Co-inoculation of indigenous Pseudomonas oryzihabitans and Bradyrhizobium sp.modulates the growth,symbiotic efficacy,nutrient acquisition,and grain yield of soybean
2022-04-16KailashChandKUMAWATInderjeetSINGHSharonNAGPALPoonamSHARMARajeevKumarGUPTAandAsmitaSIRARI
Kailash Chand KUMAWAT,Inderjeet SINGH,Sharon NAGPAL,Poonam SHARMA,Rajeev Kumar GUPTA and Asmita SIRARI
1Department of Microbiology,Punjab Agricultural University,Ludhiana 141004(India)
2Department of Plant Breeding and Genetics,Punjab Agricultural University,Ludhiana 141004(India)
3Department of Soil Science,Punjab Agricultural University,Ludhiana 141004(India)
ABSTRACT Soil microbes play a vital role in improving plant growth, crop productivity, and soil health through solubilization of essential nutrients.Present investigation was conducted to access the efficacy of Bradyrhizobium sp.LSBR-3 and the indigenous phosphate-solubilizing Pseudomonas oryzihabitans LSE-3 in improving the symbiosis,nutrient accumulation,and yield of soybean.The isolate LSE-3,selected on the basis of phosphate solubilization,was screened for beneficial traits,antagonistic activities,and pathogenicity.The levels of indole acetic acid production(50.34±2.35µg mL−1),phosphate solubilization(184.4±7.4 mg L−1),biofilm formation(optical density at 560 mm,1.389 6±0.04),siderophore production(121.46±1.61µg mL−1),and 1-aminocyclopropane-1-carboxylate deaminase activity(0.51±0.07 mmol α-ketobutyrateµg−1 protein h−1)were significantly higher with the dual inoculants(LSBR-3 and LSE-3)than with the single inoculant LSBR-3.The plant growth-promoting traits of single and dual inoculants were evaluated for the synergistic effects on soybean under field conditions.Soybean plots treated with LSBR-3+LSE-3 exhibited improvement in seed germination,plant height,plant biomass,and chlorophyll content compared with the uninoculated control.Dual inoculant treatments resulted in significantly higher symbiotic efficacy evidenced by increased nodulation(40.0±0.75 plant−1),nodule biomass(188.52±6.29 mg plant−1),and leghemoglobin content(11.02±0.83 mg g−1 fresh nodule),and significantly increased activities of phosphatase(75.16±3.17 and 58.77±6.08µg p-nitrophenol g−1 h−1 for alkaline and acid phosphatase,respectively)and dehydrogenase(32.66±1.92µg triphenylformazan g−1 h−1)compared with the control.Dual inoculation with LSBR-3 and LSE-3 enhanced the uptake of macro-and micronutrients,reduced Na content in shoots,and resulted in 10.85%higher grain yield and ca.US$96.80 ha−1 higher profit compared with the control.This is the first report on the effectiveness of combined inoculation of LSE-3 and LSBR-3 in promoting the growth,symbiotic efficacy,and yield of soybean for sustainable agriculture.
KeyWords: bacteria,biofilm formation,phosphate solubilization,plant growth-promoting trait,sustainable agriculture,zinc solubilization
INTRODUCTION
Soybean(Glycine max(L.)Merrill)is one of the most important legume crops rich in proteins,contributing approximately 30%of oil and 64%of vegetable protein produced from processed crops (Niwińskaet al., 2020).Although 85%of soybean is processed as oil and its derivatives,India continues to face scarcity of edible oil,which justifies the need for enhancing soybean production.In 2017,soybean was grown on 1.23×108ha of land area worldwide,producing a total of 3.53×108t beans at a productivity of 2 462 kg ha−1(FAOSTAT,2018).Soybean productivity in India is very low(1 219 kg ha−1)due to poor soil health and unbalanced use of inorganic fertilizers.Indiscriminate use of agrochemicals is hazardous to the environment and human health,damages soil microbial communities and their activities,and reduces the yield,leading to a hike in production costs(Mahantyet al.,2017; Luanet al., 2020).Therefore, concerted efforts are required to develop new cost-effective and environmentally sustainable long-term strategies for cropping systems that will conserve natural resources and improve soil health through biological nitrogen(N)fixation(Singh and Singh,2014).
Plant growth-promoting rhizobacteria(PGPR)can help sustain agriculture and function as eco-friendly alternatives to agrochemicals,which often pollute the environment(Muresuet al.,2019).They are natural inhabitants of plant roots and the surrounding soil,and they use plant-derived nutrients for their growth,while in return provide benefits to the plants through direct or indirect mechanisms(Anwaret al., 2016; Nagpalet al., 2020).Directly, PGPR encourage plant growth by producing phytohormones(Olanrewajuet al., 2017; Backeret al., 2018; Nagpalet al., 2019)and 1-aminocyclopropane-1-carboxylate(ACC)deaminase(Shrivastava and Kumar, 2013), or through symbiotic N fixation(Ibáñezet al.,2017;Singhet al.,2017)and solubilization of inorganic phosphate and zinc(Zn)(Rameshet al.,2014).Indirectly,they defend plants from phytopathogenic microorganismsviaantagonistic activities,i.e., release of lytic enzymes, siderophore, and antibiotics (Clúaet al.,2018;Nagpalet al.,2020).Several species ofBradyrhizobiumsuch asBradyrhizobium japonicum,Bradyrhizobium iriomotense,Bradyrhizobium lianingense,Bradyrhizobium dagingense,Bradyrhizobium huanghuaihaiense,Bradyrhizobium yuanmingense,Bradyrhizobium elkanii,Bradyrhizobium pachyrhizi, andBradyrhizobium canariensehave been reported from China,the center of origin of soybean(Yanet al., 2017).Diverse PGPR, namelyPseudomonas,Leclercia,Pantoea,Acinetobacter,Bacillus,Enterobacter,Enterococcus,Serratia,Burkholderia, andPaenibacillus,have been isolated from different tissues(root,leaf,nodule,and stem) of soybean and documented as potential novel resources for plant growth promotion, symbiosis, and antagonism(Menonet al.,2019;Sánchez-Cruzet al.,2019;Tapia-Garcíaet al., 2020).This emphasizes the role of PGPR as crucial bioagents in the process of biofertilization.Research has revealed that soil treatment with diazotrophs significantly increases the growth and yield of agricultural crops(Janget al.,2018;Raheemet al.,2018;Roychowdhuryet al., 2019).Chaudhary and Shukla (2018) documented a significant positive effect of co-inoculation ofBradyrhizobiumandPseudomonassp.on plant growth, symbiotic efficacy,nutrient acquisition,and yield components when compared to inoculation withBradyrhizobiumsp.alone.
This emphasizes the need to identify bacterial strains that will promote higher grain yield under various ecological and environmental conditions.The use of indigenous bacterial strains has an added advantage of easy acclimatization to natural conditions,ultimately enhancing the plant-microbial interactions(Zahidet al.,2015).Presently,little information is available on the utilization of microbial inoculants in the combination as consortia in India.Application of the potential microbial consortium may prove beneficial to environmentally sustainable agricultural production.Keeping in mind the beneficial effects ofBradyrhizobiumsp.andPseudomonassp.on improving plant growth,the present research aimed to:i)isolate and characterize phosphate-solubilizing bacteria(PSB)from different tissues of soybean;ii)study the biocompatibility of potential PSB with the recommendedBradyrhizobiumsp.LSBR-3 for development of a microbial consortium; and iii)study the effects of coordination betweenBradyrhizobiumsp.and the best compatible PSB on growth, symbiosis, nutrient accumulation, grain yield,and profitability of soybean in an intensive cropping system of northwestern India.
MATERIALS AND METHODS
Isolation,characterization,and phylogenetic tree construction of PSB
Plant tissue samples were collected from the Pulses Research Farm,Department of Plant Breeding and Genetics,Punjab Agricultural University(PAU),Ludhiana(30°54′N,75°48′E,247 m above sea level),located in the Central Plain zone of Punjab State of the Trans Gangetic agroclimatic zone of India.The collection was made at the flowering stage of soybean from the genotype SLE-27,wild species(Glycine sojaandG.tomentella),and a cross of SL(E)-20 during the rainy reason of 2015–2016 for isolation of PSB.
Bacterial strains were isolated from different tissues(roots,nodules,stems,and leaves)of the wild species and genotypes of soybean using the serial dilution method on phosphate-solubilizing growth medium(10.0 g glucose,5.0 g Ca3(PO4)2,0.10 g(NH4)2SO4,0.25 g MgSO4·7H2O,0.2 g KCl,5.0 g MgCl2,0.025 g bromophenol blue,20.0 g agar,1 L distilled water,and pH adjusted to 7.0±0.2 before sterilization)(Guptaet al.,1994;Aroraet al.,2014)containing tricalcium phosphate(TCP).A total of 40 morphologically distant PSB colonies capable of solubilizing inorganic phosphate were obtained(Table SI,see Supplementary Material for Table SI).Based on phosphate solubilization ability,the PSB isolatePseudomonas oryzihabitansLSE-3 from leaf tissue of soybean genotype SLE-27 was selected and characterized for phenotypic, biochemical, physiological,and plant growth-promoting (PGP) traits.RecommendedBradyrhizobiumsp.LSBR-3 was collected from the Pulses Research Microbiology Laboratory, Department of Plant Breeding and Genetics,PAU,Ludhiana,India,and subcultured on yeast extract mannitol agar medium.All the isolates were maintained in 3%–5%glycerol at−20°C until use.
The isolate LSE-3 was characterized by Gram staining reaction(Harley and Prescott,2002)and different biochemical tests,including indole,methyl red,Voges-Proskauer and citrate utilization (IMViC), oxidase, catalase, nitrate reduction,and starch hydrolysis,according to Ullahet al.(2018).The isolate was screened for sensitivity to different antibiotics of tetracycline(10µg),ampicillin(2 and 10µg),erythromycin(10µg),ciprofloxacin(5µg),chloramphenicol(10µg),gentamycin(10µg),streptomycin(25µg),penicillin(10µg),kanamycin(10µg),and amoxicillin(10µg)(Rameshkumaret al.,2016).The carbohydrate assimilation profile of the isolate was obtained using a HiMedia HiCarbohydrate kit (KB-009, HiMedia, India) according to the manufacturer’s instructions.Cell motility is a key trait for early colonization.For assay of cell motility,the test culture was stab inoculated to the bottom of trypticase soy agar plate(1% agar) and incubated at 28±2°C for 48–72 h (Parket al.,2005).
Genomic DNA of LSE-3 was extracted using the cetyltrimethylammonium bromide (CTAB) protocol with minor modifications (Huanget al., 2013) and subjected to polymerase chain reaction (PCR) amplification with 16S rRNA primers 8F1(5′-AGAGTTTGATCCTGGCTCAG-3′)and 1492R(5′-AGAGCTACCTTGTTACGACTT-3′)(Milleret al., 2013).The amplicon was separated on agarose gel(1.5%),eluted using a Qiaquick gel extraction kit(Qiagen,USA),and sent to Xcelris Lab Ltd.(Xcelris,India)for partial 16S rRNA sequencing.The obtained forward and reverse sequences were compared with 16S rRNA gene sequences available in National Center for Biotechnology Information (NCBI) GenBank (http://www.ncbi.nim.nib.gov) by employing Basic Local Alignment Search Tool (BLAST)algorithm.The sequence generated from LSE-3 was aligned with closely related bacterial sequences using ClustalW,and a phylogenetic tree was constructed using the neighborjoining algorithm of MEGA 6.0 software (Tamuraet al.,2013).
Amplification of nifH region
For amplification of a region(780 bp)of thenifHgene(encoding the nitrogenase iron protein complex), primers nifH-F(5′-TGCGATCCCCGAAAGGCCGGACTC-3′)and nifH-R(5′-ATCGGCCATCATTCTCAGCCGGA-3′)were used (Herboldet al., 2015).A 25-µL reaction mixture,including 2 µL (30–60 ng) of template DNA, 2.5 µL of 10×Taq polymerase buffer(100 mmol L−1Tris-HCl and 15 mmol L−1MgCl2), 1.0 µL (100 µmol L−1) of each primer,2.5µL of 200µmol L−1dNTPs,and 1 U of Taq DNA polymerase (Xcelris, India), was used.The amplification was conducted in a PCR thermal cycler (Thermo Fisher Scientific, USA).The PCR amplification profile included denaturation for 2 min at 94°C,followed by 40 cycles of 1 min denaturation at 94°C,50 s annealing at 55°C,1 min extension at 72°C,and a final 7 min extension at 72°C.ThenifHamplicons were separated on 1.5%agarose gel.
Biocompatibility,biofilm formation,and PGP traits evaluation
The potential LSE-3 was analyzed for its biocompatibility with the recommendedBradyrhizobiumsp.LSBR-3 using the disc plate method on Luria-Bertani (LB) agar plate (Ganeshamoorthiet al., 2008).After completion of incubation at 28±2°C for 48–72 h, the absence of any zone of inhibition around the disc indicated biocompatibility between LSE-3 and LSBR-3.Biofilm formation by bacterial isolates (single and dual inoculants) was examined using crystal violet assay according to Cassatet al.(2007).The assays were carried out in quadruplicates,and biofilm formation was monitered in terms of optical density at 560 nm.Multifunctional PGP traits such as indole acetic acid(IAA)production,phosphate and Zn solubilization,ACC deaminase activity,and siderophore and hydrogen cyanide(HCN)production were screened for the compatible consortium(LSBR-3+LSE-3)following standard protocols.
Evaluation of IAA production by single and dual inoculants of LSBR-3 and LSE-3 was carried out according to Susilowatiet al.(2002)by inoculating culture suspension in 10 mL LB broth containing 100 µg mL−1tryptophan and incubating at 28±2°C for 3–6 d.The amount of IAA(µg mL−1) was estimated quantitatively by adding 2 mL of Salkowski’s reagent(50 mL of 35%perchloric acid and 1 mL of 0.5 mol L−1ferric chloride solution)to 1 mL of the culture supernatant and incubating it in dark conditions at room temperature for 20–25 min;uninoculated broth with Salkowski’s reagent was used as reference.The assay was carried out in quadruplicate.The absorbance of the pink color was measured with an Elico UV-Vis spectrophotometer(Elico Ltd.,India)at 535 nm.
The potential of LSBR-3 and LSE-3 as single or dual inoculants to solubilize inorganic phosphate was assessedin vitroquantitatively(Murphy and Riley,1962).The assay was carried out in quadruplicate.The presence of yellow color after addition of ammonium molybdate and ammonium vanadate(1:1)to culture supernatant confirmed the phosphatesolubilizing activity of the isolates.The absorbance was read spectroscopically at 420 nm after 25 min of incubation for quantitative estimation of phosphate solubilization.
Zinc solubilization assay was performed on Tris-minimal medium supplemented with zinc oxide (1.244 g L−1or 15.23 mmol L−1) and zinc phosphate (1.988 2 g L−1or 5.0 mmol L−1) at a concentration equivalent to 0.1% Zn(Fasimet al.,2002).After the spot inoculation of log-phase cultures of single and dual inoculants,plates were incubated in the dark at 28±2°C,and formation of a clear halo zone around the bacterial growth was recorded after 7 d.The assay was carried out in quadruplicate.Zinc solubilization efficiency(SE,%)was calculated as described by Rameshet al.(2014):

Single and combined inoculants of LSBR-3 and LSE-3 were also tested for their capacity to utilize ACC as sole N source(Govindasamyet al.,2008).The quantitative assay was performed by determining the amount ofα-ketobutyrate formed through enzymatic hydrolysis of the immediate precursor of ethylene(i.e.,ACC)following the method described by Shrivastava and Kumar(2013).Bacterial cultures were grown to the exponential phase in LB broth(Himedia,India)at 28±2°C for 48–72 h.The cultures were harvested in pellet by centrifugation at 10 000 r min−1for 5–10 min,washed first with pH 7.6 and then with pH 8.5 Tris-HCl(0.1 mol L−1),and suspended in Dworkin and Foster minimal medium containing 3 mmol L−1ACC salt as sole N source.The assay was carried out in quadruplicate.Enzyme activity in the cell-free extract was inferred from the measured amount ofα-ketobutyrate(µg−1protein h−1)using the standard curve ofα-ketobutyrate(Sigma-Aldrich,USA).
Siderophore production of LSBR-3 and LSE-3 on chrome azurol S agar was evaluated by incubation at 28±2°C for 4–5 d as single or dual inoculants (Schwyn and Neiland,1987).Development of yellow orange halo zones around the bacterial colonies was considered positive for iron(Fe)-chelating compound production.To quantify catechol-type siderophore production,culture supernatant was separated by centrifugation at 10 000 r min−1for 15 min and ethyl acetate extract was prepared by extracting 20 mL of supernatant(pH 2.0)twice with an equal volume of solvent(Arnow,1937).Equal volumes of sample and Hathway’s reagent(1 mL of 0.1 mol L−1ferric chloride in 1 mL of 0.1 mol L−1HCl to 100 mL of distilled water mixed with 1 mL of 0.1 mol L−1potassium ferricyanide)were mixed,and color intensity was measured at 560 nm with sodium salicylate as standard.The assay was carried out in quadruplicate.
For assay of HCN production, exponentially grown LSBR-3 and LSE-3 were streaked separately as single or dual inoculants on King’s B(Himedia,India)medium supplemented with glycine(4%)and incubated for 24 h at 28±2°C in quadruplicate.Autoclave-sterilized filter paper strips were first soaked in 0.5%picric acid and 5%sodium carbonate solution,placed in Petri plates,and incubated at 28±2°C for 2–3 d after sealed with parafilm.Change in color from orange yellow to strong reddish brown indicated HCN production(Castric,1975).
Native potential bacterial isolates were spot-inoculated for pathogenicity test on blood agar medium containing 5%(volume/volume) sheep blood at 28±2°C for 2–3 d in quadruplicate.Absence of clear halo zone around bacterial colony was considered negative for hemolytic ability/pathogenicity for humans.
Field experiment
A field experiment was conducted for two consecutive years during the rainy seasons of 2016–2017 and 2017–2018 at Pulses Research Farm, PAU, Ludhiana, Punjab, India,with commercialized soybean variety SL-958.The soil at the field experimental site was loamy sand in texture,with 0.22 dS m−1electrical conductivity,pH 7.5,low organic carbon(2.7 g kg−1)and available N(9.31 g kg−1),medium available phosphorus(P)(1.44 g kg−1),and high available potassium(K) (10.94 g kg−1).The experiment adopted randomized block design.It consisted of four treatments,and each treatment was replicated four times.Seeds of the recommended SL-958 variety were surface-sterilized by soaking in 2%NaOCl for 7 min, followed by three washes with distilled water and soaking in broth culture of the potential single and dual inoculant treatments (107–108colony-forming units mL−1)in equal ratios.Seeds,100 g per plot,were treated with 5 mL of specific inoculum.The treatments included:i)uninoculated control,ii)single inoculation with LSBR-3,iii)single inoculation with LSE-3,and iv)dual inoculation with LSBR-3 and LSE-3.The crop was grown following recommended agronomic protocols applied to Kharif crops(Bhatti and Kaur,2020).Seed germination percentage was recorded at 10 d after sowing(DAS).Plant height(cm),plant biomass(g plant−1),and total chlorophyll content(mg g−1fresh leaf)(Arnon,1949)were measured at vegetative(55 DAS)and flowering stages(90 DAS).Symbiotic traits,including nodulation,nodule biomass,and leghemoglobin content(Wilson and Reisenauer, 1963), macronutrient accumulation, including total N (Kirk, 1950), K, and P (Bhargava and Raghupathi,1993),and total micronutrients,including sodium(Na)(Boldet al.,1965),manganese(Mn),Fe,Zn,and copper(Cu)(Ali Ehyaei and Behbahanizadeh,1993),of shoots were measured at flowering stage.Rhizosphere soil samples were collected from 0–15 cm depth of each plot and soil enzyme activities of dehydrogenase(Casidaet al.,1964) and acid and alkaline phosphatases (Eichleret al.,2004)were determined at 90 DAS.The yield components,including number of seeds per pod, number of pods per plant, 100-seed weight, and grain yield, were recorded at harvesting stage.Protein content of the seeds was estimated at harvesting.
For economic return analysis,gross return,net return,and benefit-to-cost(B/C)ratio were calculated on the basis of prevailing prices of input and output as follows:

Market price of soybean was US$0.38 and US$0.41 kg−1during 2016–2017 and 2017–2018,respectively.Economic return was calculated according to the pooled mean of the minimum support price of soybean,i.e.,US$0.40 kg−1.Cultivation cost was calculated based on the common cost spent for growing soybean(e.g.,field preparation,seeds,herbicide,irrigation,labor,fertilizer)along with particular treatment cost(e.g.,single and dual bacterial inoculants,labor),which was US$410.00,US$410.37,and US$409.00 ha−1for single inoculation(LSBR-3 and LSE-3),dual inoculation(LSBR-3+LSE-3),and the control treatments,respectively.
Statistical analysis
All the data in the present investigation are shown as mean±standard deviation.Means of the two-year data fordifferent parameters of the field experiment were compared using the analysis of variance(ANOVA)followed by Tukey’s honestly significant difference test.Significant difference between single and dual inoculation was analyzed by Duncan’s multiple range test(P <0.05).All data analyses were performed using the SPSS software.
RESULTS
Identification and characterization of LSE-3
Bacterial isolate LSE-3 from the leaf tissue of soybean had the ability to solubilize TCP on selective NBRIP agar medium.The isolate produced light yellow mucoid colonies of round-shaped raised rods;the colonies were smooth,odorless,and motile on nutrient agar medium.The isolate also produced green fluorescent pigments visible under UV light on King’s B medium.According to basic biochemical and physiological tests based on Bergey’s Manual of Systematic Bacteriology (Bergeyet al., 1984), LSE-3 isolate was Gram negative,positive for oxidase,catalase,indole,urease,nitrate reductase, cellulase, protease (casein), and citrate utilization,but negative for methyl red,Voges-Proskauer,and amylase production.The isolate was sensitive to tetracycline,erythromycin,ciprofloxacin,chloramphenicol,gentamycin,penicillin,kanamycin,and amoxicillin,but resistant to ampicillin and streptomycin.Carbohydrate utilization pattern indicated that LSE-3 utilized dextrose, mannitol, lactose,cellobiose,sucrose,sorbitol,starch,xylose,mannose,rhamnose,trehalose,raffinose,fructose,maltose,and galactose as carbon sources(Table SII,see Supplementary Material for Table SII).
On the basis of 100%sequence homology of the 16S rRNA gene(Fig.1a)of the potential bacterial isolate LSE-3 and the gene sequences available in the NCBI nucleotide database, GenBank and 16S rRNA Ribosomal Database Project III,the isolate was characterized asPseudomonas oryzihabitans.The blastn search of the 16S rRNA partial sequence showed 98.7%and 98.5%identity withPseudomonasoryzihabitansMLB-3 andPseudomonas psychrotoleransIHBB 6869,respectively.The 16S rRNA partial consensus sequence of LSE-3 isolate was deposited in NCBI GenBank under accession number MF278905.Phylogenetic tree based on 16S rRNA sequences showed that LSE-3 was closely related toP.oryzihabitansMLB-3 (Fig.2).The ability for N fixation of the bacterial isolate was confirmed by amplification of thenifHgene fragment.ThenifHregion(approximately 780 bp) was successfully amplified from LSE-3,confirming its presence in this diazotroph(Fig.1b).

Fig.1 Amplification of the 16S rRNA(a)and nifH encoding the nitrogenase iron protein complex genes(b)of Pseudomonas oryzihabitans LSE-3 and Bradyrhizobium sp.LSBR-3.Lane M is the 100-bp ladder.
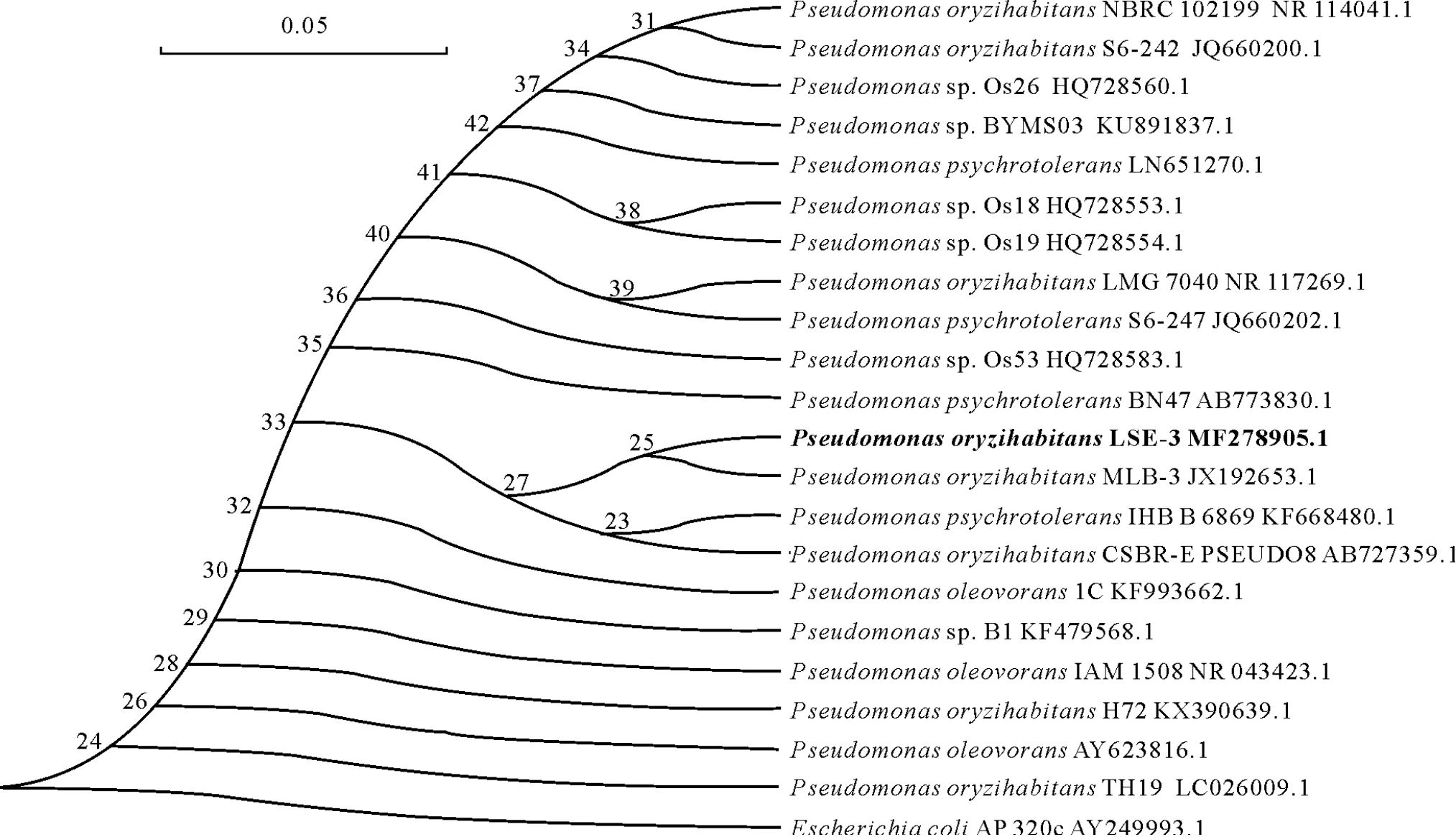
Fig.2 Phylogenetic relationship of Pseudomonas oryzihabitans LSE-3 with closely related species based on 16S rRNA gene sequences obtained from the National Center for Biotechnology Information(NCBI)GenBank database.The tree was constructed using the neighbor-joining method.Bootstrap values are shown at the nodes of branches.
Biocompatibilityof LSE-3 with LSBR-3 and biofilm formation
After assessing the non-pathogenic nature of LSE-3 on blood agar plate, we tested the compatibility of LSE-3 with the commercialized LSBR-3; the absence of zone formation around the bacterial colony on the pre-seeded plate of LB agar medium confirmed their compatibility(Fig.S1,see Supplementary Material for Fig.S1).Biofilm formation was quantitatively estimated,and a significantly higher biofilm formation in terms of optical density of crystal violet at 560 nm was observed in the treatment with combined inoculation of LSBR-3 and LSE-3 when compared with that obtained by single bacterial inoculant(Table I).
Multi-functional PGP traits of single and dual bacterial inoculants
Single inoculants of LSBR-3 and LSE-3 were able to synthesize IAA in liquid medium with Salkowski’s reagent.Intense pink color was detected in LSBR-3+LSE-3 at the 6th day of incubation in the presence of L-tryptophan(Table I).The phosphate-solubilizing ability was confirmed in LSBR-3 + LSE-3 by clear yellow halos on the NBRIP medium after two weeks of incubation.Phosphate solubilization in LSBR-3 + LSE-3 (184.4±7.4 mg L−1) was significantly higher than those in monocultures of LSBR-3 and LSE-3 (115.8±6.9 and 169.8±12.5 mg L−1, respectively)at the 9th day of incubation.Zinc solubilizationefficiency of LSBR-3+LSE-3(398.59%±20.36%)was also significantly higher than those of monocultures of LSBR-3 and LSE-3(278.46%±18.73%and 372.20%±12.64%,respectively).All the tested bacterial isolates utilized ACC as a sole N source with variable degree of efficacy.Quantitative ACC deaminase activity ranged from 0.43±0.02 to 0.51±0.07 mmolα-ketobutyrateµg−1protein h−1in single and dual inoculatants of LSE-3 and LSBR-3 on the 3rd day of incubation.Dual inoculation treatment LSBR-3+LSE-3 was more effective compared to monoculture treatment(LSBR-3 or LSE-3) for ACC deaminase activity.Both LSE-3 and LSBR-3 possessed a strong ability to produce siderophore,enhancing Fe bioavailability and inhibiting the growth of phytopathogens in rhizosphere soil.The combination LSBR-3+LSE-3 produced significantly more siderophores(121.46±1.61µg mL−1)in comparison with single bacterial treatments with LSE-3(118.9±7.83 µg mL−1)and LSBR-3(98.7±3.37µg mL−1).Both potential indigenous bacterial strains LSBR-3 and LSE-3 produced HCN in King’s B medium amended with glycine.

TABLE IMultifunctional plant growth-promoting(PGP)traits of single and dual inoculants of Pseudomonas oryzihabitans LSE-3 and Bradyrhizobium sp.LSBR-3
Beneficial effects of single and dual bacterial inoculants on soybean and soil
Isolates LSBR-3 and LSE-3 inoculated singly or in combination had the ability to promote early seed emergence in soybean (Table II).At vegetative stage, treating plants with dual inoculants of LSBR-3 and LSE-3 significantly increased plant height,shoot dry weight,root dry weight,and chlorophyll content by 17.87%,37.98%,55.97%,and 36.46%,respectively,as compared with the control.At 90 DAS,application of dual inoculants showed a similar trend regarding plant growth parameters as that of single inoculant or control treatments.The plant growth parameters in the single and dual bacterial treatments were significant different from those in the control treatment at both vegetative and flowering stages due to enhanced photosynthetic activities.
Inoculant LSE-3 alone or in combination with LSBR-3 improved the symbiotic traits in soybean.At flowering stage, the number of nodules, dry weight of nodules, and leghemoglobin content were 11.11%,33.47%,and 7.93%,respectively,higher(P <0.05)in LSBR-3+LSE-3 than in the single LSBR-3 treatment(Table III).
Significant differences in total macronutrient(N,P,and K)contents of soybean shoot were observed at 90 DAS between the treatments(Fig.3).In plants with dual inoculation(LSBR-3+LSE-3),the N,P,and K contents of the shoot were significantly higher (1.25-, 1.13-, and 1.15-fold, respectively)compared with the control.The total Na contentof soybean shoot in the dual inoculation treatment decreased significantly by 13.41%while that in the monocultures of LSBR-3 and LSE-3 decreased by 6.10%and 3.65%,respec-tively,compared with the control(Fig.4).The total Mn,Fe,and Zn contents of shoot varied nonsignificantly between the treatments at 90 DAS,though they increased in the LSBR-3+ LSE-3 treatment by 21.47%, 24.63%, and 22.54%, respectively,compared with the control.Single treatment with LSBR-3 and LSE-3 significantly increased Cu content of soybean shoot by 18.41%and 34.30%,respectively,compared with the control.The dual inoculation increased the shoot Cu content by 45.13%compared with the control.
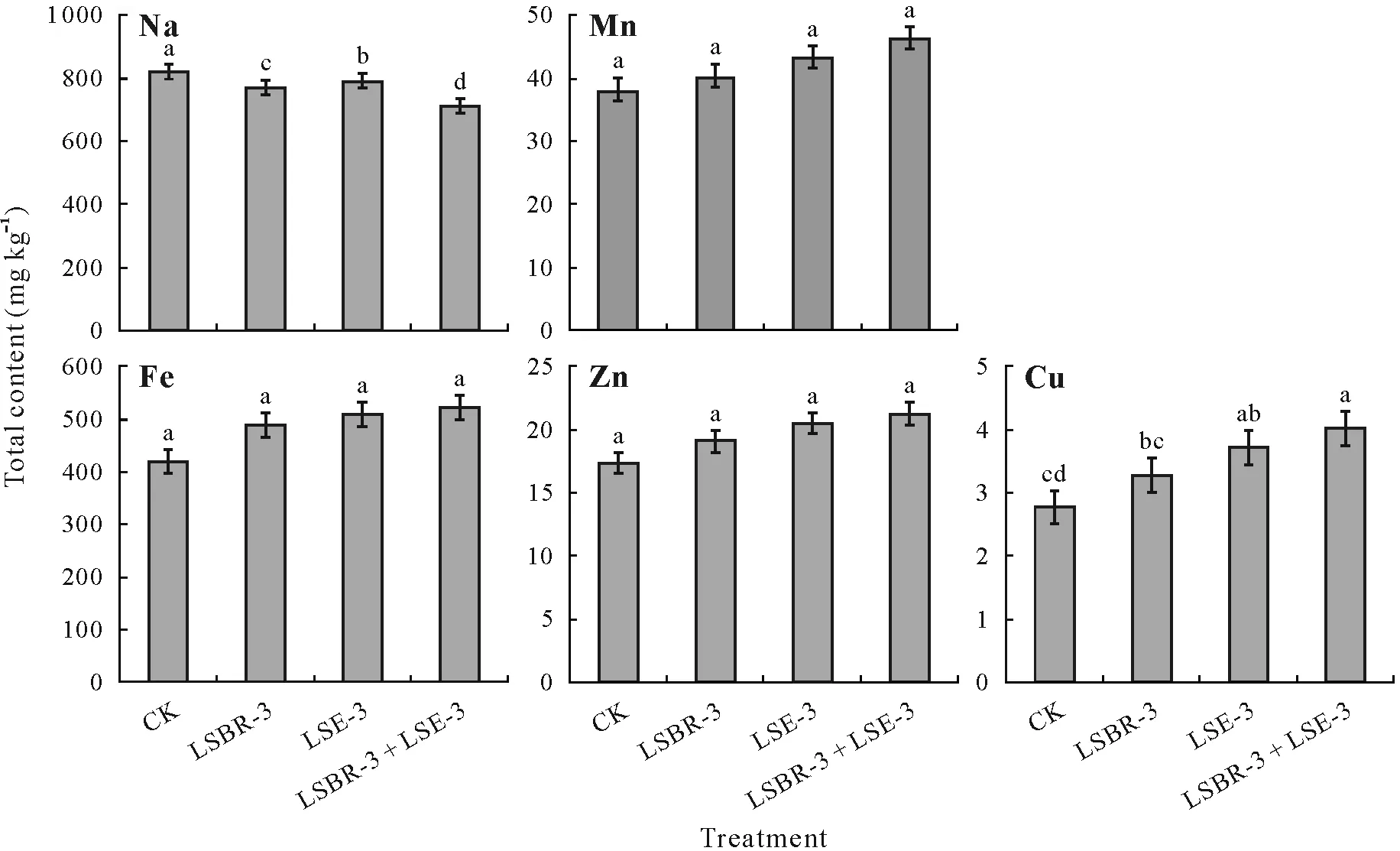
Fig.4 Effects of single and dual bacterial inoculants of Pseudomonas oryzihabitans LSE-3 and Bradyrhizobium sp.LSBR-3 on the total contents of micronutrients,Na,Mn,Fe,Zn,and Cu,in soybean shoots in a field experiment conducted during the rainy seasons of 2016–2017 and 2017–2018 at Pulses Research Farm,Punjab Agricultural University,India.Data are pooled means of two-year results.Bars are standard deviations(n =4).Different letters indicate significant differences between treatments based on Tukey’s honestly significant difference test at P <0.05.CK=uninoculated control.

TABLE IIIEffects of single and dual inoculants of Pseudomonas oryzihabitans LSE-3 and Bradyrhizobium sp.LSBR-3 on symbiotic traits of soybean at the flowering stage in a field experiment conducted during the rainy seasons of 2016–2017 and 2017–2018 at Pulses Research Farm,Punjab Agricultural University,India
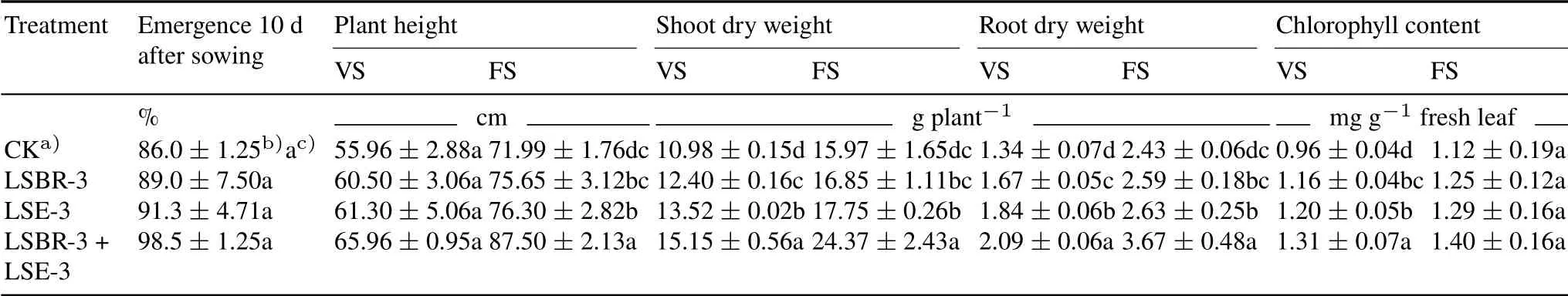
TABLE IIEffects of single and dual inoculants of Pseudomonas oryzihabitans LSE-3 and Bradyrhizobium sp.LSBR-3 on growth parameters of soybean at vegetative stage(VS)and flowering stage(FS)in a field experiment conducted during the rainy seasons of 2016–2017 and 2017–2018 at Pulses Research Farm,Punjab Agricultural University,India

Fig.3 Effects of single and dual bacterial inoculants of Pseudomonas oryzihabitans LSE-3 and Bradyrhizobium sp.LSBR-3 on the total contents of macronutrients,N,P,and K,in soybean shoots in a field experiment conducted during the rainy seasons of 2016–2017 and 2017–2018 at Pulses Research Farm,Punjab Agricultural University,India.Data are pooled means of two-year results.Bars are standard deviations(n =4).Different letters indicate significant differences between treatments based on Tukey’s honestly significant difference test at P <0.05.CK=uninoculated control.
Combination of LSBR-3 and LSE-3 significantly increased the activities of soil dehydrogenase(25.09%)and alkaline phosphatase(31.38%)compared with LSBR-3 alone(Table IV).The highest acid phosphatase activity was observed in the LSBR-3 + LSE-3 treatment, 21.28% higher than that in control.
Combined inoculation with LSBR-3 and LSE-3 improved the protein content and yield-attributing components of soybean owing to their synergistic effect.Protein content in the LSBR-3+LSE-3 treatment increased 1.02-fold compared with the control,and the yield-attributing traits of pod number,seed number,and 100-seed weight increased 1.11,1.58,and 1.14 times,respectively,compared with the control(Table V).The LSBR-3+LSE-3 treatment increased grain yield by 10.85%and LSBR-3 alone by 6.43%compared with the control.

TABLE IVEffects of single and dual inoculants of Pseudomonas oryzihabitans LSE-3 and Bradyrhizobium sp.LSBR-3 on soil enzyme activities in a field experiment with cultivation of soybean during the rainy seasons of 2016–2017 and 2017–2018 at Pulses Research Farm,Punjab Agricultural University,India
Economic return
The use of LSBR-3 and LSE-3 substantially improved the economic returns (Table VI).The highest net return was estimated to be US$592.16 ha−1in the LSBR-3 +LSE-3 treatment,followed by US$571.53 ha−1in the LSE-3 treatment and US$553.32 ha−1in the LSBR-3 treatment,and the lowest was US$495.37 ha−1in the control treat-
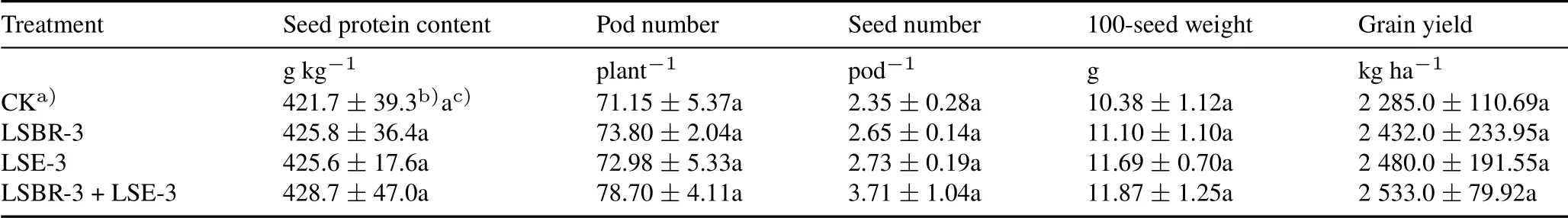
TABLE VEffects of single and dual inoculants of Pseudomonas oryzihabitans LSE-3 and Bradyrhizobium sp.LSBR-3 on protein content,yield attributes,and grain yield of soybean in a field experiment conducted during the rainy seasons of 2016–2017 and 2017–2018 at Pulses Research Farm,Punjab Agricultural University,India
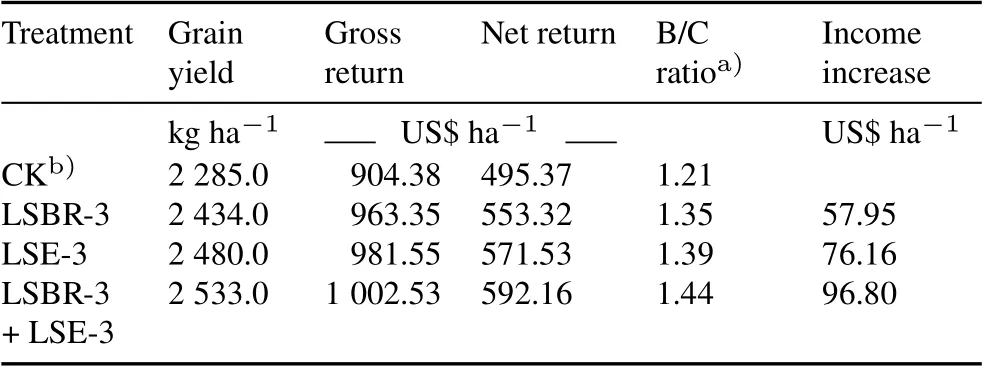
TABLE VIEconomic return of using single and dual inoculants of Pseudomonas oryzihabitans LSE-3 and Bradyrhizobium sp.LSBR-3 for soybean in a field experiment conducted during the rainy seasons of 2016–2017 and 2017–2018 at Pulses Research Farm,Punjab Agricultural University,India
ment.The application of dual inoculants(LSBR-3+LSE-3)increased the income by US$96.80 ha−1with a B/C ratio of 1.44 compared with the control.
DISCUSSION
A total of 40 PSB isolates from plant tissues of soybean genotype SLE-27,wild species(G.sojaandG.tomentella),and a cross SL(E)-20 were obtained in the present study.Of the 40 isolates, LSE-3 was selected on the basis of its morphological,physiological,and biochemical activities and its excellent potential to solubilize TCP on NBRIP medium as illustrated in previous studies(Singhet al.,2017;Menonet al.,2020).Based on 16S rRNA partial gene sequencing data and phylogenetic tree,this prospective indigenous PSB was identified asP.oryzihabitans.The nitrogenase iron protein complex genenifHis the most important metabolic functional gene involved in diazotrophic symbiotic N fixation(Dos Santoset al., 2012).In the current investigation, a sequence ofnifH(780 bp)was successfully amplified fromP.oryzihabitansLSE-3.ThenifHgene was confirmed in soybean isolatesB.yuanmingenseBR3267 andB.pachyrhiziBR 3262 (Leiteet al., 2018).Sequence of diazotrophic bacteria predicted withnifHgene showed homology with
Azospirillumsp.,B.elkani,B.japonicum,B.yuanmingense,Ensifer meliloti,Clostridiumsp.,Rhizobium cellulosilyticum,andPaenibacillussp.isolated in soybean-wheat intensive agriculture(Bromfieldet al., 2017; Gyogluuet al., 2018;Igiehonet al.,2019;Sharafet al.,2019).
Biofilm formation byPseudomonassp.enhances its establishment for nutrient availability,maintenance in favorable environment,and development of a syntrophic association with another bacteria,and promotes plant growth(Backeret al.,2018).Maximum biofilm formation(in terms of optical density)and biocompatibility on LB agar between LSBR-3 and LSE-3 were recorded when the two strains were inoculated together.Enhanced biofilm formation was reported in combined application ofStreptomycessp.R-170 andSphingomonassp.T-168 in potato rhizosphere(Santiagoet al.,2017).In an earlier study,Kumawatet al.(2019a)noticed improved biofilm formation and early colonization in soybean with combined inoculation ofBradyrhizobiumsp.andPseudomonas aeruginosacompared to single-species inoculation treatment.This enhancement in biofilm formation might be due to the production of non-inhibiting secondary metabolites in single medium with microbial consortium under natural soil ecosystem.Compatible biofilm formed by dual inoculation withTrichoderma virideandAzotobacter chroococcumshowed greater phytohormone production,phosphate and Zn solubilization,ACC deaminase activity,and siderophore production compared with single-species bacterial inoculation(Triveniet al.,2012).Compatible dualspecies bacterial inoculation improved seed germination,growth, symbiotic parameters, and nutrient accumulation in soybean compared with single inoculants(Backeret al.,2018;Kumawatet al.,2019b).
Phosphate and Zn solubilization,siderophore production,and ethylene removalviaACC deaminase activity are some of the functions ofPseudomonassp.(Ferchichiet al.,2019;Stevenset al.,2019;Nagpalet al.,2020).Hydrogen cyanide acts as a biocontrol agent and a substrate in geochemical processes(e.g.,metal chelation).Indirectly,it is known to enhance the availability of phosphate to plants and change root exudation pattern in legume plants for bacterial infection and nodulation process(Xieet al.,2016;Kamranet al.,2017;Liet al.,2017;Saikiaet al.,2018;Kumawatet al.,2019a).Our results demonstrated that better performance of the dual inoculation LSBR-3+LSE-3 can be associated with phytohormone production,ACC deaminase activity,phosphate and Zn solubilization,and siderophore and HCN production,which induced root proliferation and improved nutrient availability, plant growth, and symbiotic attributes.Jha and Saraf(2012)also reported significantly higher IAA(107µg mL−1)with the consortium ofBrevibacillus brevisMS1+Acinetobacter calcoaceticusMS5+Bacillus licheniformisMS3compared with the monoculture treatment.Higher IAA production obtained in LSE-2+LSBR-3 as compared with single culture in the presence of L-tryptophan as precursor has been reported in soybean(Kumawatet al.,2019a).Significantly higher phosphate solubilization with dual inoculation ofPseudomonas fluorescence+Bradyrhizobiumsp.LSBR-3 in soybean may be attributed to the release of organic acids as well as acid and alkaline phosphates by the PSB(Singh,2017; Kumawatet al., 2019a).Zinc in fertilizers such as ZnSO4and Zn-EDTA is available to plants as divalent cation,but most of the Zn in inorganic fertilizers is transformed into immobile complexes within 5–7 d after application in soil(Karaket al.,2005).Zinc-solubilizing bacteria are potential alternatives for Zn supplementation that convert applied inorganic Zn to available forms for plant nutrition(Rameshet al., 2014).Known as effective Zn solubilizers, PGPR convert Zn complexes into simple forms by releasing organic acids to sequester Zn cations,leading to reduction in soil pH(Kamranet al.,2017).Other mechanisms involved in Zn solubilization are related to the release of protons,chelation of siderophore ligands,and the oxido-reductase system on cell membranes(Changet al.,2005).Several potential bacterial isolates such asPseudomonas aeruginosa,P.fragi,Pantoea agglomerans,Entherobacter cloacae,Rhizobiumsp., andBacillussp.were reported as prospective Zn solubilizers for enhanced nutrition and Zn bio-fortification in soybean,wheat,and common bean rhizosphere(Kumaret al.,2016;Kamranet al.,2017;Kumawatet al.,2019a).
Under abiotic and biotic stress conditions,the level of ACC increases at high ethylene concentration,which is detrimental to plant growth.By hydrolyzing ACC into ammonia andα-ketobutyrate,PGPR with ACC deaminase activity supply N and energy required for plant growth under salt stress conditions(Gupta and Pandey,2019).Egamberdievaet al.(2017)demonstrated significantly higher ACC deaminase activity of the dual inoculants ofBradyrhizobium japonicumUSDA 110 andPseudomonas putidaTSAU1 from soybean,which utilized ACC as N source.
Improved Fe-scavenging properties ofBradyrhizobiumsp.,Pseudomonas aeruginosa,Sinorhizobium meliloti,Rhizobium cellulosilyticum,Ensifer meliloti,andLeclercia adecarboxylataare positively correlated with plant growth and nodulation effectiveness in legumes due to increased Fe solubility in the rhizosphere through siderophore and proton production(Igiehonet al.,2019; Kumawatet al.,2019b).Higher siderophore production was also reported for the bacterial strains KA-19,PF-17,and EKi(Arora and Verma,2017).Siderophore production by microbes facilitates Fe acquisition of nodules and ultimately improves the synthesis of Fe-containing proteins and Fe uptake capacity,leading to enhanced plant growth and nodulation through N fixation in legumes(Jinet al.,2014).Plant growth and yield-attributing traits ofPhaseolus vulgariswere improved by inoculation with siderophore-producing bacteria such asBacillussp.,Pseudomonassp.,and their combination withRhizobiumsp.(Hungriaet al.,2013).
Our results revealed enhanced shoot and root dry weight of soybean co-inoculated with LSBR-3 and LSE-3.This might be due to the entry of bacterial phytohormone IAA with waterviathe seed coat during embryo development,which enhances plant growth and concomitantly improves water and nutrient uptake(Egamberdievaet al.,2018).For dual inoculation,the bacteria consortium releases the phytohormone IAA into the culture medium.Strain LSE-3 produced high levels of IAA,which might regulate the distribution of plant nutrients by creating a favorable environment for LSBR-3 to form effective nodules for biological N fixation with greater nodule biomass and by increasing the number of infection sites on soybean roots.The isolates LSE-3 and LSBR-3 singly and in combination produced siderophores,which might have facilitated the uptake of Fe from soil.Iron is a major constituent of nitrogenase and leghemoglobin(Gómez-Sagasti and Marino,2015;Kumawatet al.,2019a).Bacterial siderophores might be responsible for increased leghemoglobin content in soybean in the present study.Previous results suggest that combined bacterial inoculation facilitates the absorption of nutrients through modified root architecture and formation of effective nodules in soybean(Kumawatet al., 2019a, b).Moreover, previous research showed that combination of LSBR-3 and LSE-3 resulted in the development of long taproots and adventitious roots(Kumawatet al.,2019b),improved nodulation,and enhanced N,P,and K contents of shoots in soybean.
Previous results revealed that bacterial strains increased lateral root branching and root hair density,which in turn increased the surface-to-volume ratio,directly improving the acquisition of water and essential nutrients from soil (Yuet al.,2019;Goswami and Deka,2020;Ruizet al.,2020).Htweet al.(2019)similarly reported that co-inoculation ofB.japonicumSAY 3-7,B.elkaniiBLY-3-8, andStreptomyces griseoflavussignificantly increased the N,P,and K contents of shoots in soybean as compared with the control.Combined application of MyanmarB.yuanmingenseMAS-34 andStreptomycessp.P4 also significantly augmented the growth,nutrient uptake,and grain yield of soybean byimproving soil fertility (Soeet al., 2018).In the present study, LSE-3 as a PGPR has the potential for successful plant-microbe interactions and induction of physiological processes related to mineralization and solubilization of nutrients.Dual inoculants ofRhizobium leguminosariumTAL-3612 andPseudomonas koreensisMG-209738 significantly improved micronutrient (Zn, Mn, Fe, and Cu)contents of the shoot in common bean as compared with the untreated plot(El-Nahrawy and El-Omara,2017).Used as a biofertilizer/bioenhancer in the present investigation,LSE-3 colonized the roots of soybean plants as an endophyte,solubilized the minerals,and increased available nutrients.Significantly higher soil dehydrogenase activity and grain yield of soybean were reported with combined inoculation ofB.japonicumandA.chroococcumwhen compared with the control plot(Marinkovićet al.,2018;Cassánet al.,2020).Dehydrogenase and phosphatase activities are indicators of the viable microbial community present in the rhizosphere.Enhancement in solubilization and mineralization of nutrients in soybean occurs due to significantly higher phosphatase activity and altered root system architecture caused by combined inoculation ofBradyrhizobiumsp.andPseudomonassp.(Egamberdievaet al.,2017; Kumawatet al.,2019a).Inoculation ofBradyrhizobiumsp.,Pseudomonassp.,and PGPR might increase the supply of macronutrients and micronutrients through secretion of organic acids and PGP substances, which are translocated to the photosynthetic machinery,whereby the growth,early colonization,nodulation efficiency,yield-attributing traits,and ultimately the grain yield of soybean are promoted (Hussain, 2017;Sahuret al.,2018;Singhet al.,2019).A significantly higher grain yield was observed in soybean after co-inoculation ofBradyrhizobiumsp.andPseudomonassp.along with application of a recommended dose of fertilizer(El-Nahrawy and El-Omara, 2017).Similarly, grain yield in soybean was significantly increased with combined inoculation ofB.japonicumE109 +Azospirillum brasilenseAz39 (Puenteet al., 2019) andBradyrhizobium diazoefficiensSEMIA 5080+Bacillus subtilisQST713(Morettiet al.,2020)as comparison with the control.The B/C ratio of the consortium biofertilizer LSBR-3+LSE-3 was 1.44,with additional revenue of approximately US$96.80 ha−1when compared with the control.
CONCLUSIONS
Our study shows that the tripartite bacteria-legume symbiosis can be used as a novel approach for enhancing plant growth along with improving productivity and profitability of soybean in an eco-friendly manner.The prospective indigenous diazotrophic PSBP.oryzihabitansLSE-3 is a potent microbial bioenhancer and,along with the commercializedBradyrhizobiumsp.LSBR-3,functions as a biostimulator in soybean under diversification program.This study will be useful in establishing the potency of dual bacterial inoculants in combination in soybean cultivation on a commercial scale in Indian.
ACKNOWLEDGEMENT
The first author is grateful to the Indian Council of Agricultural Research (ICAR), India for the Junior Research Fellowship (ICAR-JRF) awarded toward conducting the M.S.research project.
SUPPLEMENTARY MATERIAL
Supplementary material for this article can be found in the online version.
杂志排行
Pedosphere的其它文章
- Letter to the Editor Can sieving size affect the determination of soil available micronutrients?
- Genomic characterization of multidrug-resistant extraintestinal pathogenic Escherichia coli isolated from grain culture soils
- Soil compression influences the avoidance behavior of Allonychiurus kimi(Collembola)to cadmium and copper
- Carbon mineralization in subtropical alluvial arable soils amended with sugarcane bagasse and rice husk biochars
- Key variable for simulating critical nitrogen dilution curve of wheat:Leaf area ratio-driven approach
- CO2 emission and source partitioning from carbonate and non-carbonate soils during incubation
