Cu-X (X=B,Al,Ga,In)共掺杂对ZnS可见光吸收的影响
2022-03-04薛丽丽王卓群田列远
薛丽丽,王卓群,王 伟,田列远,王 强
(1.山东省产品质量检验研究院,济南 250102; 2.山东大学 微电子学院,济南 250101)
1 Introduction
As a kind of common semiconductor material,ZnS has been widely used in wastewater degradation,light-emitting diode,solar cell,semiconductor heterojunction,photocatalytic hydrogen production,et al.,because of its stable chemical properties,low cost and good semiconductor properties[1-5].There are two structures of ZnS: one is sphalerite structure,and the other is wurtzite structure.Under normal temperature,ZnS with sphalerite structure is more stable than wurtzite one.However,the key problem of sphalerite ZnS is the large band gap[6],which can only absorbs a small part of the ultraviolet light.In addition,the high recombination rate of photo generated carriers ( including electrons and holes) restricts the photocatalytic efficiency[7].Therefore,optimizing the structure of ZnS,and reducing the recombination rate of photocarriers are the hot issues.
Doping is recognized as one of the effective methods to adjust the band gap width.At present,many kinds of metal and non-metal doped ZnS have been studied.Tawfik[8]found that Cu can increase the dielectric constant and photoconductivity of ZnS,Huang[9]found that the reductions of band gap width of ZnS are obvious when ⅣA group elements are doped.Although doping can adjust the band gap width,it is easy to form recombination center or local state.This situation also exists in other systems,such as ZnO,TiO2[10],and so on.Co -doping can solve this problem efficiency,and has been confirmed by a large number of experiments[11,12].Because the transition metals are easy to produce impurity state in the band gap,they have a significant effect on the optical properties of semiconductor materials[13].Lee[14]found that the photocatalytic hydrogen production efficient of Cu and Co co-doped ZnS is higher than that of single doping.Prasad[15]found that Cu can enhance the absorption of visible light of ZnS.Bansal[16]found that Mn2+and Cu2+co -doped ZnS has excellent visible light absorption properties.Nikzad[17]found that ZnS with 10% Cu has the the largest absorption spectrum of visible light.Li[18]calculated Cu and C co - doping rutile TiO2and found it can improve the photocatalytic activity.Yousef[19]found that Cu - S co - doping can enhance the absorption of visible light of TiO2nanoparticles significantly.In recent years,optical absorption properties of B[20],Al[21,22],Ga[23,24]and In[25]doped ZnS have been researched.However,except Cu - In[26]and Cu-Al[27],other systems such as Cu-B,Cu-Ga coped ZnS have not been researched.Therefore,based on the first principle of density functional theory( DFT) ,the influences of Cu co-doping with B,Al,Ga,In on the optical properties of ZnS will be studied,which will provide a theoretical basis for the design of new photocatalytic materials.
2 Calculation method
In this paper,all of the DFT ( Density Functional Theory) calculations were performed using the PAW ( Projector Augmented Wave) ultrasoft pseudo potentials[27].The GGA ( generalized gradient approximation) of PBE ( Perdew - Burke - Ernzerhof)parameterization[28,29]was chosen to describe the exchange correlation function.The cutoff energy of plane wave is 400 eV; MP ( monkhorst pack) k -point network densities are 4 ×4 ×1,and single atom energy is less than 1.0 ×10-5eV/atom.The space group of sphalerite ZnS is F4 -3m,which belongs to cubic system.Fig.1 ( a) shows the structure of sphalerite ZnS.In order to verify the validity of the calculation method,the ZnS cell of sphalerite was optimized and the lattice constant a=b=c=0.5424 nm was obtained.In other papers,the lattice constant of ZnS calculated by La[31]is a=b=c=0.542 nm.It could be seen that the disparity between this paper and those obtained by other methods is very little,therefore,our calculation method is very reliable.The calculated band gap width is 2.9 eV,which is less than the experimental data.This is mainly due to the fact that the band gap width of the strongly correlated system calculated by DFT is usually narrow[33].However,this will not affect the analysis of opticalproperties.Fig.1( b) is calculation model of Cu -X( X=B,Al,Ga,In) co-doped ZnS.
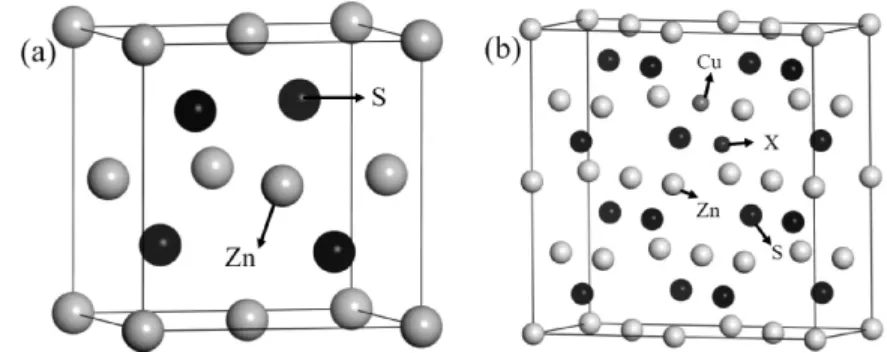
Fig.1 Models of calculation
3 Results and discussion
3.1 Binding energy
The energy releasedfor atoms combining into molecules is called chemical binding energy.If the binding energies are negative,they indicate that the binding processes need to release heat,so the structures are stable,otherwise are unstable.The binding energy can be derived from the following formula[33-34].

Ebis the binding energy; Edopedis the total energy of ZnS after doping.ECu,EX,EZnand ESare the energies of free Cu,X ( X=B,Al,Ga,In) ,Zn and S atoms respectively.The n and m are the number of Zn and S atoms,respectively.Fig.2 is the calculated binding energy of Cu-X ( X=B,Al,Ga,In) co-doped ZnS.It can be seen that the binding energies of Cu - X ( X=B,Al,Ga,In) co - doped sphalerite ZnS are - 2.213 eV, - 2.154 eV,- 1.835 eV,- 1.743 eV,respectively.After doping,the binding energies are all negative,so all of the structures are stable.Besides,the binding energies tend to increase with the increase of atomic content,which is mainly due to the lattice distortion caused by the atomic radius.

Fig.2 Binding energies of Cu -X ( X=B,Al,Ga,In) co-doped ZnS
3.2 Density of state (DOS)
Fig.3 is the calculated density of states of Cu-X ( X=B,Al,Ga,In) co - doped ZnS.Fig.3( a) are the DOSs of pure ZnS.It can be seen from Fig.3 ( a) that valence band of ZnS is mainly composed of 3p state of Zn,3p state of S,and a small amount of 3d state electrons of Zn,while the conduction band is mainly composed of 3p state,4s state and 3p state of S.Fig.3 ( b) shows the DOSs of Cu -B co-doped ZnS.It can be seen that Cu and B doping will split new energy levels at the top of the valence band.Furthermore,due to the Cu-3d state and S-3p in the band gap,p-d hybrid orbit is formed.The hybrid orbit in the band gap can promote the transition of electrons: electrons can first transit from the valence band top to the hybrid state,and then to the conduction band.Therefore,it is beneficial to the absorption of visible light.Moreover,the hybrid state in the band gap can effectively prevents the recombination of photocarriers ( including electrons and holes)and improve the absorption efficiency of visible light.Besides,the valence electrons of Cu and B,as the outer layers of doped atoms,overlap with those of Zn and S atoms.According to the solid - state physics theory,the electron overlap leads to the band broadening.Therefore,compared with pure ZnS,the band widths after doping are getting wider.Therefore,the bottom of the conduction band shifts to the low energy level,resulting in the decrease of band gap from 2.9 eV to 2.68 eV.For Cu-Al,Cu-Ga and Cu-In co-doped ZnS,the valence band tops have split into new isolated energy levels,forming a series of new valence band tops.At the same time,the conduction band bottoms continue to expand towards the lower energy level and form a series of new conduction band bottoms.The band gaps continue to decrease to 2.41 eV,2.18 eV and 1.82 eV.
3.3 Absorption coefficient
Fig.4 shows the absorptioncoefficients of Cu-X( X=B,Al,Ga,In) co-doped ZnS.It can be seen that the pure ZnS hardly absorbs visible light ( 1.64-3.19 eV) ,which is consistent with the experimental conclusion.However,for B,Al,Ga,In doped ZnS,the absorption coefficients shift to the low energy level ( red shift).Especially,an absorption peak of 50 000 cm-1is generated near 2.5 eV after In doping.What’s more,for Cu-X( X=B,Al,Ga,In)co - doped ZnS,the absorption edges have entered into the visible light range,which implies that Cu-X( X=B,Al,Ga,In) co -doped ZnS are beneficial for the absorption of visible light.
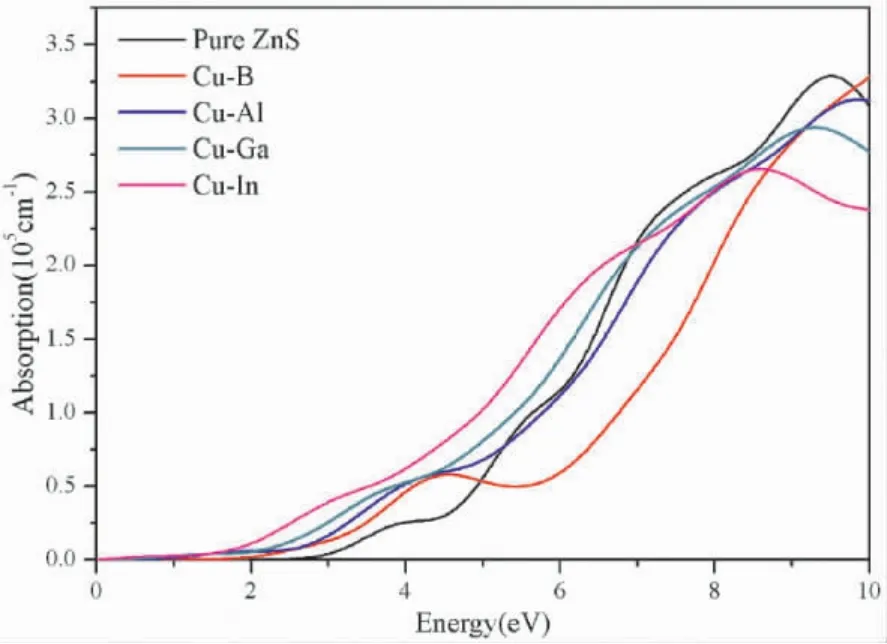
Fig.4 Absorption coefficients of Cu -X ( X=B,Al,Ga,In) co-doped ZnS
3.4 Band edge position
For semiconductors,the relationship between band edge position and redox reaction is very close.For the conditions of photodegradation water[35],the potential of the valence band should be higher than the oxidation potential of O2/H2O ( 1.23 eV vs.NHE) ,and the potential of the conduction band should be lower than the reduction potential of H+/ H2( 0 eV vs.NHE).The band edge position can be calculated by formula (2) and (3)[36],whereX is the average electronegativity of the studied system,Eeis the normal hydrogen potential ( NHE) of about 4.5 eV,and Egis the band gap width.
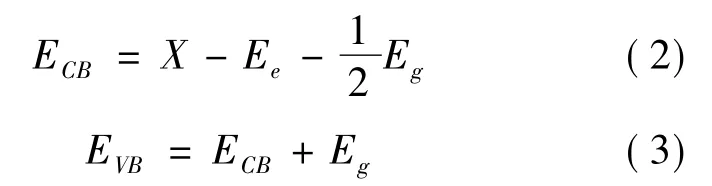
Fig.5 is the band edgepositions of Cu-X ( X=B,Al,Ga,In) co - doped ZnS.The results show that the conduction band edge positions of pure and Cu-X ( X=B,Al,Ga,In) co - doped ZnS are-1.08 eV,- 0.58 eV,- 0.56 eV,- 0.56 eV,- 0.56 eV,- 0.46 eV,respectively,while the valence band edges are 1.82 eV,2.1 eV,1.85 eV,0.56 eV,0.46 eV,respectively.After doping,the conduction band edges shift down slightly,and the valence band edges shift up except for Cu -B co -doping,which is conducive to the absorption of visible light.Although the positions of the conduction band edge shift down,all of these are less than the oxidation potential of O2/H2O,so they still have good oxidation performance.In addition,the valence band edge potentials are greater than the H+/ H2reduction potential,which implies that all of these have good reduction performance.In general,Cu-X ( X=B,Al,Ga,In) co-doped ZnS have good photocatalytic ability,which can be used to manufacture photocatalyst.
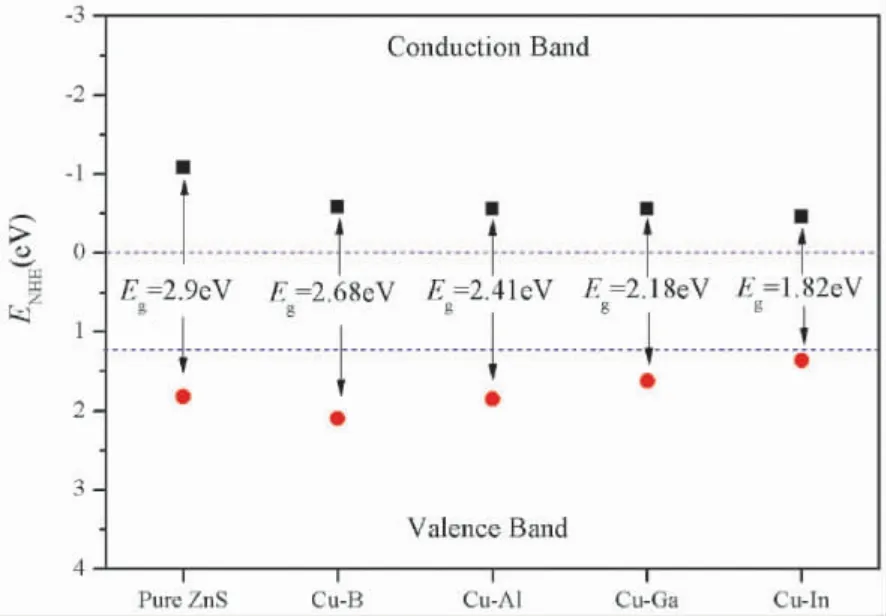
Fig.5 Band edge positions of Cu - X ( X=B,Al,Ga,In) co-doped ZnS
4 Conclusions
Theinfluences of Cu-X ( X=B,Al,Ga,In)co - dopings on the visible light absorption of ZnS were calculated by density functional theory.The conclusions are as follows.
( 1) The bindingenergies of Cu-X( X=B,Al,Ga,In) co -doped sphalerite ZnS are the negative,so the structures are stable.
(2) The band gapwidths of Cu - X ( X=B,Al,Ga,In) co-doped sphalerite ZnS decrease from 2.9 eV to 2.68 eV,2.41 eV,2.18 eV and 1.82 eV,respectively.In addition,the absorption coefficients shift to low energy level ( red shift) ,which were beneficial for the absorption of visible light.
(3) The band edge positions ofco - doped ZnS are suitable for water splitting to generate hydrogen,and will be candidate materials for water splitting driven by visible light.In general,Cu -X ( X=B,Al,Ga,In) co-doped ZnS are beneficial for the absorption of visible light.
