紫外灭活水中3种致病性曲霉的效能及其光复活控制
2022-03-29吴戈辉万琪琪徐向前曹瑞华黄廷林
吴戈辉,赵 辉,万琪琪,徐向前,曹瑞华,黄廷林,文 刚
紫外灭活水中3种致病性曲霉的效能及其光复活控制
吴戈辉,赵 辉,万琪琪,徐向前,曹瑞华,黄廷林,文 刚*
(西安建筑科技大学环境与市政工程学院,西北水资源与环境生态教育部重点实验室,陕西省环境工程重点实验室,西安 710055)
以饮用水中常见的3种曲霉(黑曲霉、黄曲霉、烟曲霉)为研究对象,紫外线作为消毒手段,研究其灭活效能与机制及紫外控制曲霉光复活的效果.结果表明,由于孢子尺寸、孢子色素、疏水性的不同,灭活结果存在差异,3种曲霉孢子对紫外的抗性为:黄曲霉>黑曲霉>烟曲霉.三者的灭活速率常数()符合Chick-Watson模型,黑曲霉,黄曲霉,烟曲霉的值分别为0.027,0.026,0.031cm2/mJ,大小顺序与灭活难度一致.紫外线通过穿透真菌孢子的细胞壁和细胞膜,阻断DNA复制和转录,最终造成细胞膜损伤以及胞内ROS水平的升高,灭活后的孢子呈现出明显凹陷并且表面表现出褶皱.3种曲霉孢子的光复活率和最大存活率:黑曲霉>黄曲霉>烟曲霉,其光复活差异是由于胞内的光解酶的数量和活性差异引起.
紫外;真菌孢子;曲霉;灭活机制;光复活
近年来,水环境的真菌爆发严重威胁了供水水质安全[1-2].研究表明,在全球各地的饮用水水源中分离出了众多的真菌属[3-7].部分真菌还可能在饮用水中造成肉眼可见颗粒物,从而利于微生物形成生物膜,产生嗅味,加速管道腐蚀等问题[8-9].
紫外线(UV)由于其在消毒过程中对各种病原体具有广谱性、形成消毒副产物(DBPs)少以及操作简易,普遍应用于水处理工艺中[10-11].紫外线(200~ 280nm)可以直接穿透病原微生物的细胞膜而直接对遗传物质DNA与RNA造成损伤,如当UV作用于DNA时,微生物细胞中的DNA链上会形成环丁烷嘧啶二聚体(CPDs)和6-4嘧啶二聚体(6- 4PPs)[12-13],从而阻止DNA的转录和复制,最终导致细胞死亡[14].
Nourmoradi等[15]研究了低压紫外(LPUV)灭活3种曲霉时发现,孢子浓度为1000CFU/mL的烟曲霉、黑曲霉及黄曲霉灭活率达到99.99%所需紫外剂量分别仅为12.45,16.6,20.75mJ/cm2,但没有对LPUV灭活3种曲霉的灭活机理及光复活控制进行研究.此外,与大肠杆菌等细菌相比,从地下水中分离的真菌孢子更耐紫外线照射,主要源于真菌孢子中存在细胞核,使紫外线照射更难到达和破坏DNA,从而导致真菌孢子具有高的抗紫外线性[16].Oliveira等[17]通过紫外发光二极管(UV-LED)灭活烟曲霉、黑曲霉和土曲霉,推断出更大的孢子会吸收更多的辐射,从而减少到达细胞核的辐射.在4种不同属(木霉、枝顶孢霉、青霉和枝孢霉)的真菌中,体积较大且有色的真菌孢子要比体积较小且透明的真菌孢子对紫外线照射的耐受性更强[18],但对曲霉属的灭活规律还没有探究.据报道,许多微生物具有2种主要的修复系统来修复紫外线(UV)消毒带来的损伤,包括光复活和暗修复[12].光复活这一现象的存在,使得紫外线消毒的最终效果下降.
本研究选择3种曲霉(黑曲霉、黄曲霉和烟曲霉)作为地下水中具有代表性的致病性曲霉物种,研究紫外对于3种曲霉的控制效能及光复活的控制,并采用流式细胞仪(FCM)评估灭活过程中细胞的损伤程度,探讨其灭活机制.
1 材料与方法
1.1 培养基的配制
曲霉的培养基采用孟加拉红培养基(DRBC),称取31.6g固体DRBC培养基于烧杯中,加入1L超纯水,搅拌均匀之后转入1L的锥形瓶中,封口.将配制好的DRBC培养基置于121 ℃高压蒸汽灭菌锅中30min,取出后摇晃均匀,倒入直径70mm的无菌培养皿中,待其凝固冷却之后使用[19].
1.2 曲霉的培养及孢子悬液的制备
曲霉的接种在超净工作台中进行,使用被酒精灯烧过的接种环将曲霉菌丝转移至新DRBC培养基上,置于28℃生化培养箱中,培养2~3周,待其生长至稳定期.
使用移液枪吸取5mL高温灭菌后的磷酸盐缓冲液(PBS)加入到稳定期的曲霉菌落表面,然后轻轻刮下曲霉孢子,将其倒入铺入3层无菌滤纸的漏斗中进行过滤.将得到的曲霉孢子过滤液,4℃, 8000r/ min离心10min,倒掉上清液,重复3次,最后得到适宜浓度的曲霉孢子悬浮液.通过光学显微镜(BX51, Olympus,日本)进行孢子计数,控制最终的孢子浓度为108,存于4℃冰箱中待用[20].
1.3 紫外灭活以及光复活实验
紫外灭活试验通过准平行光束仪来完成,采用低压汞灯(UVC 254nm),实验前开启紫外反应器预热30min至紫外灯稳定.紫外剂量通常通过公式(1)计算.
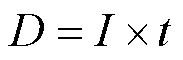
式中:为紫外线剂量,mJ/cm2;为平均紫外光强度,mW/cm2;为紫外辐照时间,s.其中紫外光强度()采用KI-KIO3光量子法测定[21].本研究中紫外光强度测定为0.120mW/cm2.
灭活实验在直径为9cm的玻璃圆盘中进行,将1mL的孢子储备液(108CFU/mL)加入99mL PBS中,摇晃均匀之后,取1mL的样品作为空白对照样品,放入紫外反应器中.在不同的时间间隔取样,经稀释之后,将0.1mL样品均匀涂布在DRBC培养基上,放在28℃的培养箱中培养2~3d后对曲霉进行计数,单位为CFU/mL.灭活实验均在室温、pH=8.0下进行.
光复活实验在灭活实验后随即进行[16],装置中有2根荧光灯(UVA365, 8W, 15W),荧光灯的辐照强度由紫外辐照计(UVA365,欣宝科仪)测得.UVA的强度为0.10mW/cm2.实验前,开启装置预热15min使荧光灯达到稳定.
UV灭活曲霉孢子2-log之后,放入光复活反应器中,开始光复活实验,分别在5, 25, 45, 60, 120, 180, 240, 360, 480min时取样.其他操作步骤同上.
1.4 流式细胞仪分析
采用流式细胞仪(Accuri C6,BD,USA)检测消毒过程中曲霉孢子DNA含量变化、膜完整性、酯酶活性以及胞内ROS的变化[22].使用的流式细胞仪检测范围为103~105CFU/mL,如有必要,将样品悬浮液用超纯水稀释.每一样品进行2次测定,取其平均值.
1.5 灭活效果评价
1.5.1 灭活动力学

式中:0和N分别是在失活时间0和时间处曲霉孢子的浓度,CFU/100mL.
灭活动力学通过Chick-Watson模型进行拟合[23],表达了灭活效率与暴露时间之间的线性关系.

式中:斜率为准一阶灭活速率常数,cm2/mJ;为紫外线通量率,mW/cm2;为灭活时间,s.
1.5.2 光复活动力学

式中:0是紫外线灭活前的孢子浓度,CFU/100mL;r是光复活一定时间后的孢子浓度,CFU/100mL.采用饱和型一阶反应模型[24]预测光复活过程:

式中:1为一阶光复活速率常数;0为紫外线失活后的存活率;为光复活时间时的存活率;m是光活化过程中的最大存活率.使用相关系数(2)评估拟合优度.
灭活结果的显著性分析通过SPSS软件进行.
2 结果与讨论
2.1 紫外灭活效果及动力学
由图1可知,随着紫外剂量增加,3种曲霉孢子的灭活数也随之增加,表明单独紫外可以有效灭活3种致病性曲霉孢子.3种曲霉的紫外抗性:黄曲霉>黑曲霉>烟曲霉,黄曲霉和黑曲霉的紫外抗性相近.通过Chick-Watson动力学模型拟合紫外灭活黑曲霉、黄曲霉和烟曲霉过程中的灭活速率常数(),分别为0.027,0.026,0.031cm2/mJ,黄曲霉的灭活速率常数()最低,烟曲霉的灭活速率常数最高.
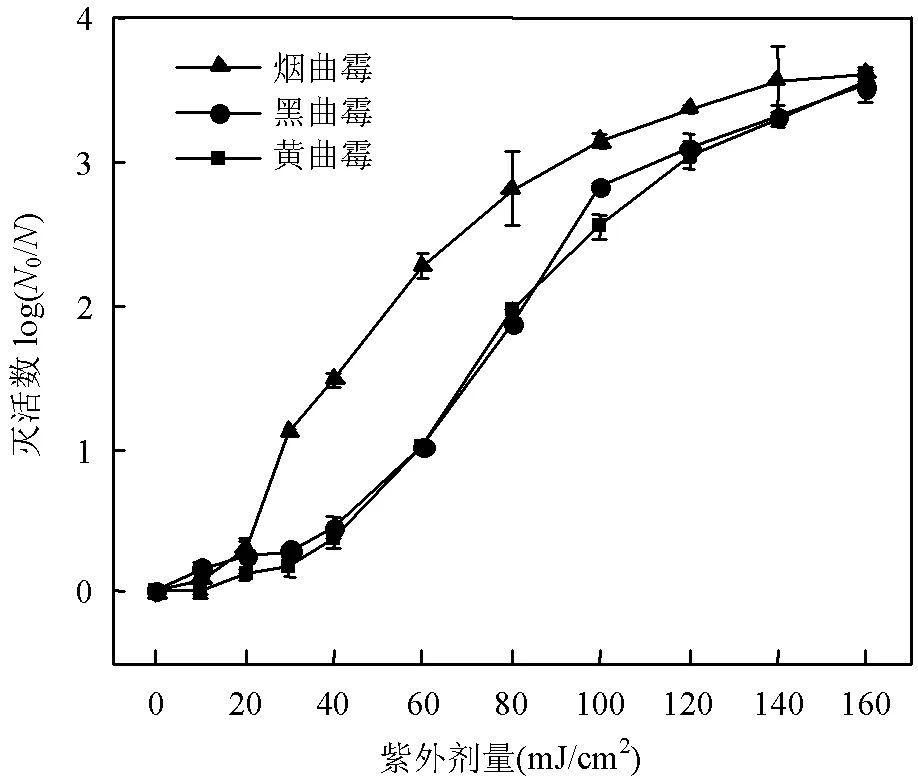
图1 紫外对3种曲霉孢子的灭活曲线
初始孢子浓度为: (1~3)×106CFU/mL, pH=8.0
紫外灭活3种曲霉存在差异性可能的原因如下(表1):第一,3种曲霉孢子的尺寸存在差异性,可能导致其对于紫外灭活表现出不同的抗性,具体表现为孢子尺寸越大其抗性越强[3,17].烟曲霉在3种曲霉孢子中的尺寸最小,因此对紫外的抗性也就最小;浓度为 (1~3) × 106CFU/mL的3种曲霉孢子在PBS中会表现出不同的颜色,分别为黑色、淡黄色、浅灰色.有研究表明,真菌孢子细胞壁中的色素具有抗氧化特性,可以保护真菌免受消毒剂的损伤[25].真菌孢子中的色素可能与紫外线发生反应,从而阻止紫外穿透孢子,影响最终的灭活结果.黄曲霉孢子中抗性色素相比于黑曲霉更多,对消毒剂的抗性也更强[26].经测定,黑曲霉、黄曲霉和烟曲霉孢子的疏水性分别为61.97%、77.00%和21.38%,而UV灭活曲霉的速率常数为烟曲霉(0.031cm2/mJ)>黑曲霉(0.027cm2/ mJ)>黄曲霉(0.026cm2/mJ),说明真菌灭活的难易程度与疏水性呈负相关,即孢子疏水性越大,灭活效果越差.这主要是由于疏水性在微生物表面粘附和相互聚集中起作用[27].不同的孢子具有不同的疏水特性[28-30],而疏水性越强的孢子之间具有更大的亲和力[31-32],越容易形成聚集.真菌孢子聚集体对消毒剂的抗性会随孢子聚集程度的增加而增加,与单分散孢子相比,聚集孢子的灭活常数会降低4~6倍[33].
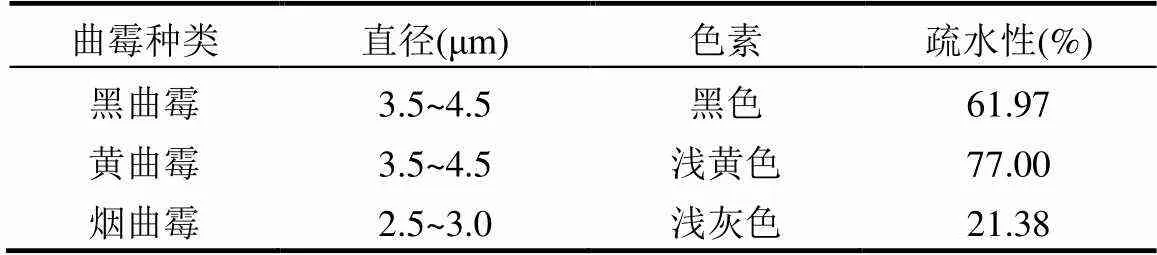
表1 3种曲霉的生理特性差异
饮用水消毒[15]规定紫外线剂量为40mJ/cm2, Lehtola等[34]研究表明,在40mJ/cm2的UV254照射下,饮用水中99%以上的细菌可以被灭活,而对木霉、顶孢霉、青霉和枝孢霉灭活率达到99%时的紫外剂量分别为45, 50, 65, 130mJ/cm2. Wen等[35]发现青霉、木霉、顶孢霉和枝孢霉的紫外灭活速率常数()分别为0.062, 0.070, 0.065, 0.019cm2/mJ,灭活的难易程度与真菌孢子的表面积和体积有关.对于较大的真菌孢子在UV照射到达细胞核之前,其细胞质对紫外线的吸收较高,导致了灭活效率较低[36].
2.2 光复活特征及动力学
如图2所示,3种曲霉孢子呈现出不同程度的光复活,前100min,黄曲霉表现出更高的光复活率,但随着时间延长,黑曲霉的光复活率高于黄曲霉和烟曲霉,这与3种曲霉的紫外抗性基本一致.曲霉的紫外抗性越弱,紫外线造成的损伤越严重,光复活率也就越低.

图2 3种曲霉孢子经2-log灭活后的光复活曲线
初始孢子浓度为: (1~3)×106CFU/mL, pH= 8.0,=25.0℃

初始孢子浓度为: (1~3)×106CFU/mL, 复活光UVA辐照强度为0.10mW/cm2,pH= 8.0,=25.0℃
由图3(a)可知,480min的光复活后,3种曲霉孢子中黑曲霉表现出了最高的光复活最大存活率.由图3(b)可知,黄曲霉光复活速率常数最小,烟曲霉与黑曲霉光复活速率常数相同.此外,曲霉孢子的光复活程度与胞内的光解酶相关,3种曲霉孢子的光复活结果的差异性可能是光解酶数量和活性不同所导致的[37].
2.3 紫外灭活三种曲霉的机理分析

图4 紫外灭活3种曲霉孢子过程中经SG染色后的二维点图
初始孢子浓度为: (1~3)×106CFU/mL, pH=8.0
2.3.1 紫外灭活对曲霉DNA含量的影响 由图4可以发现,随着紫外剂量的增加,高FL1荧光强度的孢子越来越小,越来越多的孢子呈现低荧光强度,根据SG与胞内双链 DNA 结合发出绿色荧光的原理可知,真菌孢子在紫外照射的过程中DNA受到损伤,故而呈现出比初始孢子更低的荧光值,表现为图5中3种曲霉孢子的DNA含量逐渐下降,即紫外剂量为140mJ/cm2时,黑曲霉,黄曲霉和烟曲霉的DNA含量分别下降40.67%,38.63%以及68.06%,这与3种曲霉孢子的抗性一致,说明紫外照射直接破坏了胞内DNA,导致孢子死亡.

图5 紫外灭活3种曲霉孢子过程中孢子DNA含量的变化
初始孢子浓度为: (1~3)×106CFU/mL, pH=8.0
2.3.2 紫外灭活对曲霉膜通透性的影响 由图6可知,随着紫外剂量从0mJ/cm2增加到140mJ/cm2,3种曲霉孢子的膜受损孢子数均随之增加.黑曲霉和黄曲霉对紫外抗性较高,因此膜受损孢子数未有明显升高;而烟曲霉的紫外抗性最弱,细胞膜损伤也最为严重,膜受损孢子数增加75.70%.图7反映了灭活过程中曲霉孢子的膜受损孢子数变化,3种曲霉的膜完整孢子数为:黄曲霉>黑曲霉>烟曲霉,这与曲霉孢子的紫外抗性一致.

图6 紫外灭活3种曲霉孢子过程中膜受损孢子变化
初始孢子浓度为: (1~3)×106CFU/mL, pH=8.0

图7 紫外灭活3种曲霉孢子过程中经SG/PI染色后的二维点图
初始孢子浓度为: (1~3)×106CFU/mL, pH=8.0
2.3.3 紫外灭活对曲霉胞内ROS的影响 曲霉的胞内ROS在代谢活动中形成,作为调节真菌发育过程和生理反应的信号分子,也可作为代谢指标[38].当曲霉孢子休眠时,胞内ROS水平维持在较低的水平,在曲霉孢子受到来自外界的刺激后,胞内ROS水平迅速升高,出现氧化应激反应以抵抗外界压力.曲霉孢子的胞内相应防御机制能力存在限制,当胞内ROS水平超出限值时,会引起曲霉孢子出现损伤[39-40].由图8和图9可知,随着紫外剂量从0mJ/cm2增加到140mJ/cm2,3种曲霉孢子的胞内高ROS水平也逐渐增加.其中,黑曲霉的胞内高ROS水平仅增加了5.30%,而黄曲霉和烟曲霉则分别增加了41.90%和24.25%.这表明UV照射对于黑曲霉的影响相对较小.UV照射刺激黄曲霉胞内高ROS水平升高,但胞内ROS氧化能力并未超过黄曲霉的胞内防御机制能力,因此黄曲霉表现出最强的紫外抗性.这是由于细胞内ROS水平的变化与真菌细胞内的基因和酶有关,如谷胱甘肽过氧化物酶(Gpx),最终导致紫外线照射后这些真菌孢子内ROS水平不同[39].

图8 紫外灭活3种曲霉孢子过程中胞内高ROS水平变化
初始孢子浓度为: (1~3)×106CFU/mL, pH=8.0

图9 紫外灭活3种曲霉孢子经DHE染色后的直方图
初始孢子浓度为: (1~3)×106CFU/mL, pH=8.0
2.3.4 紫外灭活前后曲霉形态的变化 由图10可知,对于黑曲霉,在160mJ/cm2剂量的紫外照射之后,其整体呈椭圆状,表面光滑,未有明显性状变化.对于黄曲霉,其整体呈椭圆状,且在灭活前表面即有一定程度褶皱,与黑曲霉光滑的表面形成鲜明对比,而在灭活结束之后其表面也并未发生明显的变化.而烟曲霉在灭活之前,呈椭圆状,表明光滑,未见褶皱.但是,在经过了160mJ/cm2剂量的紫外照射之后,其表面出现了明显凹陷并且表面表现出褶皱.相比于烟曲霉,黑曲霉和黄曲霉灭活前后的SEM图像并未发生明显变化,可能是由于黑曲霉和黄曲霉的孢子尺寸大于烟曲霉,导致紫外更难直接作用于DNA.

图10 紫外灭活3种曲霉孢子的SEM图像(30000倍)
初始孢子浓度为: (1~3)×106CFU/mL, pH=8.0, UV剂量=160mJ/cm2
2.4 能耗分析
E,N(kWh/m3)定义为UV灭活真菌孢子1-log时的能量消耗,如下式(6)所示:

式中:为UV辐照表面积,cm2;N为灭活1-log真菌孢子时所需的紫外线剂量,mJ/cm2;W与kW、s与h、mL与m3之间的换算系数为3.6×103;为样品体积,mL;为UV的转换效率;WF是水因子[41-43].
经计算可得,黑曲霉,黄曲霉和烟曲霉的E,N值分别为0.052,0.052,0.026kWh/m3.烟曲霉每灭活1-log真菌孢子所需的E,N最低,而黑曲霉和黄曲霉灭活1-log时所需的紫外剂量均为60mJ/cm2.但3种真菌孢子的E,N值均远高于大肠杆菌(0.006kWh/ m3)、铜绿假单胞菌(0.011kWh/m3)和嗜肺军团菌(0.006kWh/m3),且与HAdV2(0.057kWh/m3)和噬菌体Qb(0.049kWh/m3)等病毒相似[44-46].相比于细菌和病毒,真菌具有完整的细胞核和由几丁质组成的细胞壁,阻碍了紫外穿透细胞破坏遗传物质[47];真菌复杂的细胞结构可以使其在受到外界不良刺激时具有更强的应激防御机制;此外,病毒尺寸一般为30~200nm,细菌尺寸一般为0.4~2µm,而真菌尺寸一般为10~100µm,从而造成了真菌较细菌对紫外线的抗性更强.
3 结论
3.1 紫外灭活3种曲霉孢子的难度为:黄曲霉>黑曲霉>烟曲霉,三者的灭活速率常数大小顺序与灭活难度相同,其灭活的差异可能是由于孢子尺寸、孢子色素、疏水性而引起的.
3.2 紫外灭活2-log10之后的3种曲霉孢子的光复活率和最大存活率:黑曲霉>黄曲霉>烟曲霉,其光复活速率常数分别为0.006 ,0.004 ,0.006min-1.3种曲霉的光复活差异是由于其胞内的光解酶的数量和活性差异引起.
3.3 随着紫外剂量的增加,3种曲霉孢子的DNA含量逐渐下降,表明紫外会穿透真菌孢子的细胞壁和细胞膜,直接损坏3种曲霉孢子DNA,造成孢子死亡.
3.4 紫外灭活对3种曲霉孢子会造成不同程度的膜损伤,其中对烟曲霉的损伤最严重,膜受损孢子数占比增加75.80%,对黑曲霉和黄曲霉的膜损伤程度较低.3种曲霉的膜受损孢子数占比与对紫外的灭活抗性表现出一致性.
3.5 紫外灭活3种曲霉孢子会导致其胞内高ROS不同水平的升高,黑曲霉的胞内高ROS仅增加了5.30%,而黄曲霉和烟曲霉则分别增加了41.90%和24.25%.结合灭活结果,3种曲霉中,黄曲霉具有最强的胞内防御机制,烟曲霉的胞内防御机制最弱.
3.6 黑曲霉、黄曲霉和烟曲霉分别被紫外灭活,其灭活前后的SEM图像显示黑曲霉和黄曲霉的表面性状未出现明显变化,烟曲霉则出现了表面凹陷,表明黑曲霉和黄曲霉相对烟曲霉具有更强的灭活抗性.
3.7 相比于黑曲霉和黄曲霉,烟曲霉每灭活1-log真菌孢子所需的E,N最低,但3种真菌孢子的E,N值均远高于一些细菌,这是因为真菌孢子与细菌对紫外线的抗性不同所导致的.
[1] Pereira V J, Basílio M C, Fernandes D, et al. Occurrence of filamentous fungi and yeasts in three different drinking water sources [J]. Water Research, 2009,43(15):3813-3819.
[2] Wen G, Xu X, Zhu H, et al. Inactivation of four genera of dominant fungal spores in groundwater using UV and UV/PMS: Efficiency and mechanisms [J]. Chemical Engineering Journal, 2017,328:619-628.
[3] Oliveira B R, Crespo M T B, San Romão M V, et al. New insights concerning the occurrence of fungi in water sources and their potential pathogenicity [J]. Water Research, 2013,47(16):6338-6347.
[4] Rankovic B. Five Serbian reservoirs contain different fungal propagules [J]. Mycologia, 2005,97(1):50-56.
[5] 王 钰,刘明坤,苗小草,等.城市供水系统对水中真菌数量和群落结构的影响[J]. 微生物学通报, 2019,46(1):20-28.
Wang Y, Liu M K, Miao X C, et al. Effects of water supply system on the quantity and community structure of fungi in water [J]. Chinese Journal of Microbiology, 2019,46(1):20-28.
[6] 李群伟.真菌毒素与人畜健康的研究现状及展望[J]. 中国预防医学杂志, 2004,5(5):409-409.
Li Q W. Research status and prospect of mycotoxins and human and animal health [J]. Chinese Journal of Preventive Medicine, 2004,5(5): 409-409.
[7] Hageskal G, Gaustad P, Heier B T, et al. Occurrence of moulds in drinking water [J]. Journal of Applied Microbiology, 2007,102(3): 774-780.
[8] 韩 梅,曹新垲,王 敏,等.南水北调受水区某水厂炭砂滤池运行特性及生物安全性研究[J]. 给水排水, 2018,54(9):19-24.
Han M, Cao X K, Wang M, et al. Operation characteristics and biosafety of carbon sand filter in a water plant in the receiving area of south-to-North Water Transfer project [J]. Water and Wastewater Treatment, 2018,54(9):19-24.
[9] Kikuchi T, Kadota S, Suehara H, et al. Odorous metabolites of a fungus, Chaetomium globosum Kinze ex Fr. Identification of geosmin, a musty-smelling compound [J]. Chemical & Pharmaceutical Bulletin, 1981,29(6):1782-1784.
[10] Guo M, Hu H, Bolton J R, et al. Comparison of low-and medium- pressure ultraviolet lamps: Photoreactivation of Escherichia coli and total coliforms in secondary effluents of municipal wastewater treatment plants [J]. Water Research, 2009,43(3):815-821.
[11] 付树森,王 艺,王肖霖,等.氯和紫外消毒过程中胞外抗性基因的产生特征 [J]. 中国环境科学, 2021,41(10):4756-4762.
Fu S S, Wang Y, Wang X L, et al. Production characteristics of extracellular resistance genes during chlorine and ultraviolet disinfection [J]. China Environmental Science, 2021,41(10):4756- 4762.
[12] Friedberg E C. A history of the DNA repair and mutagenesis field: The discovery of base excision repair [J]. DNA Repair, 2016,37:35-39.
[13] Sanz E N, Dávila I S, Balao J A A, et al. Modelling of reactivation after UV disinfection: effect of UV-C dose on subsequent photoreactivation and dark repair [J]. Water Research, 2007,41(14): 3141-3151.
[14] Süß J, Volz S, Obst U, et al. Application of a molecular biology concept for the detection of DNA damage and repair during UV disinfection [J]. Water Research, 2009,43(15):3705-3716.
[15] Nourmoradi H, Nikaeen M, Stensvold C R, et al. Ultraviolet irradiation: An effective inactivation method of Aspergillus spp. in water for the control of waterborne nosocomial aspergillosis [J]. Water Research, 2012,46(18):5935-5940.
[16] Wen G, Deng X, Wan Q, et al. Photoreactivation of fungal spores in water following UV disinfection and their control using UV-based advanced oxidation processes [J]. Water Research, 2019,148:1-9.
[17] Oliveira B R, Crespo M T B, Pereira V J. Small but powerful: light-emitting diodes for inactivation of Aspergillus species in real water matrices [J]. Water Research, 2020,168:115108.
[18] Braga G U L, Rangel D E N, Fernandes É K K, et al. Molecular and physiological effects of environmental UV radiation on fungal conidia [J]. Current Genetics, 2015,61(3):405-425.
[19] 朱 红.紫外及紫外联合灭活地下水供水系统中真菌效能及机理研究 [D]. 西安:西安建筑科技大学, 2017.
Zhu H. Study on efficacy and mechanism of inactivation of fungi in groundwater water supply system by UV and combined UV [D]. Xi 'an: Xi 'an University of Architecture and Technology, 2017.
[20] Pereira V J, Marques R, Marques M, et al. Free chlorine inactivation of fungi in drinking water sources [J]. Water Research, 2013,47(2): 517-523.
[21] Rahn R O, Stefan M I, Bolton J R, et al. Quantum Yield of the Iodide–Iodate Chemical Actinometer: Dependence on Wavelength and Concentration [J]. Photochemistry and Photobiology, 2003,78(2):146- 152.
[22] Wan Q, Wen G, Cao R, et al. Simultaneously enhance the inactivation and inhibit the photoreactivation of fungal spores by the combination of UV-LEDs and chlorine: Kinetics and mechanisms [J]. Water Research, 2020,184:116143.
[23] Jung Y J, Oh B S, Kang J W. Synergistic effect of sequential or combined use of ozone and UV radiation for the disinfection of Bacillus subtilis spores [J]. Water Research, 2008,42(6-7):1613-1621.
[24] Kashimada K, Kamiko N, Yamamoto K, et al. Assessment of photoreactivation following ultraviolet light disinfection [J]. Water Science and Technology, 1996,33(10-11):261-269.
[25] Cordero R J B, Casadevall A. Functions of fungal melanin beyond virulence [J]. Fungal Biology Reviews, 2017,31(2):99-112.
[26] Zuo J, Xu X, Wan Q, et al. Inactivation of fungal spores in water with peracetic acid: Efficiency and mechanism [J]. Chemical Engineering Journal, 2022,427:131753.
[27] Mamane-Gravetz H, Linden K G. Relationship between physiochemical properties, aggregation and uv inactivation of isolated indigenous spores in water [J]. Journal of Applied Microbiology, 2005,98(2):351-363.
[28] Rosenberg M, Gutnick D, Rosenberg E. Adherence of bacteria to hydrocarbons: a simple method for measuring cell-surface hydrophobicity [J]. FEMS Microbiology Letters, 1980,9(1):29-33.
[29] Doyle R J, Nedjat-Haiem F, Singh J S. Hydrophobic characteristics of Bacillus spores [J]. Current Microbiology, 1984,10(6):329-332.
[30] 张晓敏,成卓韦,於建明,等.真/细菌对疏水性有机物的吸附及其表面特性 [J]. 中国环境科学, 2019,39(3):1268-1277.
Zhang X M, Cheng Z W, Yu J M, et al. Adsorption and surface characterization of hydrophobic organic compounds by fungus/ bacteria [J]. China Environmental Science, 2019,39(3):10.
[31] Rönner U, Husmark U, Henriksson A. Adhesion of Bacillus spores in relation to hydrophobicity [J]. Journal of Applied Bacteriology, 1990, 69(4):550-556.
[32] Faille C, Jullien C, Fontaine F, et al. Adhesion of Bacillus spores and Escherichia coli cells to inert surfaces: role of surface hydrophobicity [J]. Canadian Journal of Microbiology, 2002,48(8):728-738.
[33] Zhang H, Xu X, Tan L, et al. The aggregation of Aspergillus spores and the impact on their inactivation by chlorine-based disinfectants [J]. Water Research, 2021,204:117629.
[34] Lehtola M J, Miettinen I T, Vartiainen T, et al. Impact of UV disinfection on microbially available phosphorus, organic carbon, and microbial growth in drinking water [J]. Water Research, 2003,37(5): 1064-1070.
[35] Wen G, Xu X, Zhu H, et al. Inactivation of four genera of dominant fungal spores in groundwater using UV and UV/PMS: Efficiency and mechanisms [J]. Chemical Engineering Journal, 2017,328:619-628.
[36] Nascimento E, Da Silva S H, dos Reis Marques E, et al. Quantification of cyclobutane pyrimidine dimers induced by UVB radiation in conidia of the fungi Aspergillus fumigatus, Aspergillus nidulans, Metarhizium acridum and Metarhizium robertsii [J]. Photochemistry and Photobiology, 2010,86(6):1259-1266.
[37] Salcedo I, Andrade J A, Quiroga J M, et al. Photoreactivation and dark repair in UV-treated microorganisms: effect of temperature [J]. Applied and Environmental Microbiology, 2007,73(5):1594-1600.
[38] Mesquita N, Portugal A, Piñar G, et al. Flow cytometry as a tool to assess the effects of gamma radiation on the viability, growth and metabolic activity of fungal spores [J]. International Biodeterioration & Biodegradation, 2013,84:250-257.
[39] Gessler N N, Aver’yanov A A, Belozerskaya T A. Reactive oxygen species in regulation of fungal development [J]. Biochemistry (Moscow), 2007,72(10):1091-1109.
[40] Moreno A D, González-Fernández C, Ballesteros M, et al. Insoluble solids at high concentrations repress yeast’s response against stress and increase intracellular ROS levels [J]. Scientific Reports, 2019,9(1): 1-12.
[41] Rattanakul S, Oguma K. Inactivation kinetics and efficiencies of UV-LEDs against Pseudomonas aeruginosa, Legionella pneumophila, and surrogate microorganisms [J]. Water Research, 2018,130:31-37.
[42] Bolton J R, Linden K G. Standardization of methods for fluence (UV dose) determination in bench-scale UV experiments [J]. Journal of Environmental Engineering, 2003,129(3):209-215.
[43] Wan Q, Wen G, Cao R, et al. Comparison of UV-LEDs and LPUV on inactivation and subsequent reactivation of waterborne fungal spores [J]. Water Research, 2020,173:115553.
[44] Beck S E, Ryu H, Boczek L A, et al. Evaluating UV-C LED disinfection performance and investigating potential dual-wavelength synergy [J]. Water Research, 2017,109:207-216.
[45] Nyangaresi P O, Qin Y, Chen G, et al. Effects of single and combined UV-LEDs on inactivation and subsequent reactivation of E. coli in water disinfection [J]. Water Research, 2018,147:331-341.
[46] Rattanakul S, Oguma K. Inactivation kinetics and efficiencies of UV-LEDs against Pseudomonas aeruginosa, Legionella pneumophila, and surrogate microorganisms [J]. Water Research, 2018,130:31-37.
[47] Dai J, Qu H, Yu Z, et al. Computational analysis of AnmK-like kinase: New insights into the cell wall metabolism of fungi [J]. Journal of Theoretical Biology, 2015,379:59-65.
Inhibit the photoreactivation of three pathogenicspores in water by UV: kinetics and mechanism.
WU Ge-hui,ZHAO Hui, WAN Qi-qi, XU Xiang-qian, CAO Rui-hua, HUANG Ting-lin, WEN Gang*
(Key Laboratory of Northwest Water Resource, Environment and Ecology, Ministry of Education, Shaanxi Key Laboratory of Environmental Engineering, School of Environmental and Municipal Engineering, Xi’an University of Architecture and Technology, Xi’an 710055, China)., 2022,42(3):1173~1181
Three kinds ofspores (,and) commonly found in drinking water were used as research objects, and UV was used as the disinfection method to study their inactivation efficiency and mechanism. Control of photoreactivation for threespores inactivated by UV was also evaluated. The different inactivation degree was due to the different size, pigment and hydrophobicity. The resistance of the threespores inactivated by UV was:>>. In addition, the inactivation rate constantsof the threespores were consistent with the Chick-Watson model, in which theof,andwere 0.027, 0.026 and 0.031 cm2/mJ, respectively. The order of the inactivation rate constantof the threespores was the same as the difficulty of inactivation. UV penetrates cell wall and cell membrane, which blocks DNA replication and transcription, ultimately causing membrane damage and the increase of intracellular high ROS level. After inactivation, the spores showed obvious sinking and wrinkle on the surface. Photoreactivation rate constant and maximum survival ratio of the threespores:>>. The discrepancy of photoreactivation among the threespecies was caused by the difference of the number and activity of intracellular photolyase.
UV;fungal spores;;disinfection mechanism;photoreactivation
X523
A
1000-6923(2022)03-1173-09
吴戈辉(1998-),女,陕西西安人,西安建筑科技大学博士研究生,主要从事饮用水水质安全保障研究.
2021-08-10
国家自然科学基金(51978557);陕西省杰出青年科学基金(2018JC-026);陕西省重点研发计划(2020ZDLSF06-05,2019ZDLSF06- 03);陕西高校青年创新团队
*责任作者, 教授, hitwengang@163.com
