Improving the energy release characteristics of PTFE/Al by doping magnesium hydride
2022-03-10JixingWuQingLiuBinFengQinYinYuhunLiShungzhngWuZhongshenYuJunyiHungXinxinRen
Ji-xing Wu , Qing Liu , Bin Feng , Qin Yin , Yu-hun Li ,*, Shung-zhng Wu ,Zhong-shen Yu , Jun-yi Hung ,**, Xin-xin Ren
a College of Field Engineering, Army Engineering University of PLA, Nanjing, 210007, China
b China Huayin Ordnance Test Center, Huayin, 714200, China
c 31104 Troop of PLA, Nanjing, 210001, China
Keywords:PTFE/Al/MgH2 Mechanical properties Thermal behavior Reaction energy Ignition threshold
ABSTRACTMagnesium hydride(MgH2)was doped into PTFE/Al to improve the energy release characteristics of the material system and strive for better application in military engineering.Five types of PTFE/Al/MgH2 reactive materials with different MgH2 content were prepared by molding sintering method.The dynamic mechanical properties of the materials were studied by performing split-Hopkinson pressure bar(SHPB)tests and scanning electron microscope characterizations.The thermal behavior,reaction energy,reaction process and reaction mechanism were systematically investigated by conducting thermogravimetry-differential scanning calorimetry tests, oxygen bomb calorimeter measurements, Xray diffraction and SHPB tests.The results show that MgH2 particles less than 10% content contribute to heightening the dynamic mechanical properties of PTFE/Al system.The product Mg generated by decomposition of MgH2 can not only react with gas phase C2F4+ but also undergo a Grignard-type reaction with condensed PTFE.The reaction energy and ignition threshold of PTFE/Al/MgH2 reactive materials enhance monotonously as MgH2 content rose.With the increase of MgH2 content from 0%to 20%,the reaction time is prolonged as well as the reaction intensity is enhanced dramatically arising from the massive water vapour produced by the reaction between O2 and H2.The gaseous products generated can form a high pressure shortly after the reaction, which helps to elevate the damage effect of the PTFE/Al system.
1.Introduction
Fluoropolymer-matrix reactive materials (RMs) are promising energetic composite as a kind of impact-initiated reactive materials, which are composed by polymer with high fluorine content and active metal particles [1].Among all fluoropolymer-matrix RMs, polytetrafluoroethylene/aluminum (PTFE/Al) is the most representative and has been widely studied owing to its excellent mechanical properties, distinctive impact-induced reaction characteristics and outstanding workability [2,3].Different from the traditional metal fragments such as steel and tungsten alloy which rely on a single kinetic energy to hit the target, the reactive fragments made of PTFE/Al RMs can rapidly undergo violent exothermic reaction and give rise to combustion or explosion effects when they impact through the target at high speed, accompanied by the release of a large amount of chemical energy,bringing about multiple destructive effects containing direct kinetic energy strike,over temperature,overpressure and incendiary effect [4,5].
In recent years, extensive experimental researches have been conducted on the mechanical properties and reaction behavior of PTFE/Al for favourable application in the field of new warhead damage.The purpose of these studies is to ensure the warhead made of such material has enough strength to penetrate the target plate and possesses high energy releasing level inside the target,so as to result in sufficient damage aftereffects[6,7].It is also required that the material maintains insensitive adequately during the service treatment so that it will not ignite accidently in the process of production,machining,transportation and storage.However,it was reported that PTFE/Al specimens could react drastically under quasi-static compression [8,9], which makes the safety during production and service of PTFE/Al more concerned.Moreover, the relatively low density and strength of PTFE/Al critically place restrictions on its penetration ability and military application.In light of the outstanding tunable reactivity of fluoropolymer-matrix RMs,the mechanical properties and reactive characteristics of the materials can be purposefully adjusted by adding different types of functional fillers into PTFE/Al reactive material.Typical fillers include metals(W,Ni,Mg,Ta)[10-12],metal oxides(FeO,MnO,MoO, BiO, CuO) [13-15]and ceramic particles (SiC, AlO) [16].Zhou et al.[11]observed that the addition of W could slightly increase the reaction efficiency and diminish the overall chemical energy and destructive effect of PTFE/Al system.Lan et al.[14]compared the impact of FeO, MoOand BiOon reaction characteristics of PTFE/Al reactive material.It was found that BiOwas the most effective oxidant in improving the reaction efficiency and acted out the best penetration performance arising from its highest gas production rate.Wu et al.[16]discovered that SiC and AlOhah a significantly positive influence on reinforcing the strength of PTFE/Al and the strengthening effect of SiC was stronger than that of AlO, but inevitably reduced the reaction energy since SiC and microscale AlOdid not participate in the reaction.
The ideal functional fillers should be capable of enhancing the strength of the material while insuring high energy release level when applied in military engineering.Magnesium hydride(MgH)is a newfashioned energetic material with high hydrogen storage capacity (hydrogen content 7.7%).Previously, MgHwas generally introduced into conventional explosives and propellants to heighten the explosion heat and depress mechanical sensitivity[17-19].Considering that the decomposition of MgHcan produce Hand Mg,the reaction of Hand Ocan release substantial energy and produce a large quantity of water vapour, which helps to engender high pressure.Meanwhile, it was believed that addition of Mg contributed to accelerating the reaction rate and increasing the extent of reaction of PTFE/Al system [20].Accordingly, MgHhas great potential as an additive to improve the energy release characteristics and destructive effect of PTFE/Al.
In this work,five types of PTFE/Al/MgHreactive materials with different MgHcontent were prepared by molding sintering method.The dynamic mechanical properties of the materials were studied by performing split-Hopkinson pressure bar (SHPB) tests and scanning electron microscope (SEM) characterizations.The thermal behavior, reaction energy, reaction process and reaction mechanism were systematically investigated by conducting thermogravimetry-differential scanning calorimetry (TG-DSC)tests, oxygen bomb calorimeter measurements, X-ray diffraction(XRD) and SHPB tests.The positive influence of doping MgHon improving the energy release characteristics of PTFE/Al was ascertained.
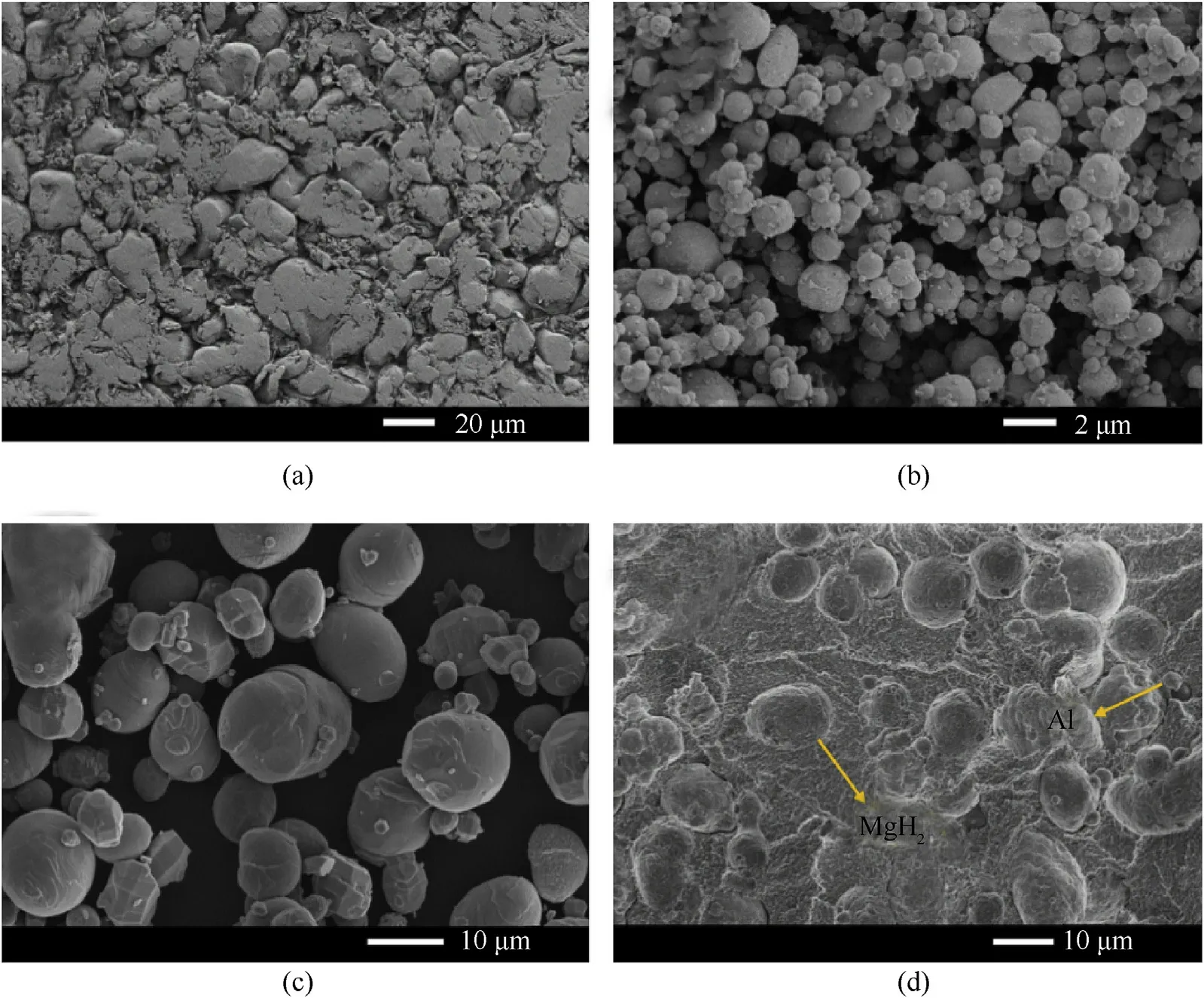
Fig.1.The initial microstructures of raw powders and PAM-10 specimen after sintering.(a) PTFE; (b) Al; (c) MgH2; (d) PAM-10 specimen after sintering.
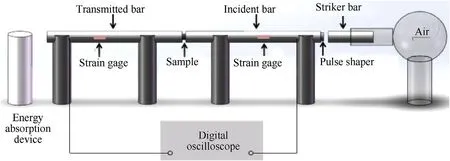
Fig.2.Schematic diagram of SHPB test.
2.Experimental setup
2.1.Specimens preparation
All Initial powders used in this study are commercially available.The mass fractions of MgHwere 0, 5%,10%, 20% and 30% respectively.The ratio of Al to PTFE in the remaining component was in accordance with the stoichiometric equilibrium (26.5%/73.5%).Table 1 tabulates the formulations of five types of PTFE/Al/MgHreactive materials, along with the corresponding theoretical maximum density (TMD).The preparation process was an application of Nielson’s work, which included mixing, cold isostatic pressing and vacuum sintering [21].All raw powders were suspended in a hexane solution and stirred mechanically for 20 min,then dried for 48 h at 60C in a vacuum oven and cold pressed by hydraulic press under a compressive pressure of 300 MPa to fabricate cylindrical specimens with size of φ10 mm × 3 mm for SHPB tests.Finally, the shaped specimens were sintered to 360C for 6 h in a vacuum oven with a heating rate of 90C/h and a cooling rate of 50C/h.The mixed powders without pressing and sintering were used for synchronous thermal analysis and reaction energy measurements.

Table 1Formulations and TMD of five types of PTFE/Al/MgH2 reactive materials.
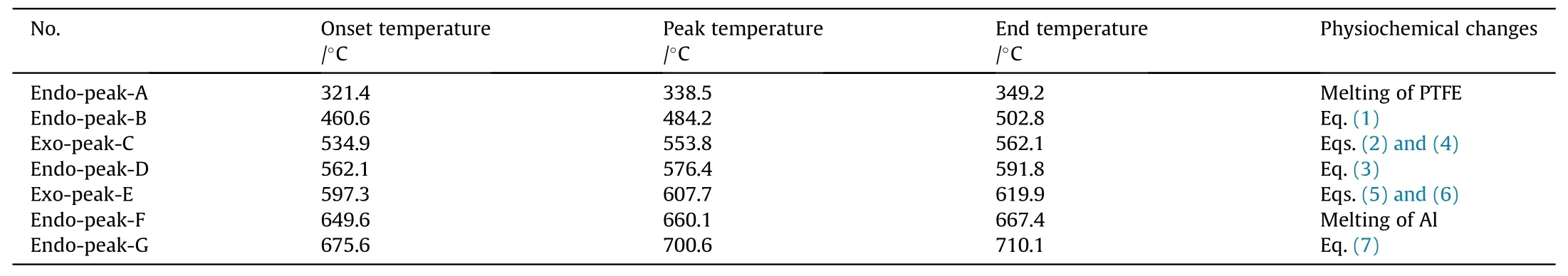
Table 2The specific parameters and corresponding physiochemical changes of each endothermic and exothermic peaks.
Fig.1 presents the initial microstructures of raw powders and PAM-10 specimen after sintering.It can be found that PTFE is soft and irregular with an average size of 20 μm,Al and MgHparticles take on spherical geometry with an average size of 1 μm and 10 μm.After sintering, Al and MgHcan be well wrapped and uniformly distributed in PTFE matrix.
2.2.Synchronous thermal analysis
The thermal decomposition and reaction process of pure MgHpowder, PA and PAM-10 samples were investigated by a TG-DSC simultaneous thermal analyzer (NETZSCH-STA449C, NETZSCH,Bavaria,Germany).The samples were placed into the crucible with a heating rate of 10C/min,covering the temperature from 100C to 800C.The tests were performed in the high-purity argon atmosphere with a flow rate of 30 mL/min to prevent air frominvolving in the reaction.Gas products produced during the tests were introduced into the mass spectrometer (MS, NETZSCHQMS403C, NETZSCH, Bavaria, Germany) through a capillary tube to determine the components of the gas products and the change with temperature.The reaction residues of the samples heated at different temperatures were collected and characterized based on the X-ray diffraction (XRD, Bruker D8 ADVANCE, Bruker, Berlin,Germany).
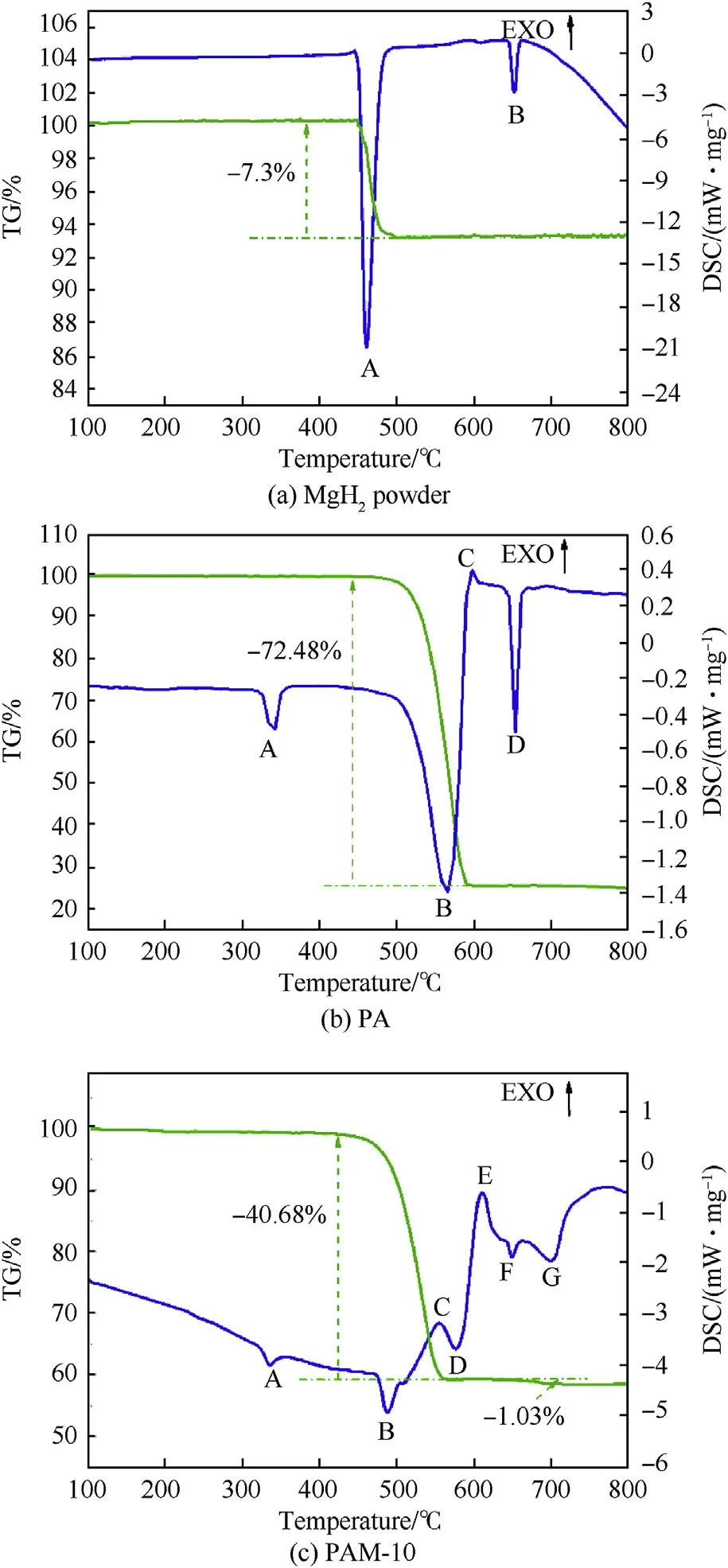
Fig.3.The TG-DSC curves of three samples.

Fig.4.The XRD patterns of PTFE/Al/MgH2 solid products at different temperatures.
2.3.Reaction energy measurements
An adiabatic bomb calorimeter (Parr 6300, Parr Inc., Moline, IL,USA)was adopted to measure the reaction energies of five types of PTFE/Al/MgHreactive materials under a highly pure oxygen atmosphere.The heat capacity of the calorimeter was calibrated by burning benzoic acid in an oxygen environment, the pressure of which was set to 3 MPa.
2.4.SHPB tests
A split Hopkinson pressure bar (SHPB) system (AOC, Hefei,China) was employed to study the influence of MgHcontent on dynamic mechanical properties and impact ignition characteristics of the materials system, as shown in Fig.2.Considering the low mechanical impedance of PTFE/Al, 20-mm-diameter aluminum bars were applied to obtain a high signal-to-noise ratio and guarantee that the one-dimensional stress wave was valid in compression bar.A rubber pulse shaper with the diameter of 10 mm and thickness of 1 mm was used to prolong the rising front time and achieve the stress equilibrium state.To probe into the impact ignition characteristics of the materials under high strain rate,all aluminum bars were superseded by steel bars.The reaction processes of five types of PTFE/Al/MgHreactive materials under impact were captured by a digital high-speed camera with a frame rate of 20,000 frames per s.
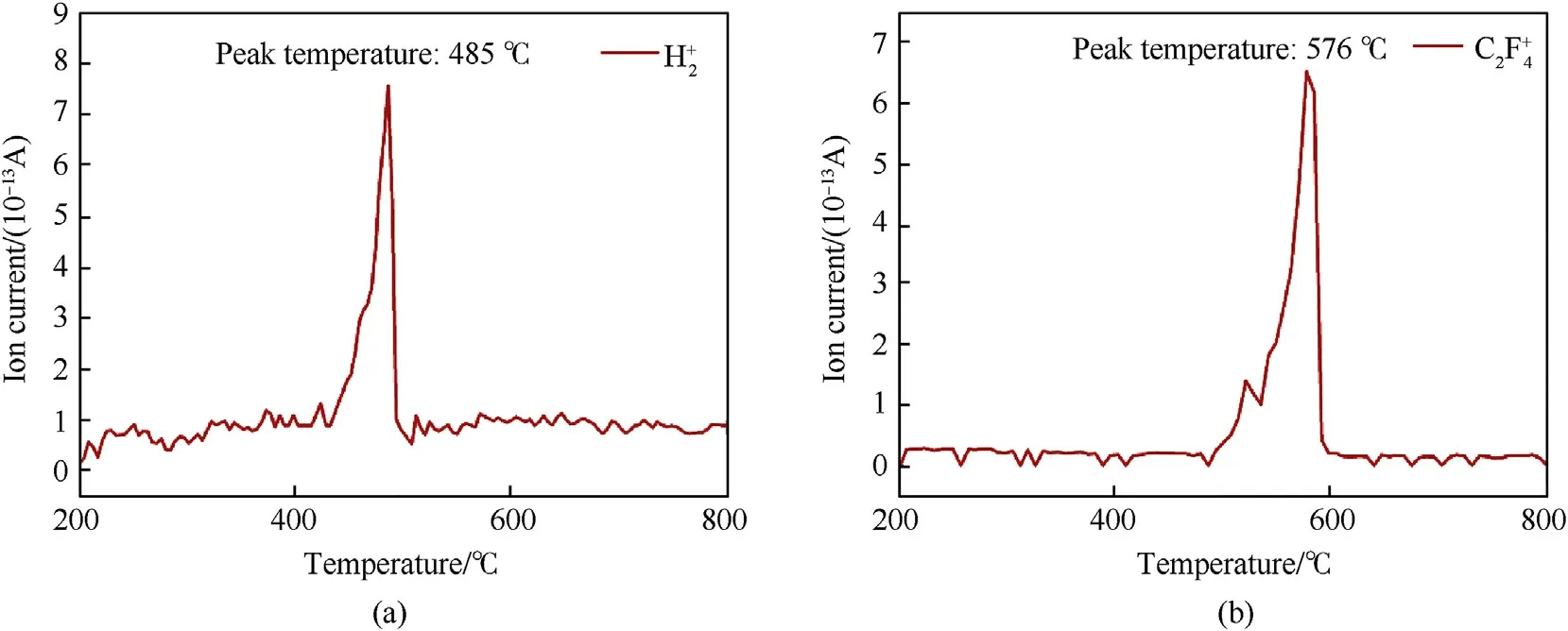
Fig.5.The gasous product mass spectrometry of PTFE/Al/MgH2 sample.(a) H2+; (b) C2F4+.
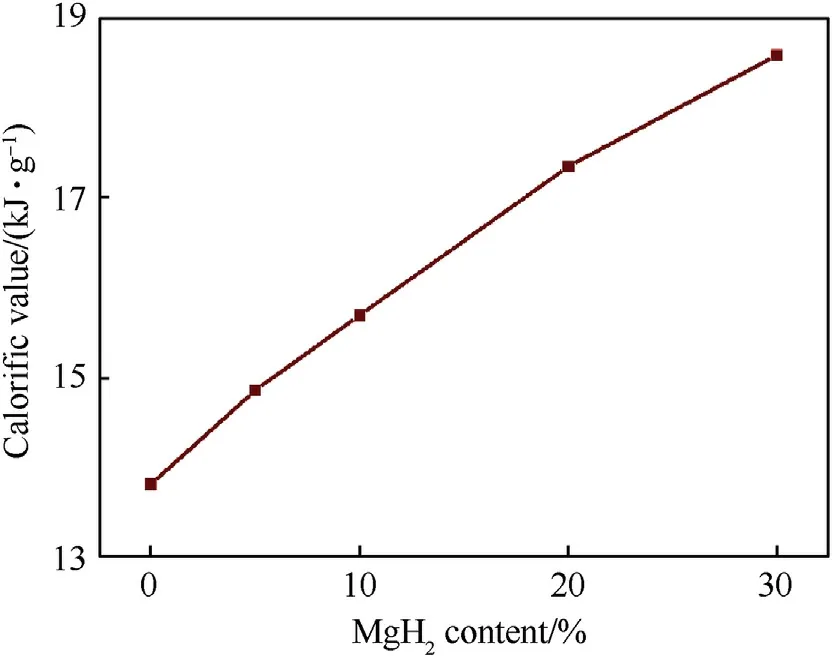
Fig.6.Reaction energies of PTFE/Al/MgH2 reactive materials versus MgH2 content.
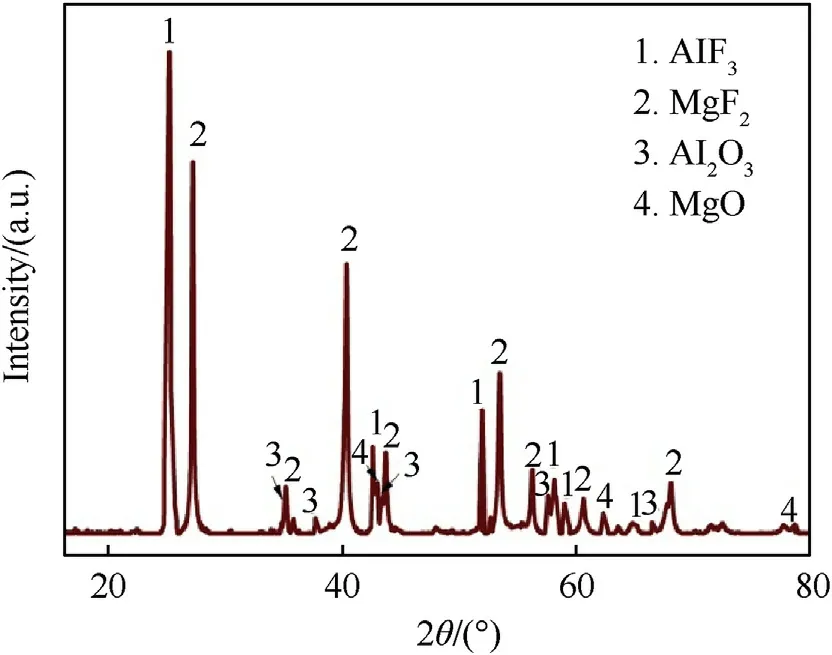
Fig.7.The XRD patterns of solid products after PAM-10 material burning under O2 atmosphere.
3.Results and discussion
3.1.Thermal behavior under TG-DSC tests
Fig.3a shows the TG-DSC curves of MgHpowder.The endothermic peak A appears apparently between 447C and 487C in the DSC curve, along with a 7.3% decrease in sample quality, indicating that the dehydrogenation reaction of MgHhas occurred at this stage.When the temperature continues to rise to around 650C, there is an endothermic peak B in the DSC curve, which corresponds to the melting point of Mg [22].
The TG-DSC curves of PTFE/Al are depicted in Fig.3b.It can be determined that the endothermic peak A is the melting endothermic peak of PTFE and the endothermic peak D is the melting endothermic peak of unreacted Al powder because of no quality change in the TG curve.The endothermic peak B covers a temperature range from 508.9C to 570.0C, where the reduction of sample mass shows up in the TG curve,bespeaking the generation of gases.It resulted from the decomposition of PTFE matrix.The fluoride ions produced from condensed PTFE can have a violent exothermic reaction with Al powder from 598.1C, leading to the formation of the exothermic peak C in the DSC curve.
Fig.3c presents the TG-DSC curves of PTFE/Al/MgHreactive material linearly heated to 800C.There are five endothermic peaks and two exothermic peak in the DSC curve.The specific parameters and corresponding physiochemical changes of each endothermic and exothermic peaks are summarized in Table 2.The XRD patterns of PTFE/Al/MgHsolid products at different temperatures are shown in Fig.4.
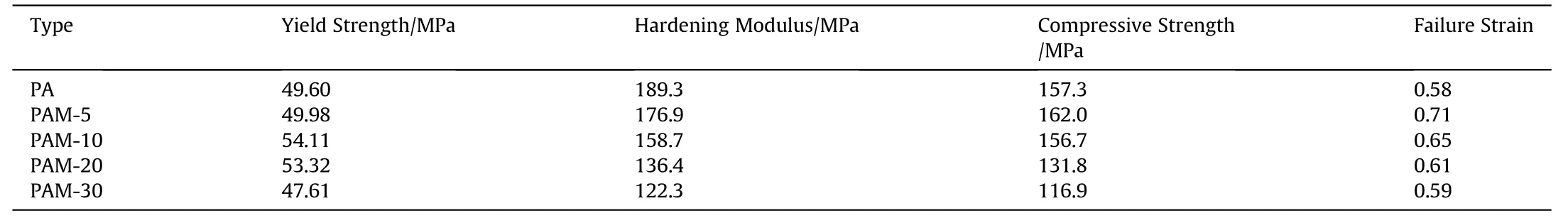
Table 3Mechanical parameters of five types of PTFE/Al/MgH2 reactive materials at the strain rate of about 3200 s-1.
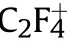
Below 600C:
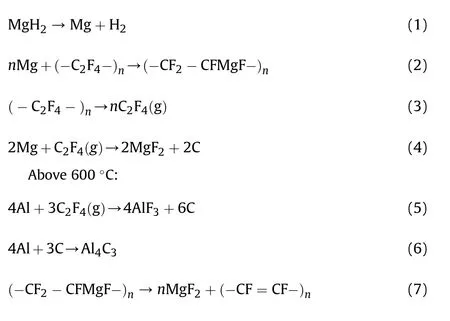
3.2.Reaction energy
Fig.6 shows the reaction energy per unit mass of PTFE/Al/MgHreactive materials as a function of MgHcontent under an oxygen atmosphere.As can be seen, the reaction energy of PTFE/Al/MgHreactive materials improve monotonously with the increase of MgHcontent,manifesting MgHplays a positive effect on the total release energy of PTFE/Al system.The reaction energy of PAM-30 material is determined experimentally to be 18.56 kJ/g, which is 34.4%higher than that of PTFE/Al(13.81 kJ/g).This is due to the fact that Hand Mg produced from the dehydrogenation reaction of MgHcan undergo exothermic reactions with O.Fig.7 presents the XRD patterns of solid products after PAM-10 material burning under Oatmosphere.In addition to the presence of AlFand MgF,AlOand MgO are also detected.
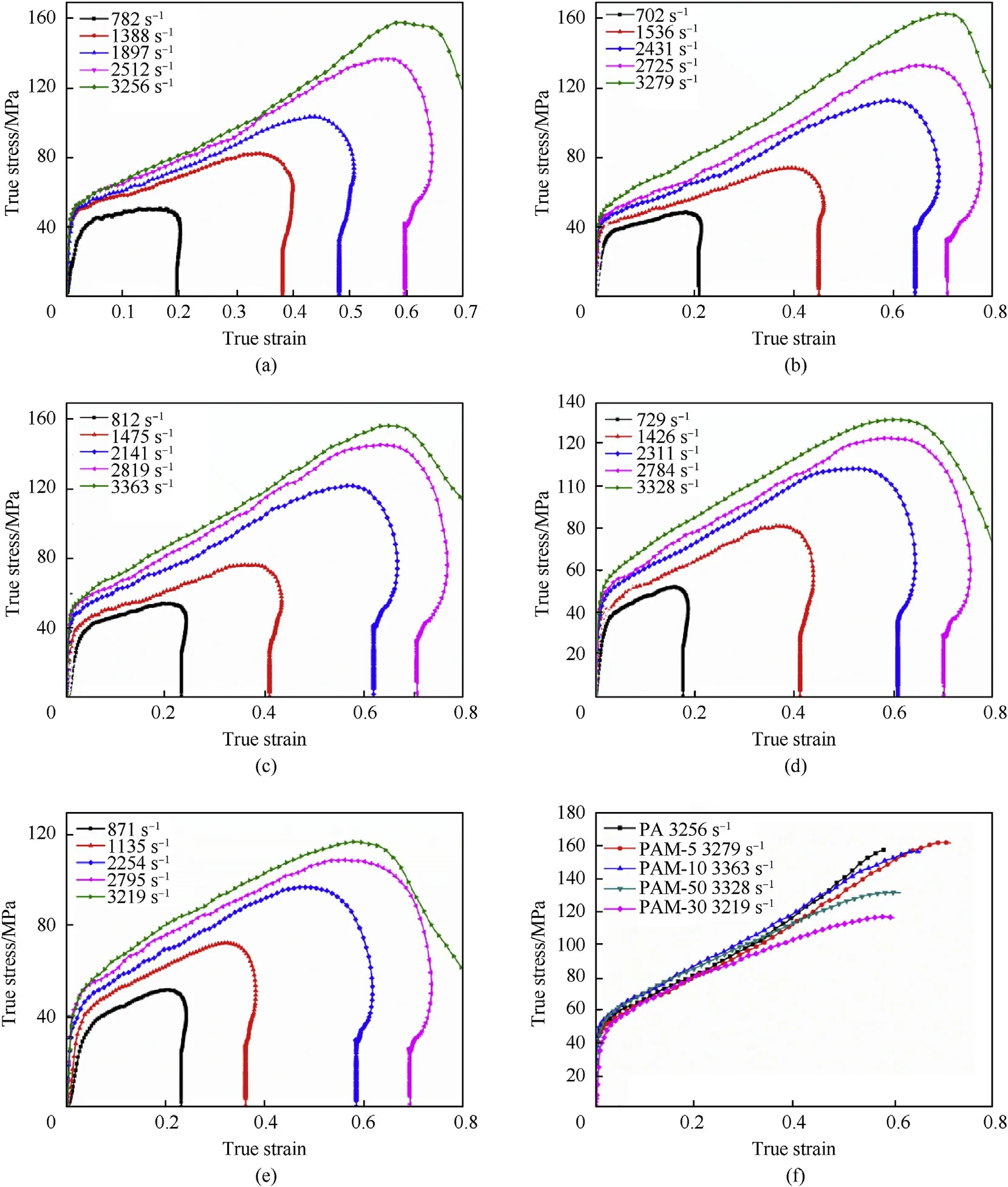
Fig.8.The true stress-strain curves of five types of PTFE/Al/MgH2 specimens under SHPB tests.(a)PA;(b)PAM-5;(c)PAM-10;(d)PAM-20;(e)PAM-30;(f)comparison at the strain rate of about 3200 s-1.
3.3.Dynamic mechanical properties under SHPB tests
The dynamic mechanical properties of five types of PTFE/Al/MgHreactive materials are determined at different strain rates in virtue of SHPB tests,as shown in Fig.8.Prominent strain hardening and strain rate hardening effects can be observed among all PTFE/Al/MgHreactive materials.The yield strength and hardening modulus of the materials take on an upward tendency with the strain rates increasing from 700 sto 3000 s.Fig.8f compares the true stress-strain curves of five types reactive materials at the strain rate of about 3200 s,corresponding mechanical parameters are calculated in Table 3.PAM-5 specimen has the highest compressive strength and PAM-10 specimen possesses the highest yield strength among all types of specimens, indicating that a proper amount of MgHparticles contribute to heightening the dynamic mechanical properties of PTFE/Al system.As the content of MgHexceeds 20%,MgHplays a negative influence on mechanical performances of materials.
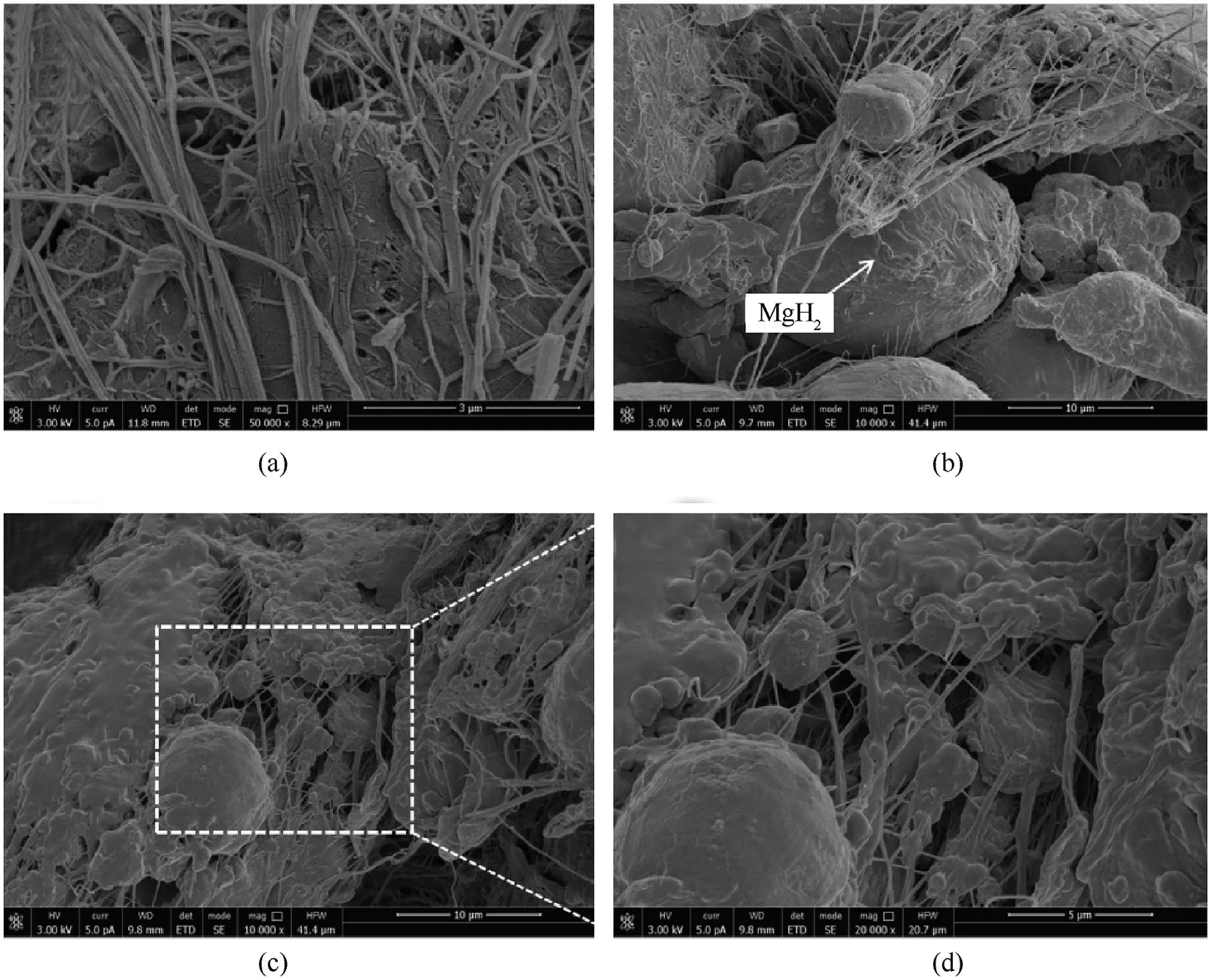
Fig.9.The microstructures of compression surface and crack fracture surface of PAM-10 specimen after SHPB test: (a) PTFE cracks and fibers in compression surface; (b) the debonding between PTFE and filler particles; (c) and (d) “pimple” morphology of PTFE.

Fig.10.Experimental data and ignition threshold of five types of PTFE/Al/MgH2 reactive materials.
To anatomize the relationship between the microstructures and dynamic mechanical performances of PTFE/Al/MgHreactive materials.The compression surface and crack fracture surface of recovered PAM-10 specimen at the strain rate of about 3200 sare characterized by SEM, as presented in Fig.9.Under dynamic loading,PTFE matrix cracks(Fig.9a)and debonding between PTFE and filler particles(Fig.9b)appear within PTFE/Al/MgHspecimen.The fracture of matrix and separation of filler particles from matrix are two major meso-scale mechanisms for failure [26].The compression upon specimen results in a large amount of local deformation in the form of fibers.The stable fibers nucleated from the stress concentration point and formed in the direction of the main stress are conducive to dispersing energy and passivating the crack tip by bridging,thus preventing the crack propagation[27].A“pimple”morphology can be distinctly observed in Fig.9(c)and 9(d).This is because the crack rapidly extend under the action of impact,and the crack tip can form a local hot spot in a short period of time [28],causing PTFE in a molten flow state.With the further increase of impact energy, the ignition reaction of specimens will be activated when the temperature of local hot spot in the crack tip reaches ignition temperature.
3.4.Impact ignition reaction under high strain rate
The input energy during the impact is too faint to excite the ignition reaction of PTFE/Al/MgHspecimens arising from the low density of the aluminum bars.For the sake of exploring the impact ignition characteristics of the materials under high strain rate,steel bars are applied in the SHPB tests instead of aluminum bars.The impact speed of the striker bar is determined by a laser velocimeter.An analogical characteristic drop method [29]is used to characterize the sensitivity of the specimens under the critical impact speed, which is defined as the ignition threshold.Experimental data recorded and ignition threshold of five types of PTFE/Al/MgHreactive materials are given in Fig.10.The ignition threshold increases monotonously as the content of MgHrose, revealing that the addition of MgHparticles has a negative effect on improving the impact sensitivity of PTFE/Al.The ignition threshold of PAM-30 is determined to be 29.91 m/s, which is 27.4% higher than that of PTFE/Al (23.47 m/s).Note that it is extremely significant for the stability evaluation of reactive materials in the process of production, machining, transportation and storage.

Fig.11.Reaction processes of five types of PTFE/Al/MgH2 specimens at impact speed of 32.04 m/s (a)PA; (b)PAM-5; (c) PAM-10; (d) PAM-20; (e)PAM-30; (f) binarized images of PAM-20 specimen after Matlab processing.
Fig.11 superimposes the reaction processes of five types of PTFE/Al/MgHspecimens at impact speed of 32.04 m/s(maximum speed of SHPB test)recorded by the high-speed camera.Matlab software is employed to analyze the high-speed photography sequences.The relative flame intensity, which is defined as the ratio of the instantaneous flame area to maximum flame area, is calculated to quantify the reaction intensity.Reaction time is defined as the duration from the ignition time to the quenching time, corresponding to the moment when the relative flame intensity decreases to 10% of the maximum point.The binarized images of PAM-20 specimen after Matlab processing are presented in Fig.11f as an example.Fig.12 shows the curves of relative flame intensity versus reaction time of five types of PTFE/Al/MgHspecimens.It can be seen that with the increase of MgHcontent from 0% to 20%, the reaction time is prolonged, as well as the reaction intensity of the materials is enhanced dramatically, which is consistent with the reaction energy measurement tendency.Different from the flame phenomenon of PA specimen, discontinuous and scattered drop-like sparks ejected from the reaction area can be observed for PAM-20 specimen.These difference are the reflection of massive water vapour produced by the reaction between Oand H.The gaseous products generated can form a high pressure shortly after the reaction[30],which elevates the damage effect of the PTFE/Al system.The ignition threshold of PAM-30 is 29.91 m/s, and the input energy is still insufficient when the specimen is loaded at the impact speed of 32.04 m/s.The chemical reaction of PTFE-based reactive materials is a typical non-selfsustaining reaction.Wang [5]reported that the fracture of reactive materials is of importance to the impact-induced reaction.The large reactive debris generated from fracture require more ignition energy, and may burn without deflagration or even quench when size is large enough.Zheng [31]observed that the deflagration behavior of reactive material fragment depends on impact velocity,target material and thickness.Based on these facts, it can be inferred that the insufficient impact velocity leads to the formation of large reactive debris for PAM-30.Therefore, the reaction intensity of PAM-30 specimen is the weakest, and the reaction duration only lasts about 600 μs.
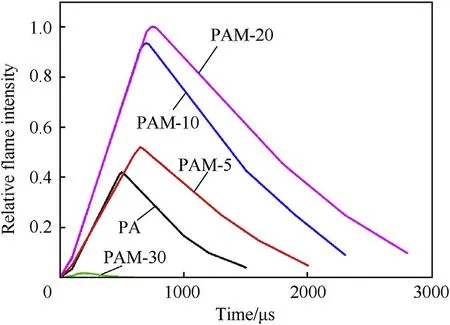
Fig.12.Relative flame intensity versus reaction time of five types of PTFE/Al/MgH2 specimens.
4.Conclusions
In this work,five types of PTFE/Al/MgHreactive materials with different MgHcontent were prepared.The dynamic mechanical properties and reactive characteristics of the materials were systematically investigated through carrying out SHPB tests, TG-DSC tests and oxygen bomb calorimeter measurements.The positive influence of doping MgHon improving the energy release characteristics of PTFE/Al was ascertained.The conclusions can be drawn as follows:
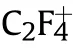
(2) The yield strength and hardening modulus of PTFE/Al/MgHreactive materials take on an upward tendency with the strain rates increasing from 700 sto 3000 s.A relatively low amount of MgHparticles contribute to heightening the dynamic mechanical properties of PTFE/Al system.As the content of MgHexceeds 20%, MgHplays a negative influence on mechanical performances of materials.
(3) The addition of MgHparticles has a negative effect on improving the impact sensitivity of PTFE/Al.With the increase of MgHcontent from 0% to 20%, the reaction time is prolonged as well as the reaction intensity of the materials is enhanced dramatically arising from the massive water vapour produced by the reaction between Oand H.The gaseous products generated can form a high pressure shortly after the reaction, which helps to elevate the damage effect of the PTFE/Al system.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
The financial support from the National Natural Science Foundation of China (General Program.Grant No.51673213) and National Natural Science Foundation of China (Grant No.51803235)are gratefully acknowledged.
杂志排行
Defence Technology的其它文章
- Effect prediction of stiffened-ring cylindrical shells subjected to drop mass impact
- Study on the influence of armature on the efficiency of reluctance accelerator
- Research on a combinatorial control method for coaxial rotor aircraft based on sliding mode
- An optimization method for passive muzzle arc control devices in augmented railguns
- Numerical and experimental investigation on aluminum 6061-Vgrooved stainless steel 304 explosive cladding
- A capture probability analytic model for the electromagnetic launched anti-torpedo torpedo
