gas sampling method and 71%±3%(66%±2%–76%±3%)for the alkali trap method(Table III).If C isotope fractionation in the processes of SOC decomposition(1.4‰–1.9‰,same as Hapludult soil)and SIC dissolution(7‰)(Szaran,1997;Chevallieret al.,2016)into gaseous CO2was considered, the contribution of SOC to emitted CO2was 65.4%±10.7%(57%±13%–71%±11%)forthe gas sampling method and 57%±7%(51%±4%–65%±7%)for the alkali trap method(Table III).The differences between the two methods were not statistically significant.As shown in Table I,the respective SOC and SIC contents were 8.6and 8.5 g kg−1.This provided further evidence that SOC decomposed faster than did SIC,consistent with the findings of many other studies(Table IV).
Similarly,we calculated the contribution of C4 and C3 plant-derived SOC to emitted CO2in Haplustalf using Eq.5.The contribution of C4 plant-derived SOC was 22.5%±1.4%(21%±2%–23%±2%)for the gas sampling method and 22.1%±1%(21%±1%–24%±2%)for the alkali trap method(Table V).If the fractionation from C3 and C4 plantderived SOC to gaseous CO2was set to 1‰,the contribution of C4 plant-derived SOC ranged from 21%±4%to 28%±6%(mean=25.2%±5.2%)for the gas sampling method and from 18%±6% to 23%±10% (mean = 20.3%±5.6%) for the alkali trap method (Table V).Considering that the ratio of C3/C4 plant-derived SOC was 117/55,this highlighted that the C4 plant-derived SOC decomposed faster than the C3 plant-derived SOC(Wynn and Bird,2007;Ponphang-Ngaet al., 2011).Another reason may be that,for both Haplustalf and Hapludult,the last crop grown prior to soil sampling was maize(a C4 plant);the newly formed SOC deriving from maize plants tends to be stored in sand particles(Amelunget al.,1999;von Lützowet al.,2007)and decomposes readily(Sixet al.,2002;Drewittet al.,2009).

TABLE IIProportion of CO2 derived from C4 plant-derived soil organic C,measured using the gas sampling(GS)and alkali trap(AT)methods,during the incubation period for Hapludult(non-carbonate soil)with and without C isotope fractionation
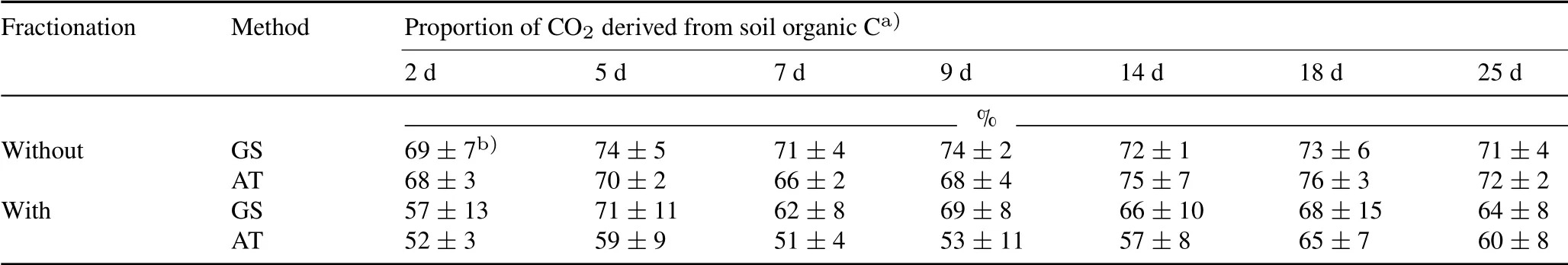
TABLE IIIProportion of CO2 derived from soil organic C,measured using the gas sampling(GS)and alkali trap(AT)methods, during the incubation period for Haplustalf(carbonate-rich soil)with and without C isotope fractionation

TABLE IVContribution of soil organic C(SOC)to CO2 emissions,measured using the gas sampling(GS)and alkali trap(AT)methods,from Haplustalf(carbonate-rich soil)under laboratory incubation conditions in different studies

TABLE VProportion of CO2 derived from C4 plants-derived soil organic C,measured using the gas sampling(GS)and alkali trap(AT)methods,during the incubation period for Haplustalf(carbonate-rich soil)with and without C isotope fractionation
In this study,there were some uncertainties in our calculations and experimental procedure:(1)SOC fractionation in Haplustalf may differ from that in Hapludult;(2)the SIC fractionation coefficient of−7‰derived from a field study(Szaran,1997;Chevallieret al.,2016)might be higher than that of SIC fractionation in our laboratory incubation conditions;and(3)the fractionation of C3 and C4 plant-derived SOC into gaseous CO2may be higher or lower than 1‰.In most laboratory studies,the highest fractionation coefficient was<1‰(Boströmet al., 2007; Breeckeret al., 2015).Using a lower fractionation coefficient in the partition calculation further supported the findings that C4 plant-derived SOC had a higher decomposition rate.In this experiment,we analyzed three replicates for each sample(n=3).Analysis of more replicates may improve the accuracy of the results.The coefficient of variation of CO2emissions(Fig.1)was<10%(Fig.S1,see Supplementary Material for Fig.S1)and that ofδ13CCO2values(Fig.3)was<8%(Fig.S2,see Supplementary Material for Fig.S2).This suggested that three replicates provided a satisfactory level of accuracy,but a larger sample size is still recommended.
CONCLUSIONS
Both alkali trap and gas sampling methods can be used to quantify CO2emissions from soils in incubation studies.For the carbonate soil,the alkali trap method may overestimate the CO2emissions due to decreasing CO2pressure within the incubation jar, while the direct gas sampling method may underestimate CO2emissions.It was found that longer sampling intervals resulted in greater differences in measured CO2values between the two gas collection methods.Interference from ambient air was a major source of error in soil incubation and CO2emission analyses,and steps should be taken to avoid this.Carbon isotope fractionation during the processes of SOC decomposition and SIC dissolution can result in variations in partitioning sources of emitted CO2.In general,SOC and C4 plant-derived SOC decomposed faster than SIC and C3 plant-derived SOC, respectively; these findings were not affected by the consideration of C isotope fractionation or not.This should be considered in future studies on CO2emissions,such as those related to land-use changes or from soils with different parent materials.
ACKNOWLEDGEMENTS
This work was supported by the National Key Research and Development Program of China(No.2016YFD0201200)and the National Natural Science Foundation of China(Nos.31370527,31261140367,and 30870414).The first author would like to acknowledge the Chinese Scholarship Council(No.201706350210)for the support of the work.
SUPPLEMENTARY MATERIAL
Supplementary material for this article can be found in the online version.
杂志排行
Pedosphere的其它文章
- Letter to the Editor Can sieving size affect the determination of soil available micronutrients?
- Genomic characterization of multidrug-resistant extraintestinal pathogenic Escherichia coli isolated from grain culture soils
- Soil compression influences the avoidance behavior of Allonychiurus kimi(Collembola)to cadmium and copper
- Carbon mineralization in subtropical alluvial arable soils amended with sugarcane bagasse and rice husk biochars
- Key variable for simulating critical nitrogen dilution curve of wheat:Leaf area ratio-driven approach
- Co-inoculation of indigenous Pseudomonas oryzihabitans and Bradyrhizobium sp.modulates the growth,symbiotic efficacy,nutrient acquisition,and grain yield of soybean
