Shoulder girdle recognition using electrophysiological and low frequency anatomical contraction signals for prosthesis control
2022-04-06EjayNsugbeAliAlTimemy
Ejay Nsugbe|Ali H.Al-Timemy
1Nsugbe Research Labs, Swindon, UK
2Biomedical Engineering Department,Al-Khwarizmi College of Engineering, University of Baghdad,Baghdad,Iraq
Abstract Shoulder disarticulation amputees account for a small portion of upper-limb amputees,thus little emphasis has been devoted to developing functional prosthesis for this cohort of amputees.In this study,shoulder girdle recognition was investigated with acquired data from electrophysiological (electromyography [EMG]) and low frequency contraction(accelerometer [Acc]) signals from both amputee and non-amputee participants.The contribution of this study is based around the contrast of the classification accuracy(CA)for different sensor configurations using a unique set of signal features.It was seen that the fusion of the EMG-Acc produced an enhancement in the CA in the range of 10%-20%,depending on which windowing parameters were considered.From this,it was seen that the best combination of a windowing scheme and classifier would likely be for the 350 ms and spectral regression discriminant analysis, with a fusion of the EMG-Acc information.The results have thus provided evidence that the two sensors can be combined and used in practice for prosthesis control.Taking a holistic view on the study,the authors conclude by providing a framework on how the shoulder motion recognition could be combined with neuromuscular reprogramming to contribute towards easing the cognitive burden of amputees during the prosthesis control process.
KEYWORDS cybernetics, electromyography, neuromuscular reprogramming, pattern recognition, prosthesis control, upperlimb amputees
1|INTRODUCTION
The loss of an upper limb is a widespread issue that affects regions worldwide, the root causes of which are varied and include vascular diseases such as diabetes, trauma sustained from accidents, and also regional conflict-related traumatic amputations [1].The losses and complications which accompany an upper-limb amputation include the following: (i)constrained level of independence,(ii) episodic phantom pain,and (iii) an imbalance of the human motor control pathway,which includes the brain, active limbs, somatosensory and motor neurons[1-3].A breakdown of upper-limb amputation statistics for the UK and Italy show that the most common form of substantial upper-limb loss is transhumeral (above elbow) amputations, closely followed by transradial (below elbow)amputations[2].According to this source,the shoulder disarticulation amputation is one of the less common types of upper-limb amputations, and as a result, functional prosthesis solutions have not been as intensely researched to the extent of transhumeral or transradial amputations[2].In addition to this,the design space for shoulder disarticulation prosthesis is constrained due to difficulties in tracking phantom motions in the absence of a stump, along with biosensor related limitations, that is sensor locations and inability to use wearables.
In recent years, shoulder disarticulate patients have been seen to favour mechanically actuated prosthesis which are mostly driven by pneumatics reliant on flexion patterns of the mid-section.In terms of myoelectric prosthesis, whichrepresents the most advanced replacement of a biological limb,research has been aimed at solving the motion recognition problem, which allows for effective prosthesis control and involves the acquisition of signals from the non-residual anatomy along the shoulder blade to interpret shoulder motions which form part of the control architecture and inform the desired actuation signals for a myoelectric prosthesis [4].Further information regarding prosthesis control architectures and sensors can be seen in review papers written by Nsugbe et al.[5] and Fougner et al.[6].
In the literature, key works by Gonzalez et al.[7], Rivela et al.[8], Soma et al.[9], and Horiuchi et al.[10] showed that shoulder motions could be identified for a group of nonamputees, whereas Kaur et al.[11] managed to differentiate shoulder motions for a group of amputees across four shoulder motions, and using the wavelet transform, obtained an accuracy of 98%.More recently,Sharba et al.[4],were able to differentiate between various shoulder motions for a group of non-amputees and amputees, where they showed that high classification accuracies (CAs) were obtainable with a slight degradation in the accuracy for the case of the amputee participants.Sharba et al.'s [4] data collection involved the combination of the commonly used electromyography sensor(EMG), which measures electrical signals associated with muscular contractions.This was combined with an accelerometer (Acc), which has the ability to monitor low frequency displacements emanating from both anatomical contractions and skeletal movements that come from designated shoulder gesture motions [4].As with Kaur et al.[11], Sharba et al.[4]extracted features from the time-frequency domain through the wavelet transform and obtained an accuracy of 87% for the case of amputees across seven shoulder motions.The wavelet has been said to be computationally intense in the scope of real-time performance, and as a result, it is mainly suited to research-based exercises and case studies at this point [4].
NeuroMuscular Reprogramming(NMR)is thought to have chiropractic roots and involves a rather tactile-based learning strategy to embed new neurological motor control programmes within the brain [12].It is seen as a key piece of rehabilitation therapy which involves a strategic repetition of novel neuromuscular‘correction’patterns to guide the muscles into learning new patterns, where the correction refers to the intervention strategy employed by the NMR practitioner to instill reprogramming of the neural circuitry in the cerebrum,which hosts the portion of the brain responsible for motor learning [12].The tactile and kinaesthetic process involved in the NMR process initiates a bi-directional flow involving the motor control centre, where stored patterns are invoked for active reprogramming, and the new patterns stored as a result of the therapeutic exercise eventually become the body's automated response[12].NeuroMuscular Reprogramming has traditionally been used for the restoration of optimal movement patterns, most notably post-trauma, ultimately making for an enhanced neuromuscular efficiency and tissue metabolism, while reducing the strain of dysfunctional neuromuscular connections [12].
In this study, we utilised the dataset collected by Sharba et al.[4], alongside a combination of advanced features, to observe the extent through which shoulder motions can be identified and differentiated.The recognition of shoulder motions will form part of an enhanced control interface,combined with therapy-based neuromuscular reprogramming,and could allow for the control of a myoelectric prosthesis arm using a range of shoulder motions,while minimising cognitive load in the process.
Specifically, the contributions of this study are as follows:
- Investigation and comparison of the CA for EMG-only,Acc-only and EMG-Acc for an extended feature vector
- Performance comparison (CA and selection time) for a set of classifiers, namely linear discriminant analysis (LDA),spectral regression discriminant analysis (SRDA) and support vector machine (SVM)
- Provide thoughts on how neuromuscular reprogramming therapy can be combined with motion recognition for prosthesis control.
2|MATERIALS AND METHODS
The data that formed the core theme of the work presented as part of this study was acquired from the work done by Sharba et al.[4].This section describes the underlying theoretical principle behind the various data collection instrumentation used by Sharba et al.[4] assuming the forward problem, along with the data collection sequence done by Sharba et al.[4] and subsequently the associated signal processing and classification methods employed as part of this study.
2.1|Mathematical models and data collection instrumentation
The theoretical models behind each instrumentation provide a numerical insight into the basis behind how an input signal is detected, alongside the accompanying dependencies.The following subsection describes the theoretical concept behind EMG and Acc.
2.1.1|EMG underlying principle
EMG signals are electrophysiological bio-potentials which are a manifestation of the firing of a motor unit action potential.The simultaneous firing of the motor neurons responsible for action potentials results in EMG signals appearing as time varying signals, which are a function of the anatomical properties from which they emanate [13, 14].Electrophysiological signals within the human body can be modelled as electrical currents flowing through tissue with the combination of a volume conductor and a multi-dimensional perspective of Ohm's law [13, 14].
Assuming a biological tissue of conductivityσirecorded atp0in thex0,y0,z0direction and a source currentIsat point P(x, y, z), a 3-dimensional electric potential can be described as seen in Equation (1).

whereVp0represents the electrical voltage potential, andriis the shortest distance betweenP0andP.From Equation (1), it can be deduced that the voltage potential acquired by a specific point is dependent on key factors such as tissue conductivityσi, and distance from the sourceri.However, it is worth mentioning that, due to the biophysical dynamics of motor units and their simultaneous action potentials,bioelectricity can be represented as a set of superimposed expressions emanating from multiple sources [13, 14].
A deeper and more comprehensive representation of biological electrical potentials can be done using the dipole theory.Dipoles are electrical sources warped by an electrical field in an extracellular medium, as proposed by Wilson et al.[15] and Plonsey and Barr [16].Consider a fibre element of lengthdxwith a current flow in the presence of an extracellular potential,denoted byp-.dx, wherep-is the dipole current/unit length,as the current freely flows from the source into unbounded space, the extracellular potential for a dipole basis can be defined as seen in Equation (2) [15, 16].

wheredφeis a changing extracellular potential,σeis the tissue conductivity of the extracellular medium, andrrepresents the distance from the source of the excitation to the recording pointPo.
If the distanceris located along the coordinates ofPandPo, the distance can be computed using Equation (3).

An integral sum across a number of action potentials from simultaneously firing motor neurons using the dipole theory basis can be seen in Equation (4).

wheretis time.
2.1.2|EMG sensors and signal acquisition
The EMG sensor used by Sharba et al.[4]was a 5-channel wet electrode system.The architecture comprised two analogue filters,namely a fourth order Butterworth low-pass filter with a cut-off frequency of 450 Hz, and a second order Butterworth high-pass filter with a 10 Hz cut-off frequency [4].The data collection was done using the USB 6009 acquisition system by National Instrument,at a sampling rate of 1000 Hz with a 14-bit resolution [17].
2.1.3|Acc underlying principle
The accelerometer operating principle works with the acquisition of an acceleration input due to an external mechanical excitation.A schematic representation of this is a mass spring damper system immersed in a case, and upon receipt of a mechanical excitation stimuli, the mass (also referred to as proof mass) receives a displacement which is converted to produce an equivalent acceleration [18].In the case of a received linear acceleration, a deflection is produced on the proof mass whose magnitude can be determined by the product of the force by the acceleration on the proof mass.This acquired deflection,along with the effect of a damping factor,is in turn converted into a respective electrical signal[18].
2.1.4|Acc sensors and signal acquisition
Three supporting Acc channels were included in the signal acquisition process; the ADXL335 Acc was used by Sharba et al.[4].The ADXL335 is a low powered 3-axis sensor with a scale range of ±3g, bandwidth in the range of 0.5-1600 Hz and power supply requirements of 1.8-3.6 V.Similar to the EMG sensor, the data was collected using the USB 6009 acquisition system and streamed at a rate of 1000 Hz per second [4, 19].
2.2|Participant information and data collection process
The data was collected by Sharba et al.[4]in a previous study.The subject cohort comprised four shoulder disarticulate amputees whose demographic information can be seen in Table 1,and six non-amputated participants[4].All amputated subjects were in the age range of 16-65 years of age [4].Subjects were briefed about and they agreed to participate in the experiments,which were conducted according to the declaration of Helsinki and its later amendments [20].
A set of wet electrodes was used for the data collection and was spread on the anatomical muscles around the dorsal and shoulder using the defined guide by Criswell and Cram [21].The anatomical locations are summarised in Table 2 and shown in Figure 1[4,21].The various muscle groups involved include the trapezius upper fibre, which is used for scapula rotation and elevation, rhomboid major for the sliding of the scapula, trapezius lower fibre for scapula depression, serratus anterior for the movement of the scapula along the thoracic wall, and pectoralis minor, which is used for depression andabduction of the scapula.The exercise commenced after the description of the exercise was given to the participants and the signing of the consent forms, and it consisted of performing seven distinct shoulder girdle motions when prompted,namely Elevation, Depression, Protraction, Retraction, Upward Rotation, Downward Rotation, and Rest (no movement).Each girdle motion was repeated eight times and was held or performed for about 5 sec, then shortly followed by a 5 sec segment of inactivity.

F I G U R E 1 Data collection process showing electrode locations on an amputee participant

T A B L E 1 Amputee demographic information

T A B L E 2 Anatomical locations of the sensors
A visualisation plot contrasting both the EMG and Acc signals for an amputee and non-amputee can be seen in Figure 2.From this, it can be seen that the EMG appears to acquire more information per motion due to its operating principle being based around the acquisition of bio-electrical signals associated with muscular contraction.However, this phenomenon has made it susceptible to electrical signals from the beating of the heart occurring at a relatively high frequency and low amplitude, as can be prevalently seen in the EMG signal of the amputee subject around the 1.4 mv amplitude.As the heartbeat interference signal was seen to be intermittent and of a much smaller amplitude in comparison to the muscular contraction signal information of the EMG sensor,itwas presumed that this would have a negligible effect on the overall signal-to-noise ratio, thus, further conditioning and filtration of the signal was not carried out.The Acc signal appears to be more stationary in time and also possesses a much smaller dynamic range in comparison with the EMG.This is largely due to the underlying principle of Accs, which acquires low frequency vibrations whose source is thought to emanate from dynamic motions and muscular contractions and has thus made its acquired waveform to be less susceptible to interference from the electrical waves from the heartbeat.

F I G U R E 2 Acquired wave forms from both electromyography (EMG) and accelerometer (Acc) for amputee and non-amputee participants
2.3|Signal processing with pattern recognition
2.3.1|Windowing
The windowing method used to separate and chunk the data involved the application of a set of separate disjointed windows of 150, 350 and 500 ms, as per the recommendation from previous literature [26].An additional window of 5000 ms,correlating to the 5 sec contraction motion time in which the participants performed the various motions, was also applied to the data, to yield a total of four windowing configurations observed within this study.
2.3.2|EMG features
The EMG features used in this study are adopted from a previous study where transhumeral phantom motions were being tracked;the features can be grouped as follows, and the mathematical framework for each feature can be seen in Nsugbe et al.[3], and Englehart and Hudgins [27].
Contraction intensity and power features
- Mean absolute value(MAV):It provides a quantification of the mean of the electrical bio-potential signal associated with muscular contraction [3, 27].
- Waveform length(WL):It provides a cumulative sum of the frequency information in a signal for a fixed time duration [3, 27].
- Zero crossing (ZC): It is viewed as a feature that is relatively robust to noise and characterises the dynamic variation of the signal by the number of times it crosses a pre-defined user threshold.Similar to previous work,the selected threshold for calculating the ZC was chosen as 1 µv [3, 27].
- Root mean square (RMS): It is a feature which helps to reflect the amount of power in an acquired signal[3, 27].
Frequency feature
- Cepstrum: The maximum cepstrum coefficient was extracted from the cepstrum as a feature, where the cepstrum is a spectral representation of a time-series deconvolved into the sum of its spectrum[3,27].
Time-series prediction and non-linear complexity features
- Autoregression (AR) coefficients: The AR model linearly combines and regresses previous sample points andsets of stochastic difference equations for a time-series.This feature is useful in characterising the pattern of a muscular contraction since the coefficient of the AR models has been seen to vary with various muscular contraction states[3,28].The fourth AR model was the model order used here since it was previously shown to be optimal for this area of study, as per the Akaike information criterion [3, 28].
- Sample entropy (SampEN): It is a varied format of the approximate entropy,and is used to assess the degree of complexity and regularity, particularly from physiological time-series [3, 28].The parameters used for the calculation for SampEn were adopted from prior studies where the subseries lengthmwas selected as 2 and the tolerance valueras 0.2 [3, 28].
The fractal features were extracted and the associated multistep procedure taken to compute the three fractal features used in this study can be seen in Nsugbe et al.[28]while a description of each of the features can be seen below.
- Maximum fractal length(MFL):It is a feature that provides insight on faint muscular contractions and allows for an enhanced characterisation of muscular contraction from amputees who typically have residual anatomical tissue around the stump[28].
- Higuchi fractal dimension(HFD):It is a feature of low order computational complexity that is used to calculate the fractal dimension, which gives an indication of the self-similarity within a signal [28].
- Detrended fluctuation analysis (DFA): It is a robust fractal feature that applies power law correlations to assess the level of repeatability in stochastic time-series signals.It is preferred over spectral methods due to noise and artefact rejection capabilities[28].
2.3.3|Acc features
Similar to the EMG, the MAV, RMS, AR, Ceps, MFL, HFD and DFA were extracted from the Acc signal, as the Acc records a vibration signal that is stationary in time.The WL,ZC and SampEN were replaced with the commonly used vibration features such as the third and fourth moments, skewness,kurtosis and the crest factor [29-31].
Given a histogram-based projection of the variability of a time-series signal spanningY1,Y2…..YN, the moment-based features can be defined as follows:
- Skewness (Skew): This feature provides the degree of symmetry of a distribution and can be mathematically expressed as seen in Equation (5) [29, 31]:

whereYis the mean of the distribution,sis the standard deviation andNrepresents the number of data points.
- Kurtosis (Kurt): It provides a quantification of the degree of skewness of the distribution in terms of the extent of the spread of the tail of the distribution and can be mathematically expressed as seen in Equation (6) [29, 31]:

- Crest factor: It is a feature which reflects the nature of the dynamics of a signal by taking into account the high amplitude peak in the signal, which ordinarily would not cause a notable change in the RMS level.For this reason, it is sometimes referred to as peak-to-RMS-ratio, and can be mathematically be expressed as seen in Equation (7) [31]:

2.4|Motion classification methods
Three different classification methods were used for this study in order to investigate the accuracy and selection time of different variants of classifiers, as described in the following subsections.
2.4.1|Discriminant analysis
Discriminant analysis is a computationally efficient classification method commonly used for gesture motion recognition.Its operating framework is centred around the projection of a multidimensional data structure into a lower dimension where class boundaries are applied,whilst the overall structure of the data is preserved[32].The LDA was used in this study,and its classification framework assumes that the data is Gaussian.An equation of the discriminant function for the LDA can be seen in Equation(8):

wheredenotes the LDA discriminant function for a feature vector given asx,is the mean vector of the training samples for a classc, Σlis the pooled covariance matrix,Cis the total number of motion classes,Nis the total number of training samples for a given classc,while Σcis the covariance matrix of a classc.
2.4.2|Spectral regression discriminant analysis
The algorithmic framework of LDA involves the eigendecomposition of dense matrices of training data for optimal projection, which is computationally taxing, and even more so for the case of high dimensional data.As a way around this, the SRDA applies spectral graph theory as a way of bypassing the eigenvector calculation, and calculates an iterative regularised least square exercise instead,thereby saving on computation time and overall memory[33, 34].
As a preamble step for the SRDA algorithm,the following is computed as a substitute for the mean centring of a dense matrix to save on computational complexity:

Absorbing the scalaraTµinto the termaby an appending process, we have Equation (11), witha'and xi'being (n+ 1)dimensional vectors, which ensures that computational complexity is minimised from not centring the data matrix[33, 34].µis the sample mean vector and xIis theith data point for a specific class.

For a set of data pointsx1,…,∈Rnforcclasses, formkwhich isthenumber ofsamplesinakthclass=m,thefull algorithm for the SRDA can be broken down as follows[33,34]:- Generation of responses:

Withy0=[1,1,…,1]T,y0is taken as the first vector alongside a Gram-Schmidt process to produce an orthogonal projection ofyk, asy0belongs in the subspace spanned byyk,and thus the vectorsc- 1 are obtained as shown in Equation (13) [33, 34].
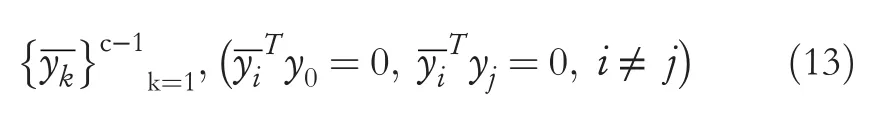
- Least square fitting with regularisation:compute the vectorsfor Equation (14), whereakis the final solution of the regularised least square equation [33,34].

where the regularisation term α controls the amount of shrinkage.
- Coordinate conversion and embedding in ac- 1 dimensional subspace: thec- 1 vectors {ak} obtained from the computation of the prior step in Equation (14) are the basis representation vectors of the SRDA.ForA=[a1,….., ac-1], it represents a transformation matrix of (n+1)×(c-1), and the subspace embedding process to ac-1 subspace is achieved through Equation (15)[33, 34].
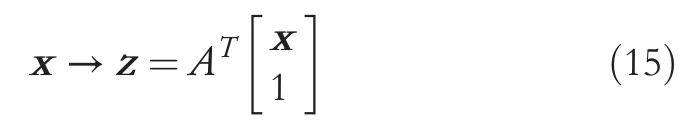
2.4.3|Support vector machine
The SVM is an iterative classifier whose performance objective is aimed around converging at an optimal separation boundary,typically regarded as a hyperplane,with the aid of a segment of the data referred to as the support vectors [35-38].
A distinct characteristic and strength of the SVM technique is based around the‘kernel trick’wherein the data is projected to a higher dimensional feature space where the hyperplane margins are set,following which the structure is preserved and projected to a lower dimensional space using a user defined kernel [35-38].The SVM's optimisation function, assuming a binary-based classification with a linear boundary, can be seen in Equation (16):

wherewis the weight vector,Φ(x) is the kernel map andbis an offset term [35-38].Presuming a feature vector that spansx, i=1,…, N,the optimisation objective can structured as seen in Equation (17) [35-38]:
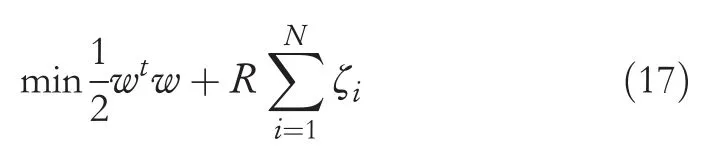
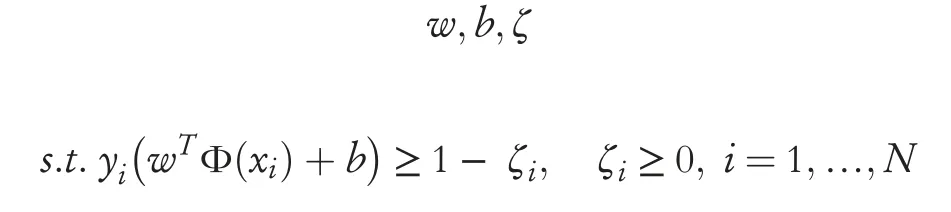
whereζis termed a slack variable to impose unique solutions to overlapping classes,Ris the regularisation term with the role to impose penalty and minimise the chances of an overfit,andyis an indication vector [35-38].The implementation of the SVM involved the use of a quadratic kernel function which would see non-linear decision boundaries,but is a low order of polynomial in order to not overfit itself round the class boundaries, with a one-vs-one multiclass method [35-38].
The LDA and SVM classification was carried out with the MATLAB classification toolbox, which automatically tunes and optimises the classifier parameters given the initial options(i.e.kernel) and data feature vector, while the SRDA classification was carried out using the open source MATLAB code for the SRDA [39].
As detailed above, a total of 10 features were extracted from the EMG-only signal per channel and Acc-only signal,while a fusion of EMG-Acc amounted to a concatenation of 20 features extracted from all channels from both the EMG and Acc sensors.The fusion was done by merging information from the feature vectors of both the EMG and Acc sensors,where a fused feature vector from both the EMG and Acc represents information from combined eight signal channels per person.In this study,in addition to a comparison between different sensor configurations,a contrast would also be made with different windowing parameters.
For all classifiers, the hold-out method was used as the validation method of the classifier which provided the CA.It is expressed as a percentage, and involves the validation of the recognition power of the classifier with an unseen sample[40].Unlike its cross-validation counterpart,it is a favoured method when a large dataset is available and is computationally effective[40].For the hold-out validation method,the data was split as 80% for model training and the remaining 20% for the model validation.
3|RESULTS AND DISCUSSION
The plots in Figures 3-6 show the results of the CA for the 150, 350, 500 and 5000 ms, for all 10 participants across all four classifiers.From the figures, it can be seen that, on average,the EMG-only sensor configuration provided a higher CA when compared with the Acc-only for all the participants across the various window schemes considered.This suggests that the electrophysiological properties are the key in conveying information regarding shoulder motion when compared with low frequency vibration signals from dynamic motions.Furthermore, five channels were used for the acquisition of the EMG signals whilst only three were used for the Acc, thereby making it challenging to carry out a rigorous investigation and draw a conclusion on this notion.The fusionof the EMG-Acc appeared to produce a considerable increase in the CA of the various classifiers for the 150-500 ms windowing scheme, but less so for the case of the 5000 ms window.The average EMG-only CA was seen to improve only by about 10% when the sensor information fusion occurred,suggesting that a larger time window reduces the uncertainty inthe data and thus allows for greater CAs by solely using an EMG sensor [3].However, the literature suggests that it is unfeasible to operate with a window scheme on the scale of 5000 ms due to computational constraints, thus a windowing scheme in the range of 150m-500 ms would be optimal for real-time purposes [26].From the viewpoint of windowing optimality, a notable increase in CA is observed when the window size is increased from the 150-350 ms, but the increment begins to flatten out and causes only a marginal difference in the CA between the 350 and 500 ms window.This suggests that,for the methods used in the data acquisition and signal processing, the 350 ms appears to be the optimal choice from the viewpoint of real-time operability.A comparison plot showing the different classifier performances for all participants in different window configuration segments can be seen in Figure 7.

F I G U R E 3 Classification results for all participants for a 150 ms window with nine classifier/electromyography-accelerometer(EMG-Acc)combinations.LDA, linear discriminant analysis; SRDA, spectral regression discriminant analysis; SVM, support vector machine

F I G U R E 4 Classification results for all participants for a 350 ms window with nine classifier/electromyography-accelerometer(EMG-Acc)combinations.LDA, linear discriminant analysis; SRDA, spectral regression discriminant analysis; SVM, support vector machine

F I G U R E 5 Classification results for all participants for a 500 ms window with nine classifier/electromyography-accelerometer(EMG-Acc)combinations.LDA, linear discriminant analysis; SRDA, spectral regression discriminant analysis; SVM, support vector machine
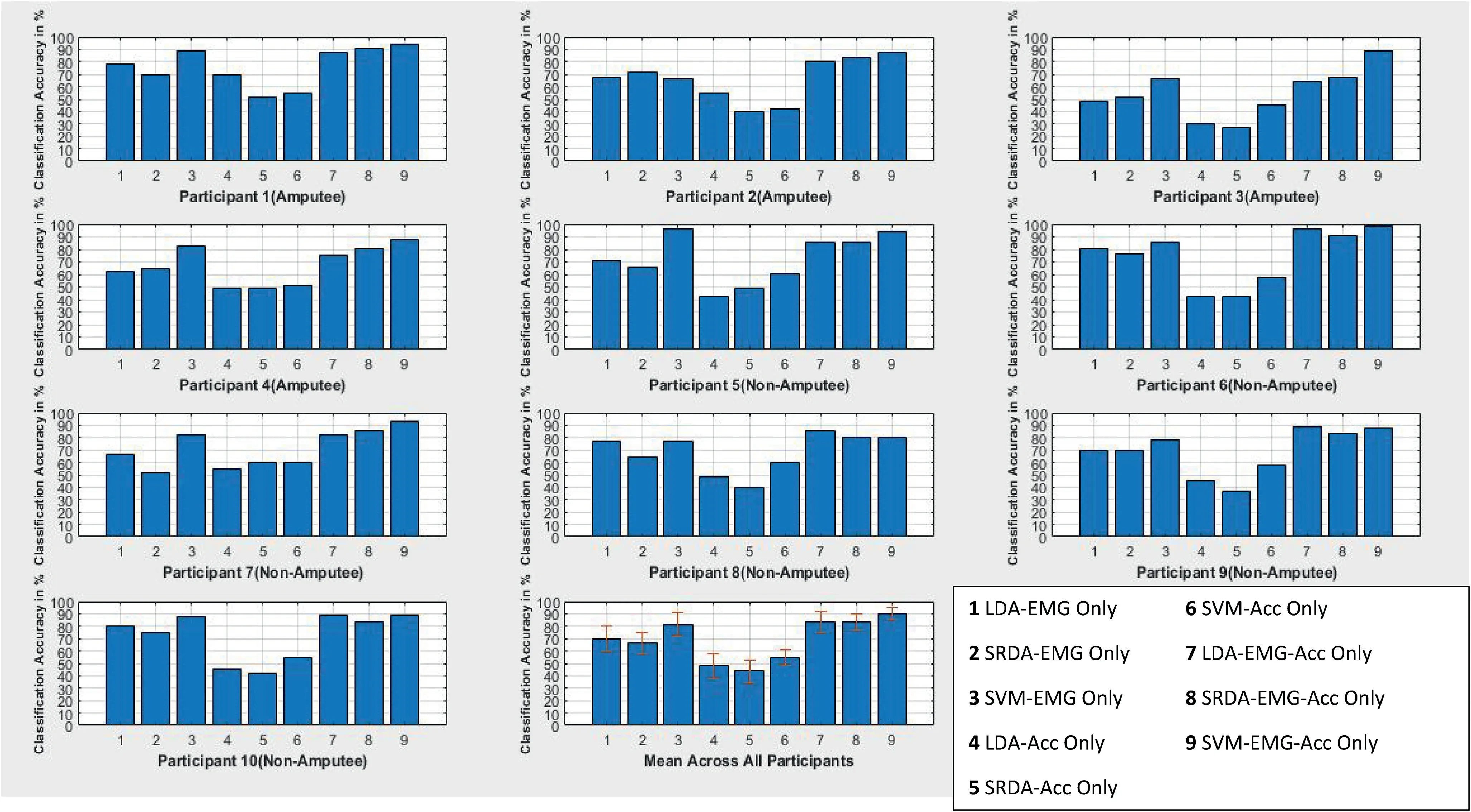
F I G U R E 6 Classification results for all participants for a 5000 ms window with nine classifier/electromyography-accelerometer(EMG-Acc)combinations.LDA, linear discriminant analysis; SRDA, spectral regression discriminant analysis; SVM, support vector machine
In terms of classifier performance, and in line with observations in previous related studies, the SVM outperformed both the LDA and SRDA, largely due to being an iterative classifier with a greater computational complexity.Table 3 summarises the selection time across all three classifiers,where the selection time can be defined as the duration it takes to map an input feature to a respective shoulder motion intent label.The LDA and SRDA can be said to have produced equivalent classification performances due to the similarities in their architectures.The likely reason why the LDA appears to marginally outperform the SRDA is due to the regularised least squared approach taken by the SRDA, which avoids the need to matrix the eigen-decomposition calculation, but at the cost of discarding information from the feature vector during the regularised least square fitting, which in the long run, slightly hinders its classification prowess.
Looking at the computational metrics in Table 3, as expected for an iterative classifier,the SVM produced the highest selection time, which provides quantifiable evidence that its classification power comes at the price of a greater computational demand.This was followed by the LDA at an order of magnitude which is less, while the SRDA recorded the lowest selection time at an order of magnitude less than that of the LDA.From this,it could be said that the best combination of a windowing scheme and classifier would likely be for the 350 ms and SRDA with a fusion of the EMG and Acc sensor information.
Figure 8 shows a set of comparison plots for the results from the amputee and non-amputee participants.The results appear to be largely similar and within the range of each other for both cohorts up until the 5000 ms window,where it begins to show that there is more variation and uncertainty associated with the amputee motions when compared with that of the non-amputees.From the literature where phantom motion of an amputated limb was tracked,it was noted that there existed a notable degradation in classification in comparison with amputated subjects[41].In this study,it can be seen that this is not the case,as the motions being recorded in this scenario are motions which can be readily produced with the intact anatomy despite the amputation.As shown in Figure 8,the results reveal that the CAs are similar to those of the healthy subjects,albeit with more uncertainty.This could perhaps present an alternative to bypassing and overcoming the limitations related to the residual tissue in the stump in other kinds of amputeessuch as transhumeral and transradial.The source of the uncertainty, which is presumed to be the cause of the reduction and variability of the CA of the amputee subjects when compared to the non-amputated subjects, is thought to stem from the issues related to balance and overall postural stability resulting from the loss of a substantial amount of the upper limb as supposed effects of phantom sensations [42].

T A B L E 3 The mean selection time in ms and classification accuracy for the three different classifiers for a 500 ms window across all participants
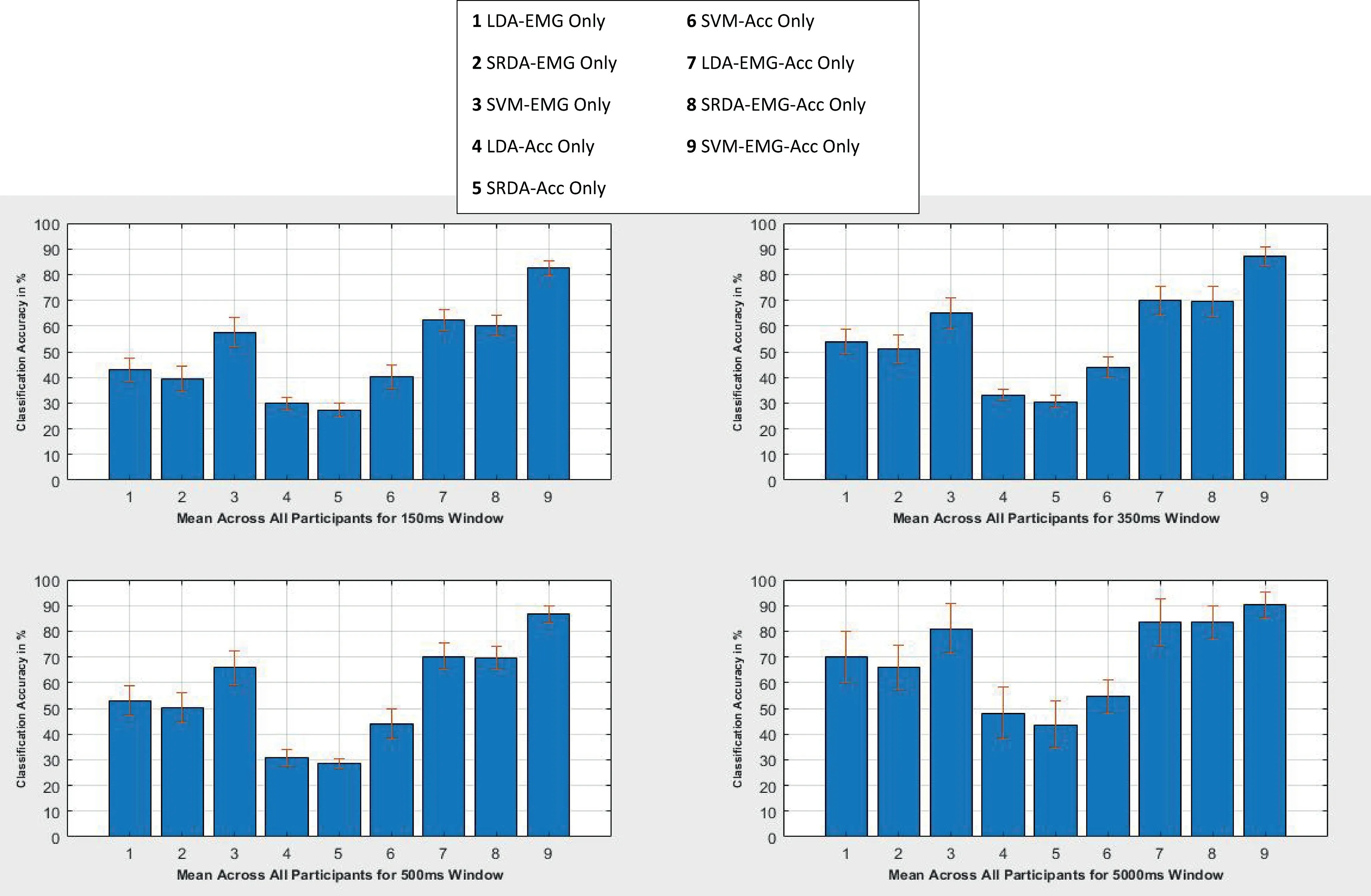
F I G U R E 7 Mean classification accuracy for all participants for various windows.Acc, accelerometer; EMG, electromyography; LDA, linear discriminant analysis; SRDA, spectral regression discriminant analysis; SVM, support vector machine
Figures 9 and 10 show the confusion plots for an amputated subject and a non-amputee for a 350 ms window classified with the SVM.It can be seen from this that aside from the No Movement (Class 7), the Shoulder Depression (Class 2), Downward Rotation (Class 6) and Retraction (Class 4)produced the higher CAs for the case of the first amputee and was generally consistent for other amputee subjects.In the case of the non-amputated subject, a slightly higher CA was obtained,and the best CAs(aside from the No Movement)were obtained for the Protraction (Class 3), Downward Rotation(Class 6) and Upward Rotation (Class 5).
From the results discussed, there is evidence suggesting that using the proposed methods alongside the given conditions, various shoulder-based motions can be recognised by a trained classifier.This section takes a wider view as to how the proposed methods can be combined with medical therapeutic interventions to facilitate a remastered means of actuation and control of a functional prosthesis limb.
The prospect of actuating and initiating control of an upper limb using shoulder muscles is thought to lack a natural level of rhythm and intuitiveness in shoulder disarticulate amputees, and thus would likely be cognitively taxing in the onset, and could ultimately result in the abandonment of the functional prosthesis limb as reported in the literature[12,43].The inclusion of NMR therapy could help adapt the motor control circuitry in the brain to learn new limb control patterns.For example, shoulder disarticulate amputees could be trained on the reprogrammed actuation of an essential upperlimb function (Hand open, Hand close) using either of thedetectable shoulder motions which have formed part of this study[12].In line with the known benefits of NMR,this would create advantages such as greater intuitive control of the functional prosthesis limb and an essential reduction in the cognitive aspects associated with the control of the limb [12].

F I G U R E 8 Mean classification results for all amputees and non-amputees for various window sizes.Acc, accelerometer; EMG, electromyography; LDA,linear discriminant analysis; SRDA, spectral regression discriminant analysis; SVM, support vector machine
The hypothesis of the combination of shoulder motions and NMR therapy for a reprogrammed control of a functional prosthesis limb is also applicable to other cohorts of amputees(transhumeral, transradial etc.) who suffer a reduction in classifier motion recognition accuracy due to the physiologically induced problems associated with tracking phantom motions[4, 12, 41].This could be seen as a means of bypassing the stump and acquisition of prosthesis actuation signals from non-residual anatomies around the shoulder whose accuracies have been seen to tally up closely with those of non-amputated subjects,as shown in this study.With this approach,the highly subjective case of being able to track phantom motion does not become a big factor in the prosthesis control process.However, the perceived shortcoming of this is thought to be around the isolation of what would ordinarily have been shoulder-specific motions to prosthesis actuation gestures once the functional prosthesis limb is worn.
It should be noted that a direct comparison with the work of Sharba et al.[4]has not been conducted as part of this study due to the difference in methods and pre-processing techniques, but the results from this study carry a greater deal of practical appeal due to the selection of methods which have a feasibility of a real-time implementation.
4|CONCLUSION AND FUTURE WORK

F I G U R E 1 0 Example of the confusion matrix for a non-amputee with support vector machine sensing 350 ms window and support vector machine classifier.Movements are as follows:(1)Elevation,(2)Depression,(3) Protraction, (4) Retraction, (5) Upward Rotation, (6) Downward Rotation and (7) Rest (no movement)

F I G U R E 9 Example of the confusion matrix for an amputee with support vector machine sensing 350 ms window and support vector machine classifier.Movements are as follows:(1)Elevation,(2)Depression,(3) Protraction, (4) Retraction, (5) Upward Rotation, (6) Downward Rotation and (7) Rest (no movement)
In this study, shoulder motion recognition for a group of shoulder disarticulate amputees and non-amputee participants has been investigated with EMG-only, Acc-only and a fusion of EMG-Acc sensing.The EMG, which is based around the acquisition of electrical signals associated with muscular contractions, showed greater motion recognition capabilities compared to the Acc, which monitors mainly low frequency vibration signals from muscular contractions and dynamic motions.The fusion from a multi-information source comprising electrophysiology (EMG) and low frequency mechanical (Acc) signals produced a large increment in the CA,and provided evidence to suggest that the two sets of sensors provide complimentary information on shoulder motions,which can lead to an enhanced CA when fused together.
An enhanced feature vector was constructed from the various sensing modules and the classification exercise was conducted, and also involved a contrast amongst three different classifiers.The classifiers were the computationallyefficient LDA, a parsimonious and optimal representation of the LDA known as the SRDA, and the SVM, which is an iterative classifier with non-linear class separation boundaries for various windowing schemes.The results showed that the SVM outperformed the other classifier variants for all the windowing and sensor configurations, but at the cost of computational complexity due to its architecture.
A comparison of the CA results from the amputee and the non-amputee participants showed a small difference between the two sets of participants,providing evidence to suggest that,unlike other aspects of amputations investigated in previous works, as the sensors are placed on healthy anatomical tissue around the shoulders and not on a stump, phantom sensation does not appear to be a driving factor in regard to the degree of CA attainable by the classifier.The immediate results combined with the NMR therapy could be explored further and applied to help promote the intuitiveness and reduce the cognitive burden associated with a possible control and actuation of a functional prosthesis limb using various shoulder input motions.The downside of this includes the primary use of wet electrodes which have ergonomic limitations and require replacing.Although embroidery electrodes are being evaluated for use in this area of research, affordable and effective wearable sensors are currently infeasible due, to a degree, to the constriction of shoulder motions primarily for actuation of the functional prosthesis limb [44].
Future work in this area would now involve a feature optimisation and comparison exercise to find out what are the key crucial features that drive the classifier accuracies, and how this can be linked back to intricate physiological principles, along with further feasibility studies around the prospect of using shoulder-based motions as input actuation for functional prosthesis control, in contrast with phantom motion for transradial and transhumeral amputees, and also potential applications of advanced machine learning models[45-47].
ACKNOWLEDGEMENTS
The authors would like to thank Dr.Carol Phillips for originally pitching the idea of how neuromuscular programming could be applicable in this area of research.They would like to thank Gaith Sharba for providing the dataset used in this study,and Brian Kerr from Kerr Editing for proofreading the manuscript.
CONFLICT OF INTEREST
The authors report no conflict of interest.
PATIENT CONSENT STATEMENT
NA.
DATA AVAILABILITY STATEMENT
Data could be available upon reasonable request.
ORCID
Ejay Nsugbehttps://orcid.org/0000-0003-0674-1611
杂志排行
CAAI Transactions on Intelligence Technology的其它文章
- Deep learning for time series forecasting: The electric load case
- Head-related transfer function-reserved time-frequency masking for robust binaural sound source localization
- A hierarchical optimisation framework for pigmented lesion diagnosis
- A spatial attentive and temporal dilated (SATD) GCN for skeleton-based action recognition
- Improving data hiding within colour images using hue component of HSV colour space
- Several rough set models in quotient space
