Expression and correlation of pyruvate kinase M2 and miRNA-122 in sepsis associated AKI
2022-03-16FUZhouLITingSONGXuLIRuxinWANGKailiWUYutaoHUNuoyaQUANKangliDUBangzhe
FU Zhou, LI Ting, SONG Xu, LI Ru-xin, WANG Kai-li, WU Yu-tao, HU Nuo-ya,QUAN Kang-li, DU Bang-zhe
1.Undergraduates of Changzhi Medical College, Changzhi 046000, China
2.Department of Physiology, Changzhi Medical College, Changzhi 046000, China
Keywords:
A BSTRACT Objective: To investigate the expression and correlation of pyruvate kinase M2 and miRNA-122 in sepsis associated acute kidney injury (S-AKI).Methods: A mouse model of S-AKI was induced by cecal ligation and perforation (CLP), and normal mice were used in the control group.Serum and renal tissue were collected from each group, respectively, and the levels of blood urea nitrogen (BUN) and creatinine (Cr) were detected by biochemical analyzer.The levels of lactate in serum and kidney tissue of mice in each group were detected by colorimetric method.Real-time quantitative PCR (RT-qPCR) was used to detect the expression of miRNA-122 and pkm2 mRNA in kidney tissue in each group.Western blotting was used to detect the expression of PKM2 protein in kidney tissue in each group.Pearson's method was used to analyze the pairwise correlation of miRNA-122, PKM2 and lactate levels.Results:(1) Compared with the control group, the levels of BUN and Cr increased significantly after 12 h and 24 h of CLP treatment (P <0.05).(2) Compared with the control group, after 12 h and 24 h of CLP treatment, the levels of lactate in the serum and kidney tissue of the mice were significantly higher than those in the control group (P<0.05).(3) Compared with the control group, the expression of miRNA-122 in renal tissue began to decrease at 4 h after CLP, and decreased significantly at 12-24 h (P<0.05).Compared with the control group, the expression of pKm2 mRNA and protein in renal tissue began to increase after 4 h of CLP, and increased significantly at 12-24 h (P<0.05).(4) Correlation analysis showed that miRNA-122 was significantly negatively correlated with lactate level (P<0.000 1, r=-0.716 7).There was a significant positive correlation between pkm2 mRNA and lactate level (P<0.000 1, r=0.681 7).There was a significant negative correlation between miRNA-122 and pkm2 mRNA expression(P<0.000 1, r=-0.812 2). Conclusion: In S-AKI, dysregulated expression of miRNA-122 may aggravate the occurrence and development of AKI by regulating the level of PKM2, and promoting aerobic glycolysis and lactate levels.
1.Introduction
Acute kidney injury (AKI) is a clinical syndrome with multiple organ and multi-system involvement caused by a variety of reasons leading to a sharp decline in renal function, of which sepsis is the most common cause of AKI[1].Studies have shown that compared with AKI caused by other causes, patients with sepsis-associated acute kidney injury (S-AKI) have a worse prognosis and higher mortality.Therefore, it will provide new ideas for the treatment of S-AKI that further elucidating the pathogenesis and finding new therapeutic targets of S-AKI.MicroRNAs (miRNAs) are a class of endogenous small molecule non-coding RNAs that specifically inhibit the translation of target mRNAs by binding to the 3'-untranslated region (3'-UTR), and it is involved in a variety of physiological and pathological processes in the body [2].The previous study of our group found that the expression of miRNA-122 was down-regulated in the kidney tissue of AKI mice induced by cecal ligation and perforation (CLP), and was closely related to the increased release of inflammatory factor IL-1β[3].However, the mechanism by which the dysregulated expression of miRNA-122 is involved in the inflammatory response remains unclear.In order to clarify the mechanism of miRNA-122, we searched for miRNA-122 target genes by miRNA prediction software, and found that pyruvate kinase M2 (PKM2) has a binding site for miRNA-122.PKM2 is an isoenzyme of pyruvate kinase (PK), an important rate-limiting enzyme in the glycolytic pathway, which catalyzes the formation of pyruvate from phosphoenolpyruvate[4].Studies have shown that PKM2 is a key gene regulating cellular energy conversion, which can convert the energy supply of cells from oxidative phosphorylation to aerobic glycolysis, thereby increasing lactate levels and aggravating inflammatory responses [4].At present, there is no report on the expression and correlation of PKM2 and miRNA-122 in S-AKI.In order to further explore the pathogenesis of miRNA-122 in S-AKI,we constructed an S-AKI model to detect the expression patterns of miRNA-122, PKM2 and lactate in the kidney tissue of S-AKI mice,and used correlation analysis to analyze them.
2.Materials and methods
2.1Materials
2.1.1Animals
8-week-old SPF grade C57BL/6 mice, weighing about 19-24 g,were purchased from Speifu (Beijing) Biotechnology Co., Ltd.,license number SCXK (Beijing) 2019-0010.Mice were housed in a clean environment with constant temperature and humidity, with free food and water, and maintained the normal time rhythm of mice with 12-hour day-night alternation.Animal experiments strictly follow the 3R principles.
2.1.2 Reagents
The lactate detection kit was purchased from Nanjing Jiancheng Bioengineering Co., Ltd.Trizol reagent was purchased from Invitrogen.miRNA reverse transcription kit, mRNA reverse transcription kit and fluorescence quantitative PCR kit were purchased from Takara Company.miRNA-122, U6, PKM2, β-actin primers were synthesized by Sangon Bioengineering (Shanghai)Co., Ltd.PKM2 antibody, β-actin antibody, and goat anti-rabbit secondary antibody were purchased from abclonal company.
2.2 Methods
2.2.1 Animal model
The S-AKI mouse model was established by the CLP method.The specific operation steps are briefly described as follows: 4% chloral hydrate (10 mL/kg) was injected intraperitoneally for anesthesia,then the abdominal cavity was opened layer by layer, the cecum was exposed, and the distal half of the cecum was ligated.A 9-gauge puncture needle was used to puncture the distal end of the ligation site once, and a little faeces were gently squeezed out.The cecum was then returned to the abdominal cavity, and the abdominal cavity was closed layer by layer.After the operation, 1 ml of normal saline was injected subcutaneously on the back of the mouse.
2.2.2 Animal grouping
The 30 mice were randomly divided into 5 groups (6 mice/group),which were the control group, the 4 h group, the 8 h group, the 12 h group and the 24 h group.The control group was normal mice without any treatment.The model group was given CLP surgery, and the serum and kidney tissue of each group were collected at 4, 8, 12 and 24 h after the operation.
2.2.3 Renal function test
After the whole blood was taken from the mice in each group,they were left at room temperature for 2 h, centrifuged at 1 000 g× 15 min, and the serum was collected.An automatic biochemical analyzer was used to detect blood urea nitrogen (BUN) and creatinine (Cr).
2.2.4 Lactic acid level detection
The lactate detection kit (colorimetric method) was used to detect the level of lactate in serum and kidney tissue of mice in each group.Prepare the enzyme working solution and chromogenic reagent according to the instructions, and properly dilute the sample.Add the sample, enzyme working solution, and chromogenic reagent in sequence according to the instructions, mix by vortex, incubate at 37 ℃ for 10 min, then immediately add a stop reagent to stop the reaction, and detect the absorbance value of each group by a microplate reader (530 nm wavelength).
2.2.5 Target gene prediction of miRNA-122-5p
Target genes of miRNA-122-5p were predicted using the Targetscan(http://www.Targetscan.org) online database.
2.2.6 RT-qPCR
Trizol was used to extract total RNA from kidney tissues in each group, and miRNA and mRNA reverse transcription kits were used to reverse-transcribe the extracted total RNA into their respective cDNAs.Real time PCR (StepOnePlus Real-Time PCR System) amplification was carried out with SYBR Green fluorescent quantitative reagent.Amplification conditions: predenaturation at 95 ℃ for 30 s, denaturation at 95 ℃ for 5 s, and annealing at 60 ℃ for 30 s, a total of 40 cycles were repeated.The 2-△△Ctmethod was used for the expression of miRNA and mRNA, and it was expressed as the relative expression corrected by the respective internal reference (U6, β-actin).Primer sequences(5'-3'): mmu-miRNA-122-5p: GGCTGGAGTGTGACAATGGTG;U6(F):CTCGCTTCGGCAGCACA; U6(R):AACGCTTCACGAATT TGCGT; PKM2(F): CAGAGAAGGTCTTCCTGGCTCA;PKM2(R): GCCACATCACTGCCTTCAGCAC; β-actin(F):C AT T G C T G AC AG G AT G C AG A AG G; β-a c t i n(R):TGCTGGAAGGTGGACAGTGAGG.
2.2.7 Western blotting
RIPA lysate (plus PMSF) was used to extract mouse kidney tissue protein, and the protein concentration was determined by BCA method.Mix an equal amount of protein with the same volume of 2×SDS loading buffer, and boil at 100 ℃ for 10 min to denature the protein.The denatured protein was electrophoresed, transferred to membrane, blocked at room temperature for 1 h, and the primary antibodies PKM2 and β-actin were added, overnight at 4 ℃.After washing, the corresponding secondary antibody was added, and after incubation at room temperature for 1 h, electrochemiluminescence reagent (ECL) was exposed for development.The bands were analyzed by grayscale scanning using ImageJ software.
2.3 Statistical processing
All experimental data were analyzed using GraphPad Prism 5 statistical software, and measurement data were expressed as mean± standard deviation (±s).One-way analysis of variance (One-Way ANOVA) was used for comparison between multiple groups, and Pearson correlation analysis was used for correlation analysis, and P<0.05 was considered statistically significant.
3.Results
3.1 Comparison of serum creatinine and blood urea nitrogen levels in each group of mice
The effects of CLP on renal injury of mice in each group were detected by renal function.The results showed that the serum urea nitrogen in mice at 12 and 24 h after CLP were: [(14.825±3.745)mmol/L], [(23.005± 6.12) mmol/L], which was significantly higher than that of the normal control group [(4.885±1.813)mmol/L] (P<0.05) (Figure 1A).The creatinine values in mice at 12 and 24 hours after CLP were: [(56.507±10.219) μmol/L] and[(66.032±21.563) μmol/L], which was also significantly higher than the normal control group [(6.47±2.233) μmol/L] (P<0.05) (Figure 1B).

Fig 1 Effects of CLP on kidney function of mice
3.2 Comparison of serum and renal tissue lactate expression levels of mice in each group
The lactic acid levels in serum and kidney tissue of mice in each group were detected, and it was found that the lactic acid levels in serum (Figure 2A) and kidney tissue (Figure 2B) increased after CLP treatment for 4 h, and gradually increased with the prolongation of CLP treatment time.Serum lactate was [(6.897±1.969) mmol/gprot] and [(7.642±2.339) mmol/gprot] after 12 and 24 h treatment,which was significantly higher than those of the control group[(2.28±0.543) mmol/gprot].(P<0.05).Lactate in renal tissue after CLP treatment for 12h and 24h was [(3.931±1.114) mmol/gprot],[(4.47±0.75) mmol/gprot], which was also significantly higher than that of the control group [(2.036±0.461) mmol/gprot] (P<0.05).
3.3 Target gene prediction results of miRNA-122-5p
The Targetscan online database was used to predict the target genes of miRNA-122-5p, and a total of 226 target genes were obtained.By reviewing the literature and incorporating a predictive software scoring system, we targeted the target gene PKM2.
3.4 Comparison of miRNA-122 and pkm2 mRNA expression levels in kidney tissue of each group
Compared with the control group, the expression of miRNA-122 in renal tissue decreased at 4 h after CLP, and significantly decreased at 12-24 h (P<0.05) (Figure 4A).The expression of pkm2 mRNA in renal tissue increased at 4h after CLP, and significantly increased at 12-24 h (P<0.05) (Figure 4B).
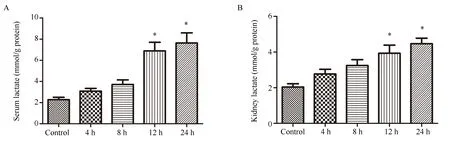
Fig 2 Effects of CLP on the level of lactate in serum and kidney of mice

Fig 3 Predicted consequential pairing of target region (top) and miRNA-122-5p (bottom)

Fig 4 Expression of miRNA-122 and pkm2 mRNA in kidney of CLP mice
3.5 Comparison of PKM2 protein expression levels in renal tissue of each group
As shown in Figure 5, there was a basal expression of PKM2 protein in the normal control group.At 4 h and 8 h after CLP, the expression of PKM2 protein in renal tissue increased, but there was no statistical difference compared with the control group (P>0.05);12 h and 24 h after CLP, the expression of PKM2 protein in renal tissue was significantly increased, and there was a statistical difference compared with the control group (P<0.05).
3.6 Correlation analysis
In order to clarify the relationship between miRNA-122, PKM2 and lactate levels in kidney tissue of S-AKI mice, Pearson correlation analysis was performed on them in this study.The results showed that miRNA-122 was significantly negatively correlated with lactate levels (P<0.000 1, r=-0.716 7) (Figure 6A).There was a significant positive correlation between pkm2 mRNA and lactate level (P<0.000 1, r=0.6817) (Figure 6B).There was a significant negative correlation between miRNA-122 and pkm2 mRNA expression (P<0.000 1, r=-0.812 2) (Figure 6C).

Fig 5 Expression of PKM2 protein in kidney of CLP mice

Fig 6 Correlation analysis
4.Discussion
Studies have shown that miRNAs are significantly altered in various types of AKI and may regulate inflammation, programmed cell death, and the cell cycle during the damage and repair phases of AKI by binding to the 3'-untranslated regions of their target genes[5].It has been shown that many miRNAs have certain therapeutic potential in AKI[5].The previous study of our group found that miRNA-122 was dysregulated in the kidney of S-AKI mice[3].Previous studies have mostly described miRNA-122 as one of the liver-specific miRNAs, which plays a role in regulating lipid and cholesterol metabolism[6].However, in recent years, more and more studies have shown that miRNA-122 is also closely related to kidney disease.For example, Abdelsalam et al.found that the expression of miRNA-122 in the blood of healthy people was significantly higher than that in patients with end-stage renal disease (ESRD)[7].Decreased expression of miRNA-122 in kidney disease may attenuate the inhibitory effect of transforming growth factor-β(TGF-β) and exacerbate the accumulation of fibroblasts and matrix proteins, which in turn leads to progressive nephropathy[8,9].Kagawa et al.found that the expression of miRNA-122 is generally downregulated in acute kidney injury induced by various drugs, and its change is earlier than that of traditional biomarkers such as plasma urea nitrogen and creatinine, which suggests that the down-regulation of miRNA-122 may be an an early marker of tubular and glomerular injury[10].In addition, miRNA-122 was down-regulated in the serum of patients with uric acid nephropathy and in uric acid-treated renal tubular epithelial cells, which promotes the activation of the NLRP3 inflammasome and exacerbats renal injury[11].The above studies show that the down-regulation of miRNA-122 is involved in acute and chronic kidney injury caused by various reasons.Consistent with this, the previous study of our group found that the expression of miRNA-122 was down-regulated in the kidneys of S-AKI mice,and it may aggravate renal injury through pro-inflammatory effects.PKM2 is a key enzyme that regulates cell metabolism and growth under different physiological conditions[12].As one of the important rate-limiting enzymes in glycolysis, PKM2 catalyzes the formation of pyruvate from phosphoenolpyruvate[12].In normal cells, when the oxygen supply is sufficient, pyruvate enters the tricarboxylic acid cycle and provides energy through oxidative phosphorylation, when the oxygen supply is insufficient, it provides energy through glycolysis[13].In the last century, German scientist Warburg discovered that tumor cells still supply energy through glycolysis and produce lactic acid even in the presence of sufficient oxygen.This phenomenon is called the Warburg effect, also known as aerobic glycolysis[13].Although less ATP is produced by aerobic glycolysis than the net energy produced by oxidative phosphorylation in mitochondria, cancer cells are more inclined to metabolize glucose through the Warburg effect.This allows cancer cells to produce ATP at a faster rate to accommodate the increased energy demands of cancer cells, while also providing cancer cells with intermediates necessary for multiple anabolic pathways[14].At present, the Warburg effect has become one of the important features of tumor cell metabolism, and PKM2 is considered to be an important regulator of the Warburg effect in tumor cells.PKM2 is highly expressed in various tumor cells and plays an important role in tumor cell metabolism by promoting the Warburg effect [12,15].Recent studies have found that, similar to the metabolism of tumor cells, the Warburg effect is also enhanced in the metabolic process of the inflammatory response, which aggravates the occurrence and development of the inflammatory response.For example, the expression of PKM2 is significantly increased in LPS-activated macrophages and promotes the release of the inflammatory factor IL-1β [16,17].PKM2-dependent glycolysis promotes the activation of inflammasomes NLRP3 and AIM2 in macrophages during sepsis,which is dependent on lactate production[18].Conversely, inhibition of PKM2 expression can effectively alleviate sepsis injury[18].
In order to clarify the dynamic expression of PKM2 in the kidney tissue of S-AKI mice, the mRNA and protein levels of PKM2 were detected in this study.The results showed that the mRNA and protein expressions of PKM2 increased at 4 h after CLP, and gradually increased with the prolongation of operation time.In addition, this study detected the levels of lactic acid in the serum and kidney tissue of the mice respectively.The results showed that both serum and renal tissue lactate levels gradually increased with the prolongation of operation time.The correlation analysis of pkm2 mRNA and lactate levels in kidney tissue showed that they were significantly positively correlated.It is suggested that PKM2 may be involved in the occurrence and development of S-AKI by promoting aerobic glycolysis.Consistent with this, Tan et al.[19] also found that the expression of pkm2 mRNA was increased in the kidney tissue of septic mice, and the aerobic glycolysis inhibitors could significantly reduce the level of PKM2 and alleviate renal injury in S-AKI mice.Wu[20] and other studies have shown that inhibiting the expression of PKM2 can effectively alleviate LPS-induced renal tissue damage.Therefore, rational inhibition of PKM2 may be an effective means to prevent sepsis and its induced AKI.
Although studies have shown that the expression of PKM2 is increased in various inflammatory lesions and plays a role in proinflammatory and pro-injury, the mechanism of the up-regulation of PKM2 in these diseases is still unclear.Based on the previous study, this study verified the decreased expression of miRNA-122 in the kidney tissue of S-AKI mice again, and analyzed the correlation between miRNA-122 and PKM2.The results showed that they were highly negatively correlated, which means that the down-regulation of miRNA-122 may be one of the reasons for the increased expression of PKM2.
In conclusion, the expression of miRNA-122 was significantly decreased in the renal tissue of S-AKI mice, while the expression of its target gene PKM2 were significantly increased.In addition,lactate levels were significantly increased in serum and kidney tissue of S-AKI mice, suggesting that downregulation of miRNA-122 may promote the expression of PKM2, which in turn increases lactate production by regulating aerobic glycolysis, and exacerbats renal impairment of S-AKI mice.Next, we will use miRNA-122 overexpression and intervention methods to observe the expression of PKM2 at the cellular level, and further search for evidence that miRNA-122 regulates PKM2.
Author's Contribution:
This experiment was designed by Fu Zhou and directed by Li Ting.Li Ting and Fu Zhou were responsible for modeling and sampling.Fu Zhou, Li Ruxin and Wang Kaili used RT-qPCR to detect the expression levels of miRNA-122 and pkm2 mRNA in kidney tissues of each group.Song Xu and Wu Yutao detected the lactic acid level in mouse serum and kidney tissue.Hu Nuoya, Quan Kangli and Du Bangzhe detected the expression level of PKM2 protein in kidney tissue of each group.The experimental data was analyzed by Fu Zhou and Li Ting.Fu Zhou wrote the paper, and Li Ting reviewed the paper.
All authors declare no conflict of interest.
杂志排行
Journal of Hainan Medical College的其它文章
- Advances in pharmacological action of bergamot lactone
- Progress of improvement of pain and joint function of knee osteoarthritis treated with thunder-fire moxibustion in the last five years
- Abnormal expression of TGFβ1 in acute myeloid leukemia and its regulation effect on leukemia cells
- Analysis of key pathogenic target genes of ovarian cancer and experimental verification of cells in vitro
- Investigation of paeonol-geniposide on acute alcoholic liver injury based on uniform design method
- Effect of Drynaria total flavonoids on the expression of NMDAR1,GluR2 and CaMK Ⅱ in the brain of hydrocortisone model mice
