Influence of Doped Ions on Persistent Luminescence Materials: a Review①
2022-03-12ZHANGLiuWeiSHENRuiChenTANJieYUANQuan
ZHANG Liu-Wei SHEN Rui-Chen TAN Jie YUAN Quan
(Institute of Chemical Biology and Nanomedicine, State Key Laboratory of Chemo/Biosensing and Chemometrics, College of Chemistry and Chemical Engineering, Hunan University, Changsha 410082, China)
ABSTRACT Persistent luminescence materials (PLMs) are potential luminescent materials which can remain emitting light after stopping the excitation. PLMs can avoid the autofluorescence of biological tissues, and play an important role in biosensing, targeted imaging and other fields. However, the applications of PLMs are often restricted by their weak persistent luminescence and short decay time after excitation. Doped ions will directly affect the luminescence centers and trap levels of PLMs, thereby leading to great differences in the optical performance of PLMs. Given this, the selection of doped ions to improve the optical performance of PLMs has become a fascinating research direction in recent years. At present, the published reviews mostly focus on the surface modifications and applications of PLMs. However, the influence of doped ions on the structure and optical performance of PLMs is seldom summarized. In this review, the influence of doped ions on the structure and optical performance of PLMs is introduced from three aspects: the type of doped ions, the number of types of doped ions, and the content of doped ions. Furthermore, we highlight recent achievements and mechanisms in the development of PLMs. Finally, we also propose and discuss the future opportunities and current challenges of ion-doped PLMs.
Keywords: persistent luminescence, doped ions, structure and optical performance;
1 INTRODUCTION
Persistent luminescence materials (PLMs) can absorb excitation energy and continue to emit light after the excitation is stopped[1-7]. In ancient China, natural minerals with luminous properties were made into luminous cups and night pearls. In 1866, Sidot et al. first prepared the sulfide PLMs ZnS:Cu2+, making PLMs enter the vision of researchers[8]. In 1968, Pailil et al. observed the persistent luminescence of SrAl2O4:Eu2+, pushing the researches of PLMs into a new stage[9,10]. Subsequently, Matsuzawa et al.found that the phosphorescence intensity and phosphorescence time of SrAl2O4:Eu2+, Dy3+phosphors were more than ten times those of early PLMs[11]. Since then, many PLMs have been developed and widely used in transportation,military facilities, biosensing and other fields[12-15].
The persistent luminescence inherence makes PLMs proper candidates in material applications. Given this, many researchers are devoted to finding ways to improve the phosphorescence intensity and phosphorescence time of PLMs. The introduction of doped ions provides a possibility to improve the optical performance of PLMs[16-23]. For example, Yamamoto et al. discovered that the phosphorescence intensity and phosphorescence time of the materials were improved after introducing Dy3+ion into SrAl2O4:Eu2+[11]. Liu et al. enhanced the phosphorescence intensity and prolonged the phosphorescence time exceeding 13 hours by adjusting the contents of Nd3+ion in Zn2Ga3-x-yGe0.75O8:Crx, Ndy[24]. Studying the mechanisms of ion doping to enhance the optical performance of PLMs can help design PLMs with stronger phosphorescence intensity and longer phosphorescence time, thereby expanding the applications of PLMs.
Recently, reviews focus on synthesis methods, persistent luminescence mechanisms and applications of PLMs have been published. Li et al. systematically summarized the synthesis techniques, persistent luminescence mechanisms,characterizations and applications of PLMs[9]. Wang et al.summarized the persistent luminescence mechanisms,synthesis methods and biomedical applications of PLMs[25].Singh et al. outlined the surface modifications, bioimaging applications of PLMs, and future research directions[26].Doped ions play a vital role in improving the optical performance of PLMs. However, the influence of doped ions on the structure and optical performance of PLMs is seldom summarized. In this review, we mainly focus on how the optical performance of PLMs is affected by the type of doped ions, the number of types of doped ions, and the content of doped ions. Furthermore, the opportunities and challenges of PLMs in ion doping are also presented.
2 INFLUENCE OF DOPED IONS ON THE STRUCTURE AND OPTICAL PERFORMANCE OF PLMs
With the introduction of doped ions, different energy levels are produced in PLMs, resulting in different structures and optical performance. Herein, we classify and discuss how doped ions affect the structure and optical performance of PLMs based on the number of types of doped ions, the type of doped ions and the content of doped ions. To better demonstrate the influence of doped ions on PLMs, a
summary of the main influence of doped ions on some common ion-doped PLMs is shown in Table 1.
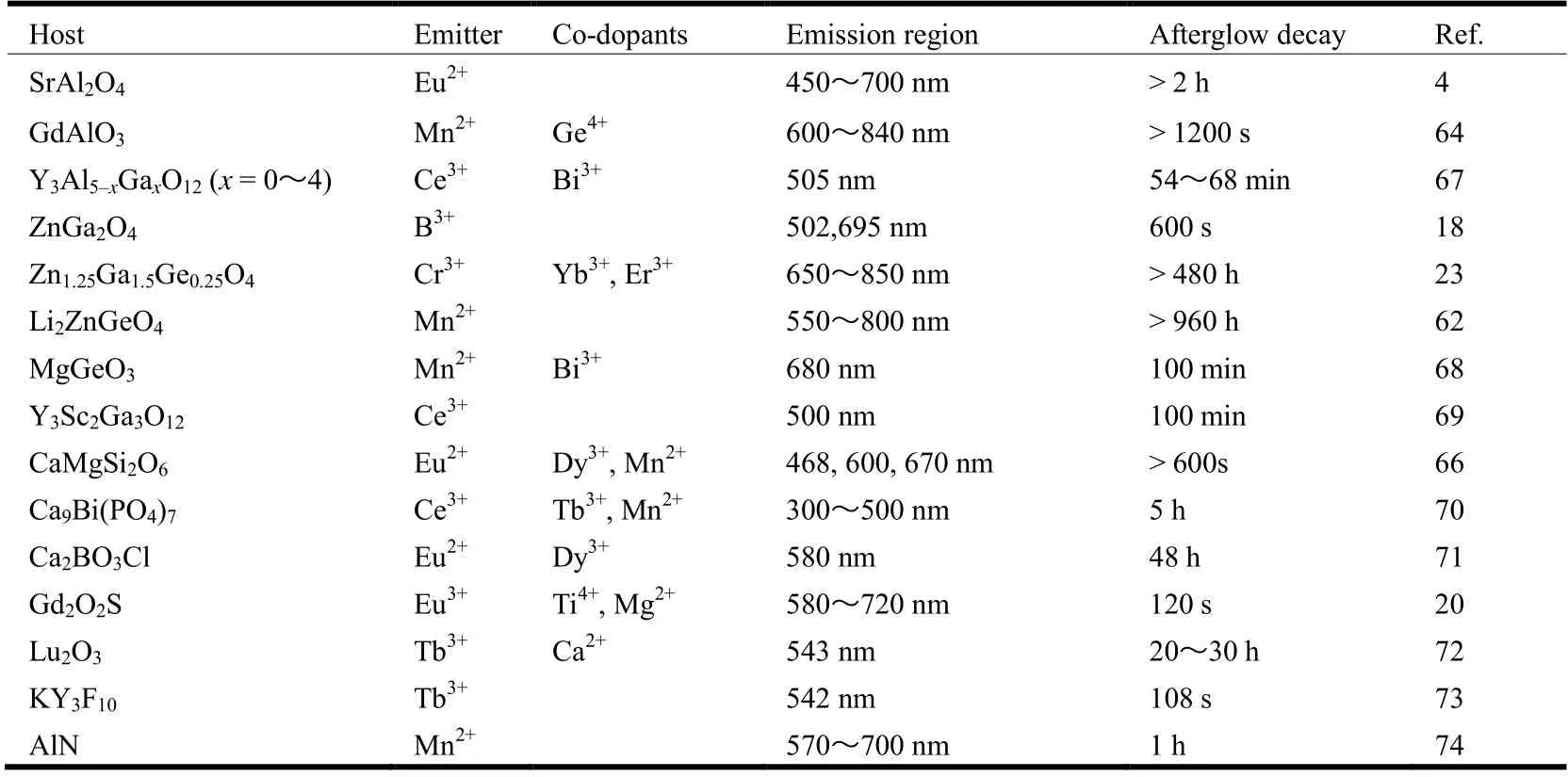
Table 1. Details of Host, Emitter, Co-dopants, Emission Region and Afterglow Decay of PLMs
2. 1 Influence of doped ions type on the structure and optical performance of PLMs
Due to the difference in the atomic structure and energy levels, various doped ions have different effects on the structure and optical performance of PLMs. Thus, the relationship between the type of doped ions and the structure and optical performance of PLMs is introduced in this section.
2. 1. 1 Cr3+-doped PLMs
Cr3+ion is usually introduced into PLMs to obtain red or near-infrared persistent luminescence resulting from its unique electronic transitions. Among them, Cr3+-doped ZnGa2O4is one of the research hotspots in PLMs. The ZnGa2O4crystal exhibits a cubic spinel structure, in which the Zn2+ion occupies the A-sites of the tetrahedron, and the Ga3+ion the B-sites of the octahedron[27,28]. Cr3+ion tends to replace Ga3+ion in the distorted octahedral coordination for the reason that Cr3+ion has the same valence electron and ion radius as Ga3+ion, which makes the Cr3+-doped ZnGa2O4materials produce strong near-infrared phosphorescence at 696 nm[2,29,30]. Hao et al. found that the Cr3+-doped ZnGa2O4exhibits excellent optical performance, which can maintain phosphorescence for 10 hours after stopping the ultraviolet light irradiation[31]. Moreover, the author observed that the excitation spectrum of Cr3+-doped ZnGa2O4has three excitation bands of 250~350, 350~487, and 487~650 nm,which are caused by the host excitation band of ZnGa2O4, the charge transfer between ZnGa2O4and Cr3+ion, and the electronic transitions of Cr3+ion, respectively. Based on previous researches, Yan et al. further comprehensively studied the persistent luminescence mechanism of Zn2.94Ga1.96Ge2O10:Cr3+[19]. As shown in Fig. 1, after the Zn2.94Ga1.96Ge2O10absorbs incident photons, the electrons of Zn2.94Ga1.96Ge2O10move to the conduction band and are trapped by native defects by means of nonradiative relaxation.When the ultraviolet light excitation is finished, the combination of holes and electrons released by native defects produces short phosphorescence. Since the absorption spectrum of Cr3+ion fully overlaps with the emission spectrum of Zn2.94Ga1.96Ge2O10, the energy absorbed by Zn2.94Ga1.96Ge2O10is transferred to Cr3+ion by means of nonradiative energy[32]. The continuous energy transfer causes the electrons of Cr3+ion to transform into three different excited states. Then, the electrons are trapped by the shallow electron traps or deep electron traps by means of nonradiative relaxation[33]. At the same time, the electron (t2e) fills the energy-matched traps in the form of4T1and4T2through the tunneling process. When the continuous energy transfer is stopped, the electron traps release the electrons which recombine with Cr3+ion, thereby producing a strong or ultra-long phosphorescence[19].
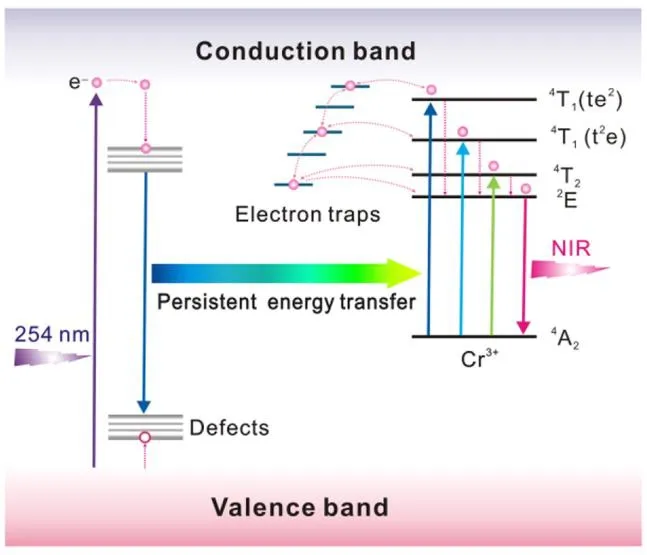
Fig. 1. Persistent energy transfer mechanism of Cr3+-doped ZnGa2O4 materials
2. 1. 2 Mn2+-doped PLMs
Mn2+ion has a typical 3d5electronic configuration. The energy transitions of Mn2+ions are quite sensitive to the ligand/crystal field due to the participation of thedshell.Mn2+ions surrounded by anions can have different geometric structures, such as linear, octahedron, spherical, tetrahedron,or square plane. The environment of Mn2+ion at different crystal fields leads to different emission energy distributions and produces an emission band of 450~750 nm. For example, tetrahedral coordination of Mn2+ion produces green emission[34], while octahedral coordination of Mn2+ion produces orange to red emission[35,36]. Tan et al. researched the size, crystal structure and optical performance of Mn2+-doped ZnGa2O4with a typical rod-shaped structure in different environments[21]. The size of Mn2+-doped ZnGa2O4rapidly decreases as the pH increases from 6 to 7.5. In comparison, when the pH further increases to 9.5, the size of the Mn2+-doped ZnGa2O4materials slightly increases to 80 nm. The detailed crystal structure of the Mn2+-doped ZnGa2O4shows that the materials have lattice fringe (110)parallel to the direction of the crystal rod and lattice fringe(113) at an angle of 66° to the direction of the crystal rod[37].Their interplanar spacing is 0.71 and 0.29 nm, indicating that the Mn2+-doped ZnGa2O4grows along thecaxis[38]. The optical performance of ZnGa2O4:Mn2+materials was further researched by researchers[39]. The photoluminescence spectrum of Mn2+-doped ZnGa2O4materials shows that two emission peaks are detected at 450 and 480 nm, which are resulted from the native defects, for example, interstitial Zn and oxygen vacancies (Fig. 2a). When the pH is below 7.0,the intrinsic luminescence of the materials and the emission of Mn2+ions have a serious overlap. As the pH increases, the intrinsic luminescence intensity gradually decreases, and the emission band intensity of the Mn2+ion increases. The intrinsic luminescence almost disappears and is dominated by the green emission band of Mn2+when the pH increases to 9.5. In this case, the phosphorescence time of Mn2+-doped ZnGa2O4materials exhibits more than 100 s (Fig. 2b).According to the above results, a possible persistent luminescence mechanism of Mn2+-doped ZnGa2O4materials is proposed (Fig. 2c)[40,41]. The excited electrons and holes produced under ultraviolet light excitation are trapped by electron traps and hole traps, respectively[42]. Then, some electrons get away from the electron traps under thermal stimulation and transfer to native defects, thereby resulting in the formation of emission peaks from ZnGa2O4materials[43].The other part of the electrons escape and transfer to the excitation level of the Mn2+ion[44]. Subsequently, the recombination of holes and electrons causes Mn2+ion to emit green light[45].
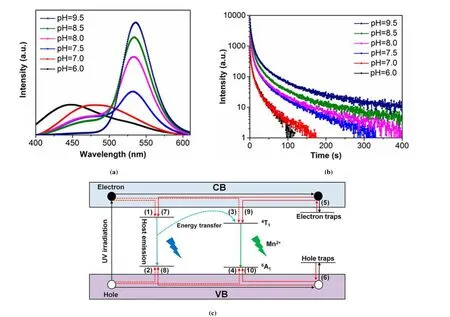
Fig. 2. (a) Photoluminescence spectrum, (b) phosphorescence decay curve,(c) persistent energy transfer mechanism of ZnGa2O4:Mn2+ materials
2. 1. 3 Eu2+-doped PLMs
Eu2+ion is a common doped ion in PLMs. The introduction of Eu2+ion usually produces yellow, orange and red emissions for the reason that the crystal field reduces the emission energy of 4f65d1electron configuration of Eu2+ion[46]. Yang et al. researched the structure and optical performance of Eu2+-doped SrAl2O4materials[4]. It is found that the crystal phase of the Eu2+-doped SrAl2O4materials corresponds to the standard pattern, indicating that the structure of the SrAl2O4materials has not obviously changed after the introduction of Eu2+ion. Moreover, Eu2+-doped SrAl2O4shows a strong emission band of 450~700 nm and exhibits a phosphorescence time that exceeds 2 hours.Considering the structure and characteristics of SrAl2O4:Eu2+,a possible persistent luminescence mechanism of SrAl2O4:Eu2+is deduced. The SrAl2O4material as the host lattice absorbs the soft X-ray photon energy and stores it in electron traps. Then, the energy gets away from the electron traps and transfers to the 4f65d1energy level in the Eu2+ion,which causes the Eu2+ion to produce radioactive transition luminescence.
2. 1. 4 B3+-doped PLMs

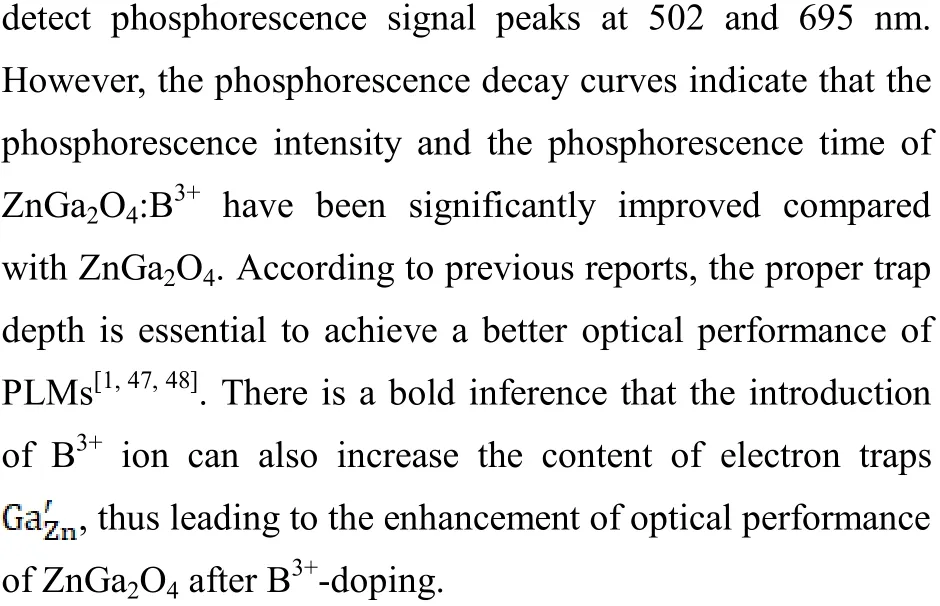
2. 2 Influence of the number of types of doped ions on the structure and optical performance of PLMs
As above mentioned, the structure and optical performance of PLMs are closely related to the type of doped ions. Most PLMs used in practical applications need to be doped with multiple ions to enhance optical performance. The simultaneous introduction of multiple ions will affect not only the host of PLMs, but also the interaction among different ions.Therefore, we introduce the influence of the number of types of doped ions on the structure and optical performance of PLMs in this section.
2. 2. 1 Ge4+ and Cr3+ co-doped PLMs
The introduction of Ge4+ion will change the width of the material bandgap due to its unique electronic structure. Thus,the change of structure and optical performance of Ge4+, Cr3+co-doped PLMs is discussed[5]. Wang et al. found that the crystal phases of the Cr3+-doped ZnGa1.995O4and Ge4+, Cr3+co-doped ZnGa1.995O4materials are corresponding to the standard pattern, indicating that the structure of the materials has not obviously changed after Ge4+, Cr3+co-doping. In addition, the emission spectra of ZnGa1.995O4:Cr3+and Zn1.25Ga1.5Ge0.25O4:Cr3+both present a near-infrared emission band of 650~900 nm[49,50]. The phosphorescence decay curves show that the optical performance of Zn1.25Ga1.5Ge0.25O4:Cr3+is better than that of ZnGa1.995O4:Cr3+[5]. Based on the above results, the introduction of Ge4+improves the filling efficiency of electron traps,thus increasing the phosphorescence time of the materials[23].
2. 2. 2 Pr3+, Cr3+ co-doped PLMs
The number and depth of the trap energy levels of PLMs are changed after the introduction of Pr3+ion because of the special 4f5delectronic structure and abundant electronic transition types of Pr3+ion. Therefore, the changes in structure and optical performance of Zn2.94Ga1.96Ge2O10after the introduction of Pr3+and Cr3+ions are discussed[19]. By analyzing the XRD pattern, the structure of Zn2.94Ga1.96Ge2O10:Cr3+, Pr3+has not obviously changed, and the pure spinel phase is still maintained. Later, the author does in-depth studies on the optical performance of Zn2.94Ga1.96Ge2O10:Pr3+, Cr3+. The electrons recombine with holes in the natural defects, thereby leading to a wide emission band of 350~660 nm. In addition, the Cr3+ion provides a near-infrared emission band of 695 nm through the2E→4A2transition[27], while the Pr3+ion increases the phosphorescence time by adjusting the depth and density of traps based on the energy levels of 4felectron configurations[51-55]. Similarly, Yu et al. did further research on the persistent luminescence mechanisms of Zn3Ga2GeO8:Cr3+, Pr3+to clarify the function of Pr3+ion[56].The introduction of Pr3+ion deepens the trap energy level and increases the number of traps. Given this, a possible persistent luminescence mechanism of Zn3Ga2GeO8:Cr3+,Pr3+is proposed (Fig. 3). TrapAforms more traps than trapA'by introducing Pr3+ion, thereby improving the phosphorescence intensity and the phosphorescence time of Zn3Ga2GeO8:Cr3+, Pr3+materials.

Fig. 3. Persistent luminescence mechanism of Zn2.94Ga1.96Ge2O10:Pr3+, Cr3+ materials
2. 2. 3 Yb3+, Er3+, Cr3+ co-doped PLMs
Yb3+ion and Er3+ion are rare earth element ions used as luminescence centers, which are usually introduced into PLMs to change the density and depth of the trap levels resulting from their unique electronic structure. Consequently,the structure and optical performance of Zn1.25Ga1.5Ge0.25O4:Yb3+, Er3+, Cr3+(ZGGO:Cr3+, Yb3+, Er3+)with a pure spinel phase is discussed[23]. Yan et al. found that the structure of Zn1.25Ga1.5Ge0.25O4has not obviously changed after doping with Yb3+, Er3+and Cr3+ion. Moreover,ZGGO:Cr3+, Yb3+, Er3presents an excellent optical performance, and the phosphorescence time exceeds 20 days. Later,the author studied the persistent luminescence mechanism of ZGGO:Yb3+, Er3+, Cr3+(Fig. 4). The excited electrons are captured by electron traps under ultraviolet light irradiation,and then moves to deep traps by means of nonradiative relaxation. Subsequently, the combination of the excited electrons and the Cr3+ion leads to the formation of a persistent phosphorescence signal after the ultraviolet radiation is stopped. The introduction of Yb3+and Er3+ions can not only provide the materials with additional electrons and energy levels, but also adjust the density and depth of traps[51,57,58]. The excited electrons are trapped in the deepest 4fenergy levels of Yb3+and Er3+ions, thereby extending the time for the trapped electrons to return to the2E energy level of Cr3+ion (Fig. 4a, b)[58], which also obviously prolongs the phosphorescence time of materials.

Fig. 4. Persistent luminescence mechanism of (a) ZGGO:Cr3+, (b) ZGGO:Cr3+,Yb3+,Er3+,(c) ZGO:Cr3+,Yb3+,Er3+. ZGO refers to a zinc gallate matrix
2. 2. 4 Eu3+, Ti4+ and Mg2+ co-doped PLMs
Viana et al. researched the structure and optical performance of Eu3+, Ti4+, Mg2+co-doped Gd2O2S materials with a pure hexagonal Gd2O2S phase[20]. The study found that Ti4+and Mg2+ions are trapping centers, and Eu3+ions are the emission centers. The Gd2O2S:Eu3+, Ti4+, Mg2+has an emission band of 580~720 nm due to the electronic transitions of Eu3+ion. Therefore, it can be inferred that the Eu3+ion plays the role of the luminescence center in the materials. Moreover, Gd2O2S: Eu3+, Ti4+, Mg2+has a longer phosphorescence time compared with Gd2O2S: Eu3+for the reason that Ti4+and Mg2+ions play an important in improving the density of trap energy levels. However, the particle size and crystal structure of Gd2O2S materials have not obviously changed after the introduction of Eu3+, Ti4+and Mg2+ions.
2. 3 Influence of the content of doped ions on the structure and optical performance of PLMs
As mentioned above, some doped ions play the role of the emission center of PLMs, while other doped ions will affect the number and depth of trap levels. Therefore, it is of great necessity to understand how the content of doped ions influences the structure and optical performance of PLMs.This section will discuss how the content of doped ions affects the structure and optical performance of PLMs.
2. 3. 1 Pr3+, Cr3+ co-doped PLMs
Normally, Cr3+ion serves as the luminescence center of PLMs, while Pr3+ion affects the number and depth of trap levels. Yan et al. studied how the contents of Pr3+and Cr3+ions influence the structure and optical performance of PLMs.It is found that the increase of Cr3+content promotes the energy release of Zn2.94Ga1.96Ge2O10:Cr3+, Pr3+. Therefore, as the amount of Cr3+ion doping increases, the phosphorescence intensity and phosphorescence time of the material decrease[19,27,59]. Similarly, Zhang et al. studied the changes in particle size of Pr3+, Cr3+co-doped Zn2Ga2.98-xGe0.75O8materials with different Pr3+contents[22]. It is found that the average size of the materials decreases with the increase of Pr3+contents. Considering that the doped ions can strongly affect the crystal growth rate through surface charge modification[60], the size reduction of Zn2Ga2.98-xGe0.75O8:Cr3+, Pr3+may be related to the influence of Pr3+ion on the surface charge of the material. Specifically,the surface charge distribution of the materials will be changed when the Pr3+ion is introduced into the materials,thereby reducing the growth rate. Subsequently, the optical performance of Pr3+, Cr3+co-doped Zn2Ga2.98-xGe0.75O8materials is researched. As shown in Fig. 5a, the phosphorescence intensity and the phosphorescence time of the materials increase as the increase of Pr3+doping contents due to the changes in the trap levels of the materials caused by the introduction of Pr3+ion (Fig. 5b). When the content of Pr3+ion is too high, the luminescence performance will decrease due to the concentration quenching effect.

Fig. 5. (a) Phosphorescence decay curves of Pr3+, Cr3+ co-doped Zn2Ga2.98-xGe0.75O8 materials with different Pr3+ doping contents.The inset presents the phosphorescence intensity of the materials after the ultraviolet light irradiation is stopped,(b) Thermo-luminescence spectra of Pr3+, Cr3+ co-doped Zn2Ga2.98-xGe0.75O8 materials with different Pr3+ doping contents
2. 3. 2 Bi3+, Cr3+ co-doped PLMs
Bi3+ion can significantly reduce the bandgap energy of PLMs and improve the efficiency of electron trap filling resulting from its unique 6sorbital. Tuerdi et al. researched the influence of crystalline structure, emission peak wavelength and phosphorescence time of ZnGa2O4:Bi3+, Cr3+materials with different Bi3+doping contents[16]. The Bi3+ion tends to replace Ga3+ion in ZnGa2O4with a similar ion radius, which makes the material produce higher periodic lattice distortion, thereby making the materials exhibit a longer phosphorescence time. When the doped ratio of Bi3+ion is low, the crystal phase of Cr3+, Bi3+co-doped ZnGa2O4materials corresponds to its standard spectrum, indicating that the structure of ZnGa2O4materials has not obviously changed after the introduction of Bi3+ion. However, when the doped ratio of Bi3+ion increases to 0.03, a strong peak related to the rhombohedral Bi3+structure at 28.01° is observed in the XRD pattern (Fig. 6a), illustrating that the original crystal structure of the materials is destroyed. Correspondingly, its optical performance is also reduced. The phosphorescence emission spectrum of ZnGa2O4:Bi3+, Cr3+materials with different Bi3+doping contents shows a red-shift of emission peak wavelength, which indicates that Bi3+ion reduces the crystal field intensity of the Cr3+ion (Fig. 6b).
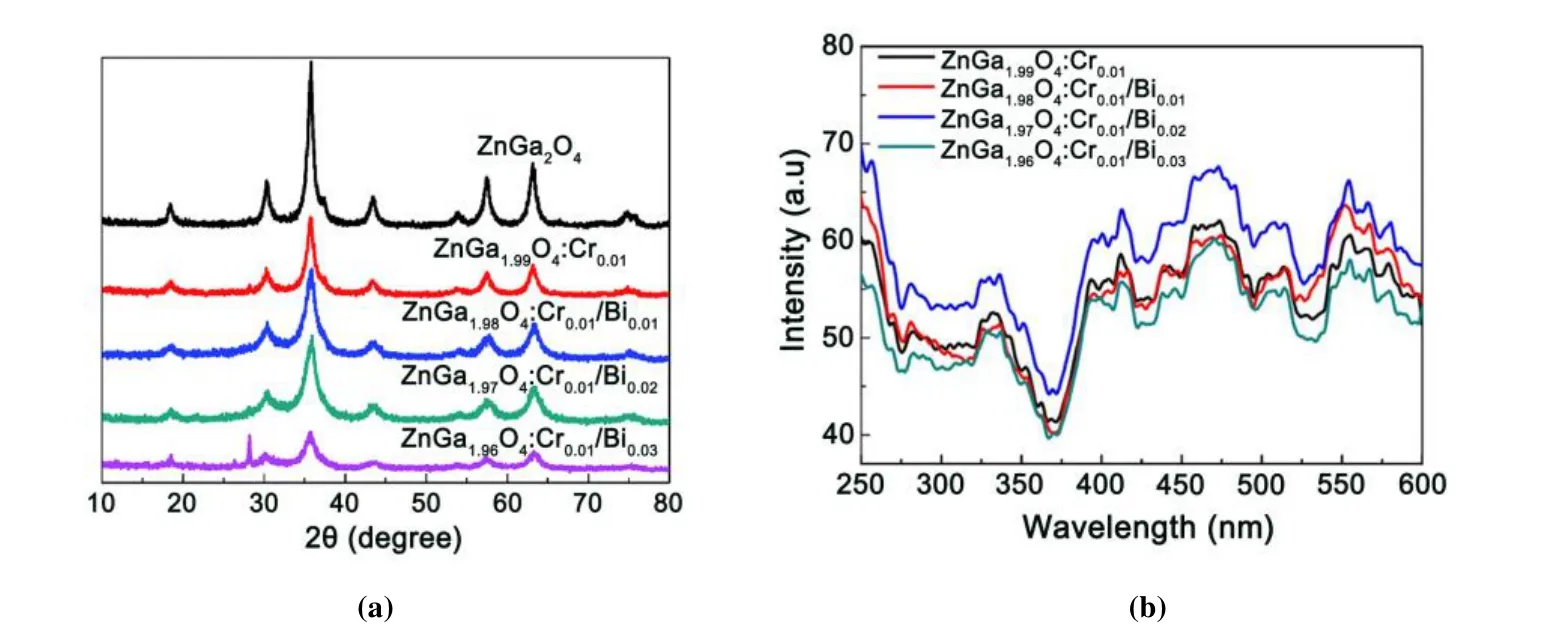
Fig. 6. (a) XRD patterns of ZnGa2O4, ZnGa2O4:Cr3+ and ZnGa2O4:Cr3+, Bi3+,(b)Phosphorescence spectra of ZnGa2O4, ZnGa2O4:Cr3+ and ZnGa2O4:Cr3+, Bi3+
2. 3. 3 B3+, Cr3+ co-doped PLMs
As mentioned above, the introduction of B3+ion can improve the optical performance of ZnGa2O4. On this basis,the researchers studied the influence of doped ion content on PLMs. For example, Yan et al. synthesized ZnGa2O4:B3+,Cr3+materials by hydrothermal method, and studied the optical performance of ZnGa2O4:Cr3+with different B3+doping contents[17]. The XRD pattern presents that the structure of the material has not obviously changed after doping with the B3+and Cr3+ions. Besides, the emission wavelength of the materials has not obviously changed when the B3+ion is introduced into the materials, which indicates that the B3+ion does not play the role of luminescence center in the materials. At the same time, the improvement of the optical performance of materials shows that the introduction of B3+ion injects new electron traps into the materials and increases the content of electron traps.
2. 3. 4 Eu3+, Ti4+ and Mg2+ co-doped PLMs
Eu3+, Ti4+and Mg2+ions will act as luminescence centers or trap levels to affect the optical performance of the material.Therefore, studying the optical performance of Gd2O2S:Eu3+,Ti4+and Mg2+materials with different doping contents will help us better understand the role of these ions in the material[20]. The study found that Eu3+ion provides trap levels for electron-hole recombination, and Ti4+ion provides electron traps to achieve the formation of the phosphorescence signal. Since Ti4+ion replaces Gd3+ion and destroys the charge conservation of the materials, it is necessary to introduce Mg2+ion to maintain charge balance.In addition, as the doping contents of Mg2+ion increase, the phosphorescence intensity of the material increases. This indicates that the introduction of Mg2+ion can induce the formation of intermediate trap levels in the material, which prolongs the storage time of the carriers in the material, thus realizing the improvement of the persistent performance of the materials[61].
Apart from the ion-doped PLMs mentioned above, other doped ions also have an impact on the structure and optical performance. For example, Li2ZnGeO4:Mn2+[62],SrAl2O4:Eu2+, Dy3+[63], GdAlO3:Mn4+, Ge4+[64], LaAlO3:Mn4+,Ge4+[65], CaMgSi2O6:Eu2+, Dy3+, Mn2+[66], Y3Al5-xGaxO12:Ce3+,Bi3+(x= 0~4)[67], MgGeO3:Mn2+, Bi3+[68],Y3Sc2Ga3O12:Ce3+[69], Ca9Bi(PO4)7:Ce3+, Tb3+, Mn2+[70],Ca2BO3Cl:Eu2+, Dy3+[71], Lu2O3:Tb3+, Ca2+[72], KY3F10:Tb3+[73]and AlN:Mn2+[74].
3 CONCLUSION
In this review, we mainly focus on the impact of the type of doped ions, the number of types of doped ions, and the content of doped ions on the structure and optical performance of PLMs. In the past decade, although various doped ions have been widely used to improve the optical performance of PLMs, there is still much work to do in the future. First, in the actual preparation process, the optical performance of PLMs will be affected by the symmetry of matrix lattice, the radius of activated ions, and the electronegativity and distribution of external electron cloud.This should be paid more attention to by researchers. Second,exploring the relationship between doped ions and the host lattice and investigating the influence of the defects caused by doped ions on the storage time of the carriers. At present,these issues still have no exact quantitative relationship, and further researches are needed. Third, understanding the role of trap types, trap contents and probability of trapping electrons in the researches of the persistent luminescence mechanisms. Forth, exploring new doped ions and substrates.For example, most current PLMs are based on aluminates,gallates and silicates matrices. It is necessary to find new matrices with excellent properties. Collectively, with the deepening research of PLMs, ion-doped PLMs with higher luminescence intensity and phosphorescence lifetime will have a broad development prospect.
杂志排行
结构化学的其它文章
- Synthesis, Crystal Structure and Fungicidal Activity of 3,4-Dichloro-5-(6-chloro-9-(4-fluorobenzyl)-9H-purin-8-yl)isothiazole①
- Synthesis, Crystal Structure and Antifungal Activity of New Furan-1,3,4-oxadiazole Carboxamide Derivatives①
- Syntheses, Crystal Structures, Anticancer Activities and DNA-Binding Properties of the Dibutyltin Complexes Based on Benzoin Aroyl Hydrazone①
- Synthesis, Crystal Structure, Fungicidal Activities and Molecular Docking of Acyl Urea Derivatives Containing 2-Chloronicotine Motif①
- Molecular Design and Property Prediction of High Density 4-Nitro-5-(5-nitro-1,2,4-triazol-3-yl)-2H-1,2,3-triazolate Derivatives as the Potential High Energy Explosives①
- A New Borate-phosphate Compound CsNa2Lu2(BO3)(PO4)2:Crystal Structure and Tb3+ Doped Luminescence①
