Functionalized Graphene Materials:Definition,Classification,and Preparation Strategies
2022-03-04YingjieMaLinjieZhi
Yingjie Ma ,Linjie Zhi
1 CAS Key Laboratory of Nanosystem and Hierarchical Fabrication,CAS Center for Excellence in Nanoscience,National Center for Nanoscience and Technology,Beijing 100190,China.
2 University of Chinese Academy of Sciences,Beijing 100049,China.
Abstract:Since its emergence in 2004,graphene has attracted enormous attention because of its unique and fantastic properties,which signals the birth of two-dimensional (2D) nanomaterials.The strictly atomic-layered 2D structure endows graphene with unconventional optical,electronic,magnetic,and mechanical properties.Owing to these extraordinary features,graphene has exhibited great potential in various fields,such as biology,medicine,chemistry,physics,and the environment.Notably,when graphene is used in these fields,it is always functionalized to facilitate its manipulation or meet the different area demands.After functionalization,the properties of graphene,such as its composition,size,shape,and structure,are modified,leading to changes in its electronic structure,surface chemistry,solubility,and mechanical and chemical properties.Functionalization of graphene can be achieved through various approaches,including chemical oxidation,doping,covalent and non-covalent modification,and hybridization with other materials,yielding various products (i.e.,graphene oxide,nano graphene,graphene nanoribbons (GNRs),graphene nanomeshes,and graphene-polymer hybrids).However,these resulting products have not been systematically classified or strictly defined until now; although they have been classified as covalent and non-covalent functionalized graphene,graphene-based polymer composites,and graphene-based composites.Systematic classification and exact definition will benefit research on functionalizing graphene.In this review,based on research on functionalization of graphene,we propose a systematic classification of the products from graphene functionalization,their corresponding definitions,and preparation strategies,which are illustrated by representative examples.All the products from graphene functionalization are defined as functionalized graphene materials,which fall into two categories:functionalized graphene and functionalized graphene composite.Functionalized graphene is the product of modifying graphene by tuning its composition,framework,dimension,and morphology,and functionalized graphene composites are hybrids of graphene (or functionalized graphene)with other materials,including small molecules,polymers,metals,inorganic compounds,and carbon nanotubes (CNTs).Functionalized graphene materials are prepared through two strategies:“top-down” and “bottom-up,” each of which has its advantages and shortcomings and includes many corresponding preparation methods.The selection of preparation strategies depends on the application requirements,as different applications require different types of graphene.Both strategies are elucidated with detailed examples through an extensive analysis of the literature.Finally,the major challenges and perspectives of functionalized graphene materials are discussed.This review presents the proposed systematic classification and exact definition of functionalized graphene materials,which can enhance their development.It is believed that functionalized graphene materials will achieve significant progress in the future.
Key Words:Graphene functionalization; Functionalized graphene materials; Classification; Definition;Preparation strategy
1 Introduction
Graphene is an allotrope of carbon,which is a single-layered hexagonal lattice made of sp2-hybridized carbon atoms.Each sp2-hybridized carbon atom connects its three neighboring carbon atoms by σ-bonds,and pz orbitals of all the sp2-hybridized carbon atoms form the delocalized network of π orbitals,giving a fully π conjugated 2D structure.The long-range 2D πconjugation in graphene endows it with extraordinary physical and chemical properties1.For instance,its delocalized π orbitals in which the π electrons are free to move throughout its 2D plane gives it the highest known electron conductivity(200000 cm2·V-1·s-1)2; the inherent strong σ bonds between each carbon atom give rise to outstanding thermal conductivity(~5000 W·m-1·K-1)3,and high Young’s modulus (0.1 TPa,fracture strength of 130 GPa)4; single atomic layer graphene has a physical thickness of ~0.35 nm,and thus possesses the theoretical specific surface area as high as 2630 cm2·g-15.Besides,graphene also has other remarkable properties such as the quantum Hall effect6and optical transparency with an absorption of approximately 2.3% toward visible light.In consequence,after it emerges in 20047,all these inherent properties enable graphene to have great potential for multiple applications in biomedical8,catalysis9,water desalination10,electrochemistry11,electronics12,energy storage13,environment14,fuel cells15,gas sorption and separation16,electromagnetic interference (EMI)17,optoelectronic devices18,etc.
In actual,it is difficult to produce large-area perfect graphene in bulk quantity as defects,wrinkles and domain boundaries are usually introduced when preparing graphene or fabricating graphene-based materials.Besides,it should be noted that graphene always needs to be functionalized by chemical or physical engineering to give “functionalized graphene materials”,and thus facilitate its manipulation and meet the demands of practical applications when it is applied in these areas19.It has been found that graphene cannot been dissolved in most solvents except for the solvents with surface tension close to 40-50 mJ·m-2,such as N,N-dimethylacetamide (DMA),g-butyrolactone,and 1,3-dimethyl-2-imidazolidinone20.In addition,isolated graphene sheets tend to reaggregate due to ππ stacking and van der Waals interactions between them,which also hinders manipulation of graphene.Consequently,graphene is always functionalized to improve its solubility.For example,graphene is oxidized into graphene oxide (GO) to make it soluble in aqueous solution21,and graphene is modified with organic groups to give it remarkable solubility in organic solvents19.On the other hand,graphene needs to be functionalized to acquire properties that can meet the demands of special applications.For instance,when graphene is used in transistors,functionalized graphene,such as GNRs22,graphene nanomesh23,and graphene quantum dots (GQDs)18,should be prepared to open a considerable and tunable bandgap as graphene does not have an intrinsic electronic bandgap24.Apparently,via functionalization,the features of graphene such as composition,size,shape,and structure can be modified,consequently tuning its electronic structure,solubility,mechanical and chemical properties,and even endowing it new properties.
Until now,much effort has been devoted to graphene functionalization,and many approaches have been applied to functionalize graphene,such as chemical oxidation25,heteroatom doping26,covalent and non-covalent modification27,and hybridizing with other materials28.As a result,various products of graphene functionalization have been fabricated,such as graphene oxide21,reduced graphene oxide29,GNRs30,graphene nanosheets31,graphene foam32,graphene membranes33,etc.Some products have been classified and defined1,34-37,for example,doped graphene38,graphene-based nanomaterials39,graphene-based materials9,graphene-based composite40,covalent and non-covalent graphene27.However,there are no systematic classification and definition for all these products from graphene functionalization,which will,obviously,benefit the research and development of graphene materials.
Therefore,this review will propose the systematic classification and exact definition of all the products from graphene functionalization,which are illustrated by several representative examples (Section 2).Additionally,the corresponding preparation strategies of functionalizing graphene will be summarized and be demonstrated by some typical approaches (Section 3).As shown in Fig.1,the classification,definitions,preparation strategies,and typical products of graphene functionalization are outlined.The review will be devoted to present the classification,definition,and main preparation strategies of graphene functionalization,instead of providing an exhaustive list of all the products and methods.
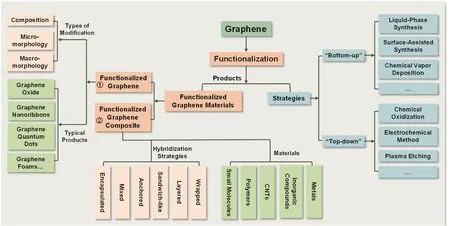
Fig.1 Graphene functionalization:classification,definition,and preparation strategies.
2 Definition and classification
2.1 Definition
“Functionalizing graphene” is to modify graphene by chemical or physical approaches or both to improve the properties of graphene or endow it with new ones,facilitating its manipulation or/and meeting the demands of applications.All the products of graphene functionalization are collectively called“functionalized graphene materials” and fall into two categories:“functionalized graphene” and “functionalized graphene composite”.
2.2 Functionalized graphene
Graphene is functionalized by modifying its composition,micromorphology (dimension,framework,and arrangement),and macromorphology,producing “functionalized graphene”.The representative samples of functionalized graphene are heteroatom doped graphene41,GNRs42,GQDs43,44,graphene nanomeshs45-48,bilayer graphene49-54,graphene fibers55,56,3D graphene sponge57,graphene oxide58,covalent functionalized graphene59.All of them are prepared through modifying one or more aspects of graphene,including composition,micromorphology or macromorphology.
2.2.1 Composition
Graphene consists of only carbon atoms,and its chemical and physical properties rely on the 2D hexagonal lattice formed by carbon atoms.It is known that graphene is a semimetal with zero bandgap arising from the long-range 2D π-conjugation.Thus,its electronic structures and properties will be tuned if its composition is changed via surface-transfer doping with functionalized groups or substitution doping with heteroatoms(Fig.2)60.
When functionalized groups are introduced into the graphene sheet via covalent27,these groups will donate or withdraw electrons from graphene,which depends on the electronegativities of these groups (Fig.2).Compared to carbon,heteroatoms,such as nitrogen (N)62,63,boron (B)64,oxygen(O)65,sulfur (S)66and phosphorus (P)26atoms,have different valence electrons and electronegativities.If the graphene is substituted with these heteroatoms (Fig.3b),electrons will be injected or extracted into or from graphene,causing a Fermi level lift and consequently producing n-type or p-type doped graphene (Fig.3a).After being doped with functionalized groups or heteroatoms,the lattice symmetry of graphene is broken,resulting in a gap between π and π* bands (Fig.3a) and converting graphene into a semiconductor.In addition,these heteroatoms in graphene usually serve as active sits for catalysis65or energy storage and conversion67,because they have different electronegativities and will create unbalanced charged areas.It should be noted that the 2D hexagonal lattice of graphene will be disturbs when graphene is doped with functionalized groups or heteroatoms,inducing defects or/and disorders which will decrease the electronic mobility of graphene.
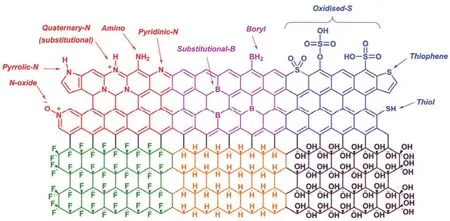
Fig.2 Illustration of the structure of heteroatom doped graphene.

Fig.3 (a) p-/n-type doped graphene; (b) illustration of heteroatom doped graphene.
2.2.2 Micromorphology
Graphene’s properties are also determined by its micromorphology,including dimension,carbon framework,and arrangement.In consequence,adjusting its micromorphology can also tune the properties,giving corresponding functionalized graphene.
Dimension:graphene’s electronic and optical properties can be tuned by adjusting its dimension due to quantum confinement,which can be observed when the dimension of graphene is of the same magnitude as the Bloch electron wavelength24.Graphene is a 2D semimetal with zero bandgap.However,if adjusting the dimension of graphene to produce one or zero-dimensional nanosized graphene,which are GNRs and GQDs (Fig.4)68,69,respectively,it will open graphene’s bandgap70.It has been reported that armchair GNRs with widths smaller than 10 nm serve as semiconductors with non-zero bandgaps,and larger bandgaps will be obtained when decreasing the width of GNRs71-73.A bandgap (~0.7 eV) comparable to that of germanium would be got via GNRs as narrow as 2-3 nm72.GQDs are graphene fragments with diameters below 20 nm,and thus have non-zero bandgaps and luminesce on excitation because of quantum confinement effects.Bandgaps of GQDs depend on the size and surface chemistry of GQDs.Theoretical calculation by density functional theory (DFT) shows that a bandgap of approximately 2 eV would be realized via a GQD consisting of 20 aromatic rings.Until now,as a main kind of functionalized graphene,a variety of GNRs and GQDs have been synthesized with different features.
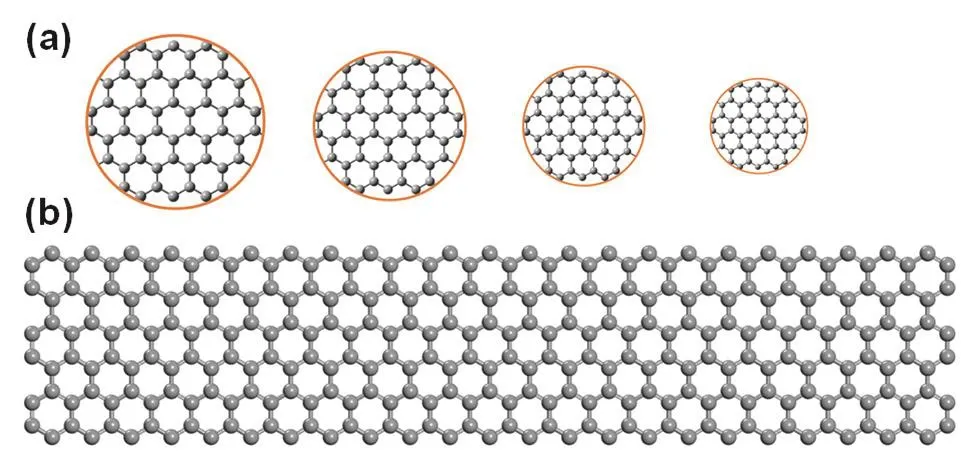
Fig.4 Illustration of structures of GNRs and GQDs.
Carbon framework:the unique properties of graphene come from the long-range 2D π-conjugated framework formed by sp2-hybridized carbon atoms.As a consequence,functionalizing graphene can be achieved by modifying the carbon framework of graphene.As mentioned above,graphene’s electronic properties depend on its width.Besides,edge structures of graphene also determine its electronic properties74.There are two kinds of typical edge structures in graphene,armchair and zigzag(Fig.5A),which exhibit different electronic and magnetic properties.For instance,armchair GNRs are non-magnetic.In contrast,zigzag GNRs have unique magnetic properties75.Additionally,zigzag edges are particularly reactive in graphene because molecular orbitals are mainly localized at zigzag edges76.In consequence,little difference in edge configuration results in large variation in graphene properties.On the other hand,introducing defect,such as vacancy (Fig.5B),into the basal plane by removing carbon atoms are also applied to functionalize graphene to tune its mechanical,thermal,electronic,and magnetic properties.The single vacancy in graphene leads to quasi-localized states near the Fermi level,giving the magnetic graphene77.For the same reason as zigzag edges,vacancies created in the basal plane of graphene are very reactive as well.The representative examples are graphene nanomeshes which have unique physical and chemical properties derived from plenty of vacancies in their carbon frameworks46-48.

Fig.5 Illustration of (A) zigzag and armchair edges,(B) monovacancy(the dangling s bonds are shown),and (C) local structure (the dashed lines represent overlap between pz orbitals) of a curved graphene sheet.
Arrangement:besides the above aspects,the arrangement of bi-/multi-layer graphene in nano scale also affect its physical properties,which has been consequently used to functionalize graphene.The number of graphene layers determines the thickness,specific surface area,and electronic properties.The arrangement of multi-layer graphene,especially bilayer graphene,is closely related to the electronic properties of graphene,by adjusting which bilayer graphene with several unique physical properties have been obtained49-54.
As shown in Fig.6a,there are three different stacking modes in bilayer graphene78:the first one is AA-stacking,where A (B)atoms of the top layer sit directly above A (B) atoms of the bottom layer; the second is AB- or the equilibrium stacking Bernal,where A (B) atoms of the top layer are above the underlying B (A) atoms,and their B atoms are above the center of the un derlying hexagons; the third is the twisted variation,where the two layers are rotated with respect to each other.Each stacking mode is able to impact the electronic properties of the bilayer graphene.And the twisted bilayer system has angledependent properties.The twisted bilayer graphene with large angles of rotation mostly has properties of single layer graphene,while the one with small angles of rotation has much more complex properties.For instance,the twisted bilayer graphene with an angle of about 1.1° displays intrinsic unconventional superconductivity49,50.
In addition,bi-/multi-layer graphene will be also endowed with unique properties through formulating it into various nanostructures.For instance,flower-like few-layer graphene nanosheets (GNSs) grown on silicon nanocone arrays show robust adhesion toward water,exhibiting super-wettability,which originates from intrinsic wetting features of graphene and large-area plentiful edges of graphene nanosheets that can be tuned by conical nanostructure (Fig.6b)79.Moreover,submicron-sized,paper ball-like graphene particles of reduced graphene oxide (rGO) have been used as scaffolds to stabilize Li metal anodes because of their unique properties,including aggregation and mechanical stress resistant,easy solutionprocessing,high external/internal surface area and high lithiophilicity (Fig.6c)80.

Fig.6 (a) Different possible stacking modes of bilayer graphene:AA-stacking,AB- or Bernal stacking,and emerging moiré pattern when rotating two layers of graphene by 8° or (d) 15°; (b) SEM image of flower-like few-layer graphene nanosheets (GNSs) grown on silicon nanocone arrays (SNAs); the inset shows high-magnification SEM image of GNSs grown on SNAs; (c) SEM image of paper ball-like graphene particles of reduced graphene oxide (rGO).
2.2.3 Macromorphology
Assembling individual two-dimensional graphene sheets into various macroscopic structures (Fig.7)81,such as graphene fibers55,56,conductive transparent films82,sponges57and foams83,can also extend graphene’s functions and achieve special applications.Functionalized graphene with macroscopic structures can not only retain the intrinsic properties of 2D graphene,but also possess unique properties of its own81,such as tunable pores,mechanical properties,and liquid absorption behaviors.

Fig.7 Functionalized graphene fibers; (b) graphene sponge;(c) graphene foam by tuning micromorphology.
2.2.4 Typical products
In practice,when preparing functionalized graphene,more than one aspect of graphene,including composition,micromorphology (dimension,framework,and arrangement),and macromorphology,are usually be tuned.The typical products are GO and covalent functionalized graphene.
GO is prepared by oxidizing the graphite materials,and its structure depends on the oxidation conditions and the starting graphite materials86.There may many structural models of GO,but the most widely adopted one is Lerf-klinowski model (Fig.8a)29,84-87.In GO structure,there are two regions:the first one is graphene-like,where sp2-hyridized carbon dominates; the other one is highly oxygenated,where sp3-hybridized carbon atoms dominate.In this model,hydroxyl and epoxide functional groups decorate the basal plane,while carbonyl groups,carboxylic acids,are present on the edges of the sheets.Apparently,in GO,the aspects of pristine graphene,including the composition,conjugated framework and edges have been modified.
Furthermore,GO can also work as starting materials to fabricate other functionalized graphene.It is well-known that GO is always used to prepare rGO by reduction via various approaches such as thermal annealing,electrochemical reduction,and chemical reduction82.After reduction,most of the oxygen groups of GO are removed and most defects in GO are eliminated to give rGO,however,which still contains residual oxygen and some structural defects and thus cannot fully regain the unique properties of graphene such as ultrahigh conductive properties (Fig.8b).
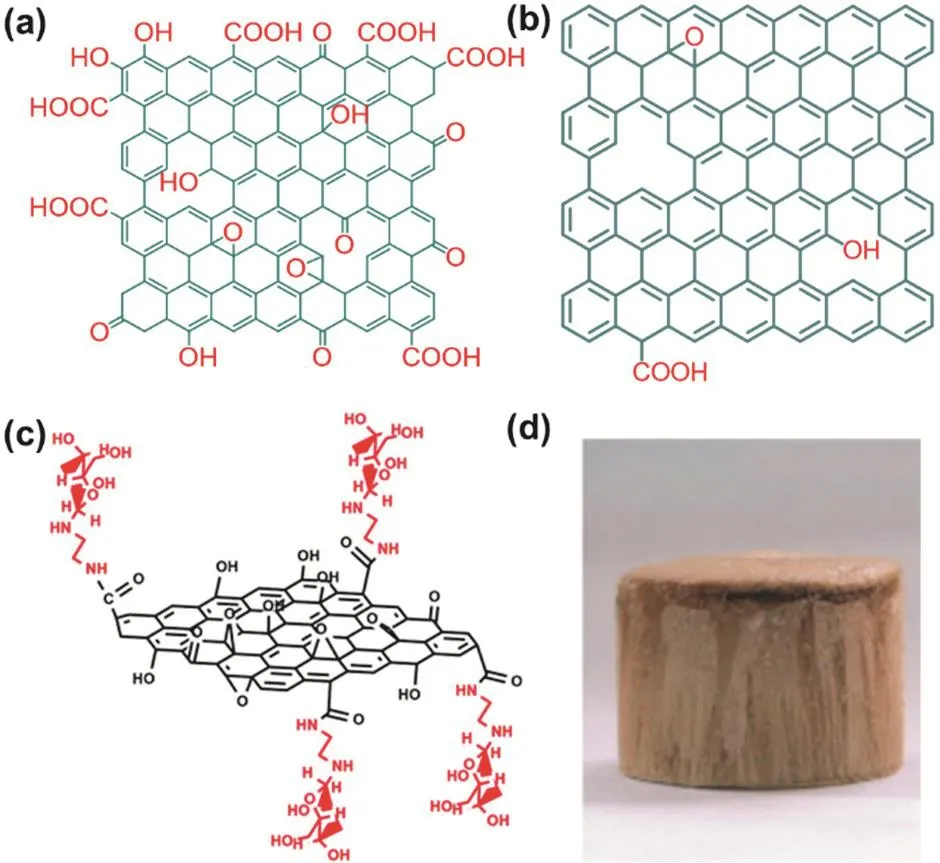
Fig.8 Illustration of structure of (a) GO (Lerf-klinowski model) and(b) rGO; (c) functionalization of GO with mannosylated ethylenediamine; (d) optical image of the GO aerogels.
GO can be also functionalized like graphene to give the second generation of functionalized graphene because of its excellent dispersibility in water,amphiphilicity and possibilities for surface functionalization.As shown in Fig.8c,GO has been covalently functionalized with mannose using mannosylated ethylenediamine,obtaining mannose-GO that shows higher affinity to human cells84.
As mentioned above,GO bears plenty of oxygen groups and thus it shows high affinity toward to cations such as metal ions and is easy to manipulate by solution process.As a result,GO are always to formulate into functionalized graphene with special macroscopic structures.For example,GO aerogels prepared by a unidirectional freeze-drying method are porous and possesses unidirectional connected pores and high surface areas (Fig.8d)85.Consequently,the GO aerogels show good adsorption ability for Cu2+in water.
The other representative example is covalent functionalized graphene which is prepared by covalently modifying the sp2carbon network of graphene with organic functional groups via traditional synthetic chemistry methods (Fig.9).Covalent functionalization can improve the solubility and processability of graphene,and allows for the combination of unique properties of both graphene and functional groups.Covalent functionalization requires the formation of covalent bonds on the basal plane of graphene,which converts sp2to sp3hybridization and consequently introduces defects into the basal plane.Bearing sp2carbon network,graphene is aromatic,and thus it can be covalent functionalized with reactions that are suitable for arene59,88,such as nucleophilic addition,cycloaddition,free radical addition,Friedel-Crafts acylation,and hydrogen-lithium exchange.Moreover,covalent functionalization of graphene is also realized with GO instead of pristine graphene because of oxygen containing groups besides sp2carbon network,which can connect functional groups through nucleophilic substitution,electrophilic substitution,condensation,and addition reactions.

Fig.9 Covalent functionalization of graphene via different reactions:(a) free radical addition with aryl diazonium salts; (b) [3 + 2]cycloaddition; (c) nucleophilic addition; (d) Friedel-Crafts acylation.
2.3 Functionalized graphene composite
Graphene or functionalized graphene or both can be hybridized with other materials,such as small molecules,polymers,metals,inorganic compounds,and carbon nanotubes(CNTs),giving the “functionalized graphene composite” where there are no covalent bonds between (functionalized) graphene and other materials.
2.3.1 Materials
Hybridization can improve the properties of (functionalized)graphene or other materials,or combine the properties of both graphene and other functional materials to give more robust composite materials.
As shown in Fig.10a,the electronic properties of graphene is tailored by hybridizing with the small molecule,N,N’-didodecylsubstituted 3,10-diazapicenium bromide89.The N,N’-didodecylsubstituted 3,10-diazapicenium bromide is electron-deficient,and has rigidity and planarity of an extended p-conjugation paired with inherent redox activity.Thus,it can form a complex with graphene in EtOH through π-π interaction.In the complex,charge density is shifted from the basal plane of graphene to the positively charged 3,10-diazapicenium,giving the p-doped graphene.Besides,the inherent positive charge of 3,10-diazapicenium enable graphene to be dispersed in ethanol via charge repulsion.
Hybridizing graphene with polymers has been applied to improve its properties as well.For instance,poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate)(PEDOT:PSS)has been introduced into the thermoelectric graphene fiber for wearable energy harvesting to improve its performance due to the high electrical conductivity,outstanding biocompatibility and excellent mechanical behavior of PEDOT:PSS (Fig.10b)90.In the this hybrid fiber,PEDOT:PSS was applied as a conductive filler to enhance the fracture elongation and electrical conductivity simultaneously.
Functionalized graphene composites have been also prepared by combing metals and/or inorganic compounds with graphene.As shown in Fig.10c,graphene-supported Ni-CeOxnanocomposites display efficiently catalytic activity for hydrolytic dehydrogenation of ammonia borane due to the combination of unique features of CeO2,nickel,and graphene91.In the nanocomposites,Ni is the active site for the hydrolytic dehydrogenation of ammonia borane,CeOximproves the catalytic performance of the Ni through a strong Ni-CeOxinteraction,and graphene provides a large scaffold for anchoring the nano particles of Ni-CeOx,owing to its large specific surface area and two-dimensional planar conjugation structure.
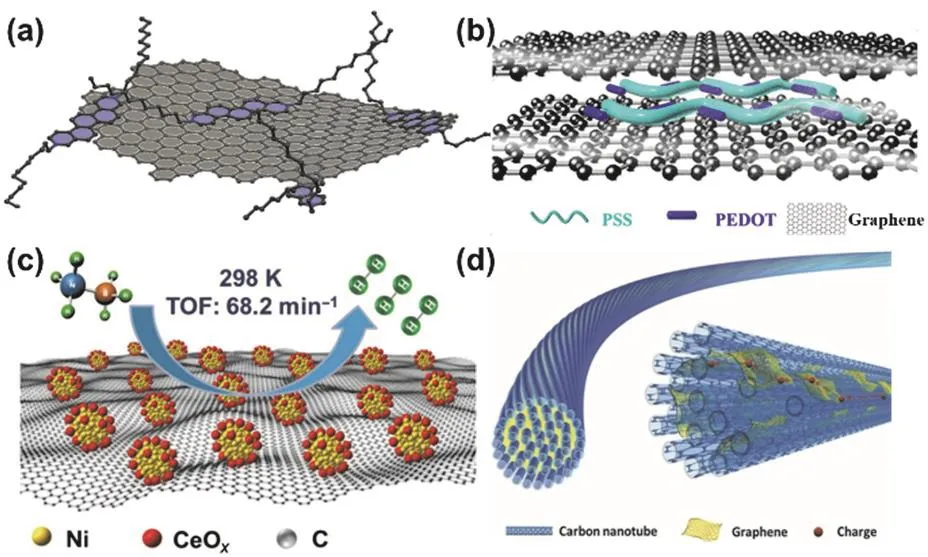
Fig.10 (a) Schematic illustration of a graphene flake functionalized by the diazapicenium dications; (b) structure of a graphene/PEDOT:PSS hybrid fiber for thermoelectric devices; (c) Ni-CeOx/graphene composite for hydrolytic dehydrogenation of ammonia borane;(d) Schematic illustration to the structure of the graphene/CNT composite fiber.
Additionally,CNTs and graphene have been used to prepare functionalized graphene composites,which have the advantages of CNTs and graphene simultaneously.For example,graphene/CNT composite fibers have been made to achieve both high electrocatalytic activity and conductivity in wire-shaped dye-sensitized solar cell (Fig.10d)92.CNT fibers show many advantages,including low density of 1 g·cm-3,high tensile strength up to 103MPa with specific strength to be 5.3 times of T1000,and high electrical conductivity up to 103S·cm-1,but relatively low electrocatalytic activities.In contrast,graphene fibers demonstrate higher loading capability for catalytic active maters such as platinum nanoparticles,but lower electrical conductivity of 10-102S·cm-1.While the graphene/CNT composite fibers simultaneously achieve both high electrical conductivity and electrocatalytic activity by combination of the advantages of CNTs and graphene,thus showing highperformance as electrodes in wire-shaped dye-sensitized solar cells.
2.3.2 Hybridization strategies
Hybridization is usually achieved through non-covalent interactions between (functionalized) graphene and other materials.The non-covalent interactions include π-π,cation-π,anion-π,and C-H···π interactions,which arise from the fully πconjugated structure of graphene (Fig.11a)93.Compared to pristine graphene,GO is more often used to hybridize with other functional materials because of hydroxyl,carboxyl,and epoxide groups bearing on their basal plane and edge as well as good dispersibility in aqueous solutions.The oxygen moieties on GO aide in binding with other functional materials through noncovalent interactions28,33,94,95,including hydrogen bonding,ionic bonding,and π-π interactions (Fig.11b).Besides,graphene’s ultrathin 2D structure provides an ideal platform for encapsulating functional materials.
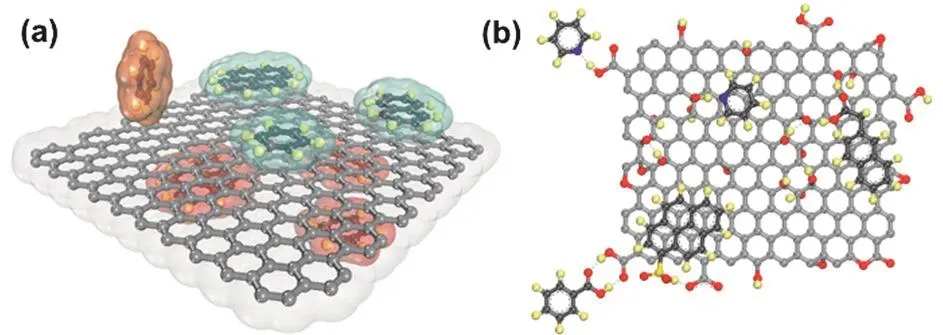
Fig.11 (a) Idealized π-π or C-H···π interactions from benzene,naphthalene,or pyrene molecules above and below the basal plane of graphene; (b) hydrogen bonds and/or π-π stacking between GO and other materials.
The structures of (functionalized) graphene and functional materials in functionalized graphene materials are proposed the structure models of graphene composites in electrochemical energy-storage devices (Fig.12)96.There are six types of structure models——encapsulated:functional materials are encapsulated by (functionalized) graphene; mixed:(functionalized) graphene and functional materials are synthesized separately and mixed mechanically; wrapped:functional materials are wrapped by multiple (functionalized)graphene sheets; sandwich-like:(functionalized) graphene is used as a template to generate (functionalized) graphene/functional material/(functionalized)graphene sandwich structures; layered:functional materials are alternated with(functionalized) graphene sheets to form a composite layered structure.
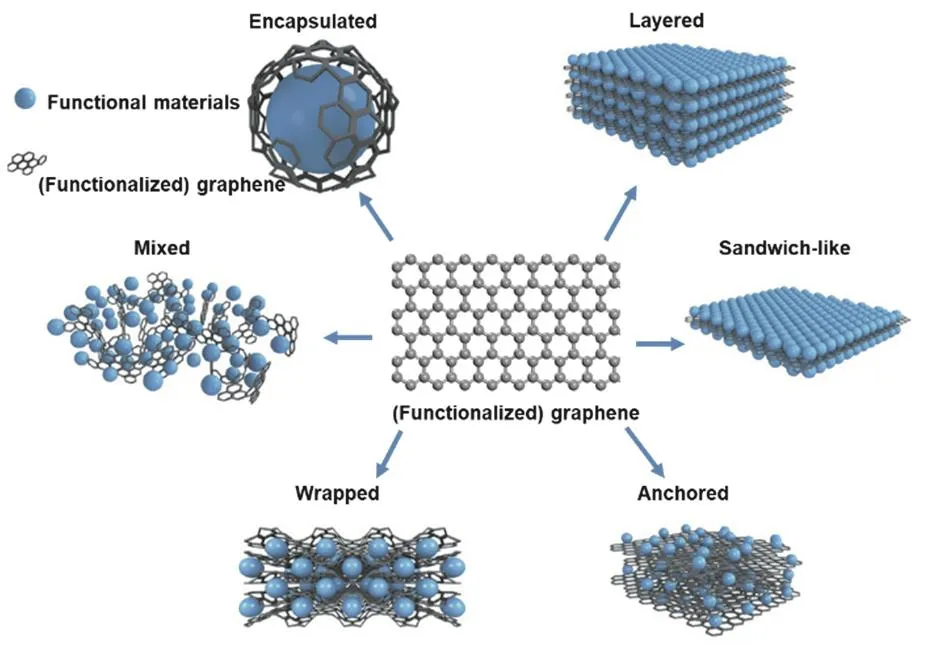
Fig.12 Structural model of functionalized graphene materials.
3 Preparation strategies
There are two preparation strategies for functionalized graphene materials,including “top-down” and “bottom-up”(Fig.13).
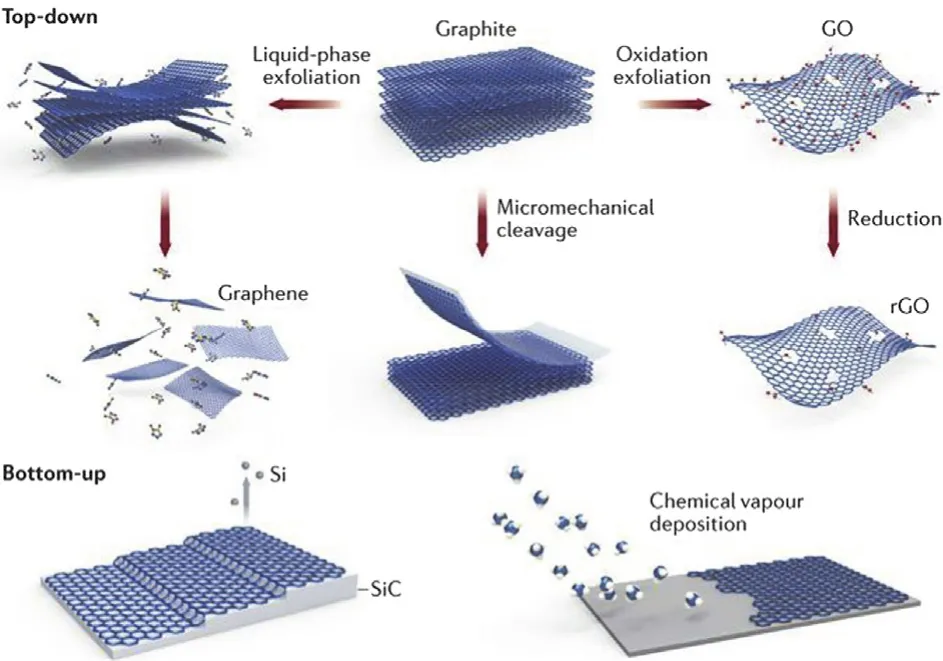
Fig.13 Preparation strategies of functionalized graphene materials.
3.1 “Top-down”
The “top-down” fabrication process for functionalized graphene materials is to break or split carbon materials,such as graphite,graphene,CNTs,fullerenes,by chemical or/and physical techniques into small fragments,during which functionalization is achieved simultaneously——or the prepared small fragments themselves are functionalized graphene materials,such as graphene nanoribbons obtained by unzipping CNTs.
The representative example is to fabricate GO from graphite(Fig.13).Multi layered graphite is oxidized by typical Hummers’ method (sodium nitrate,sulfuric acid and potassium permanganate),and then is exfoliated into single-layered GO.
Another sample is to prepare small GQDs (~2-3 nm) by cageopening of C60through chemical oxidation with strong acid (Fig.14a).C60is a well-defined carbon material with a diameter of ~1 nm,thus it can serve as starting materials to produce GQDs via a top-down wet chemistry approach98.
Via a top-down strategy,pristine few-layer nanoribbons can be produced by unzipping mildly gas-phase oxidized multiwalled carbon nanotubes via mechanical sonication in an organic solvent (Fig.14b)99.Through this strategy,covalent functionalized graphene has been prepared as well (Fig.14c)100.As shown in Fig.14c,covalent functionalized graphene nanosheets were prepared via solvent-free 1,3-dipolar cycloaddition (1,3-DC) between graphite flakes and 4-methyl-2-phenyl oxazolone (MPO) or 4-methyl-2-p-nitrophenyl oxazolone (MNPO) in a top-down approach.There reactions were carried out by heating the mixture of graphite flakes and MPO or MNPO at mild temperatures (70-120 °C),directly functionalizing graphite flakes and exfoliating them into few layers of graphene nanosheets.
In addition,the composite of graphene and N,N’-dimethyl-2,9-diazaperopyrenium dication (MP2+) has been fabricated by exfoliating graphite through π-π interactions between graphene and MP2+in aqueous media (Fig.14d)101.MP·2Cl has good solubility in water,and MP2+is electron-deficient organic dication.Therefore,in aqueous solution,MP2+can intercalate into the graphite layers,and exfoliate graphite into graphene sheets by sonication.More importantly,MP2+can stabilize the exfoliated graphene sheets in water via strong π-π interactions between them,giving the graphene/MP2+composite.Various“top-down” approaches have been reported to fabricate functionalized graphene materials,such ball milling102,electrochemical method103,direct current pulse104,micro fluidization44,and plasma etching105.Most of “top-down”approaches have advantages on efficient preparation of functionalized graphene materials in bulk,but usually suffer from drawbacks such as uncontrollable sizes and irregular edge structures.
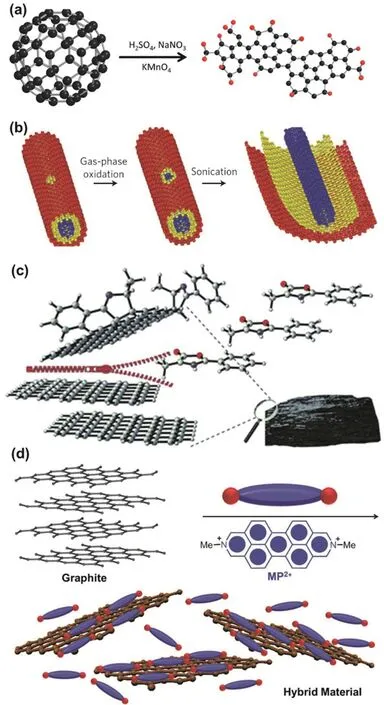
Fig.14 (a) preparation of GQDs by cage-opening of C60;(b) preparation of graphene nanoribbons by unzipping CNTs;(c) covalently functionalizing graphene with mesoionic oxazolones;(d) preparation of the functionalized graphene composite with small molecule via exfoliation of graphite.
3.2 “Bottom-up”
Different from the “top-down” strategy,the “bottom-up”strategy can be used to controllably create functionalized graphene materials,especially functionalized graphene.Using suitable building blocks,such as polycyclic aromatic hydrocarbon (PAH),functionalized graphene are precisely built up via a “top-down” strategy30,70,106-117.As shown in Fig.13,functionalized graphene with various macroscopic structures can be prepared on well-designed substrates via typical chemical vapor deposition (CVD) using CH4 as carbon source.Besides CVD,the other two main “bottom-up” approaches for fabrication of functionalized graphene,particularly graphene nanoribbons and nanographene,are liquid phase synthesis and surface-assisted synthesis (Fig.15).
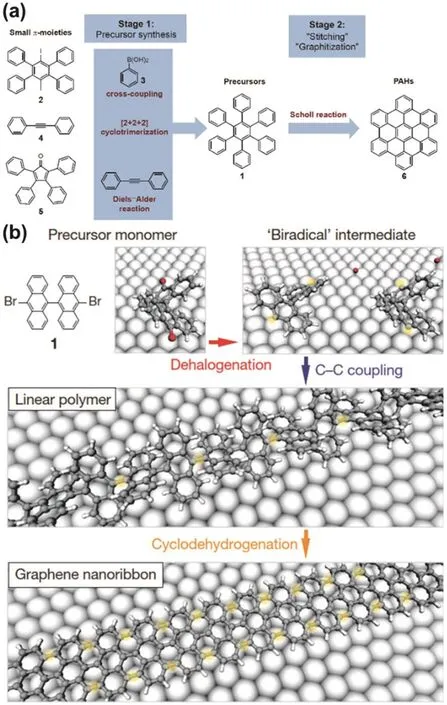
Fig.15 (a) Illustration of two-stage synthetic protocol of PAHs with p-HBC as an example; (b) Bottom-up fabrication of atomically precise GNRs.Illustration of structure of GO (Lerf-klinowski model).
The two approaches have similar processes,which contain two key stages:formation of precursors (conjugated oligomers or polymers) and further ring closure (“stitching” or “graphitization”)to produce the fused ring systems118.However,there are several differences in the two approaches——PAHs (starting materials)for liquid phase synthesis should have good solubility in organic solvent,which is not necessary in surface-assisted synthesis; In liquid phase synthesis (Fig.15a),the precursors are usually synthesized by cross-coupling reactions,such as Yamamoto polymerization,Suzuki polymerization and Diels-Alder addition,while in surface-assisted synthesis precursors are usually fabricated by radical addition through annealing at high temperature (200 °C) (Fig.15b); Ring closure in liquid phase synthesis is usually achieved by Scholl reaction in the presence of chemical oxidants,but ring closure is revealed by cyclodehydrogenation at high temperature (400 °C) in surfaceassisted synthesis; liquid phase synthesis is suitable for bulk preparation118,but the yield of surface-assisted synthesis is usually low although it can generate high-quality functionalized graphene,including graphene nano ribbons116,nano graphene and graphene nano mesh113.
Other functionalized graphene materials,such as covalent functionalized graphene and functionalized graphene composites,can be prepared via the bottom-up strategy as well.For the synthesis of covalent functionalized graphene,the building blocks are functional molecules and graphene or functionalized graphene (for example,GO and rGO).As shown in Fig.16a119,graphene-dicarboxylic acid was prepared by reacting fluorographene with diethyl bromomalonate followed by hydrolysis in basic solution.Then,graphene-dicarboxylic acid was conjugated with iron tetraaminophthalocyanine (FePc-NH2),giving the covalent functionalized graphene.Likewise,the complex covalent functionalized graphene has been also fabricated through bottom-up strategy.By combining lignosulfonate (SL) with reduced graphene oxide (rGO) through covalent bonds,a negatively charged multi-function graphene separator (rGO@SL) was fabricated to effectively suppress the shuttling of the negatively charged polysul fide ions via a“charge-repulsion” approach (Fig.16b)120.rGO@SL was prepared by heating a mixture of GO,SL,and hexamethylene diisocyanate (HDI) at 140 °C for 4 h under nitrogen.After reaction with HDI and SL at high temperature,GO was reduced into rGO.Simultaneously,rGO and SL was linked by the linear chain compound (HDI) via condensation of isocyanates in the HDI with the hydroxyl groups in the rGO and SL.
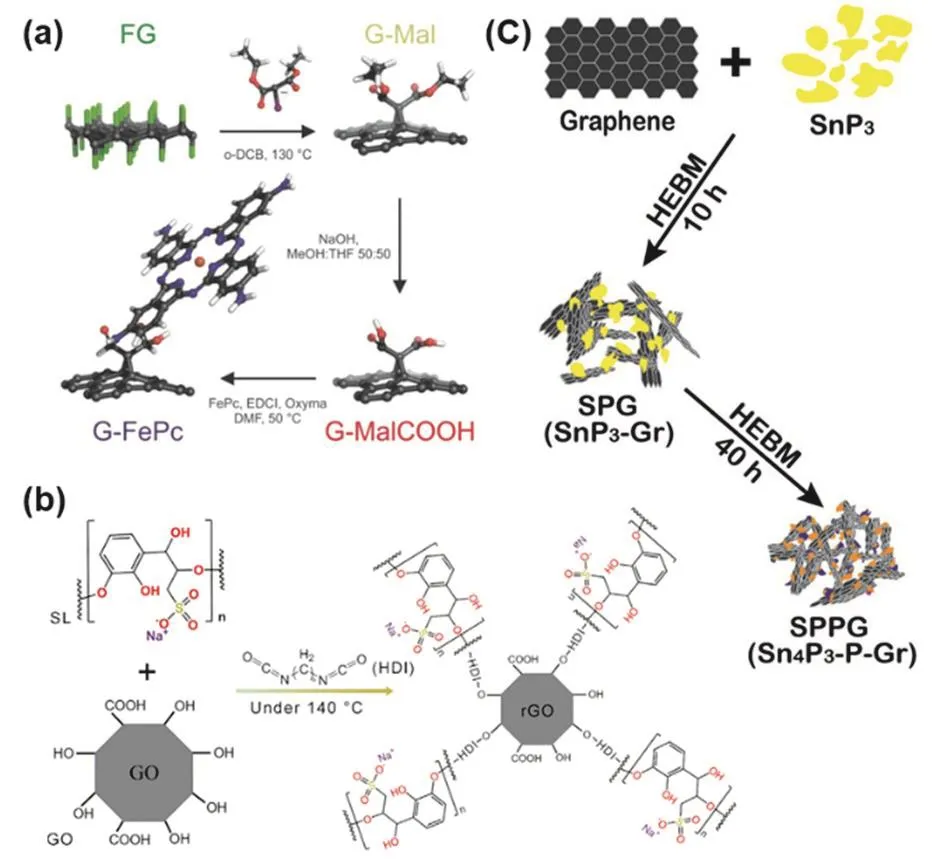
Fig.16 (a) Covalently functionalized graphene with iron tetraaminophthalocyanine; (b) synthetic steps to produce rGO@SL;(c) the mechanochemical synthesis of Sn4P3-P@Graphene Nanocomposite (HEBM:high energy ball milling).
Functionalized graphene composite can be also fabricated by the “bottom-up” strategy,in which the building blocks are(functionalized) graphene and other functional materials.For instance,via this strategy,a Sn4P3-P@graphene nanocomposite was synthesized through a facile mechanochemical approach(Fig.16d)121.Initially,SnP3was mechanochemically synthesized from Sn and red P by mechanically milling the mixture of tin and red phosphorus with an atomic ratio of 1:3 under Ar.Subsequently,the mixture of the as-synthesized SnP3 and graphene stacks (≈ 700 m2·g-1) with a mass ratio of 7 :3 was mechanically milled at the same conditions to give the Sn4P3-P@graphene nanocomposite.
4 Conclusions and perspectives
Graphene possesses fantastic properties and shows great potential in various areas.However,it always needs to be functionalized to improve the manipulation,processability and properties of graphene as well as to meet the requirement of different applications,but not is directly applied.Until now,lots of fabrication approaches have been developed and tremendous products of graphene functionalization have been prepared.However,the systemic classification of these products and corresponding exact definitions are still lack,which would lead to confusion and misleading information.Thus,based on summarizing the reported work in this area,we give the systemic classification of products from graphene functionalization and their exact definitions as well as the summarization of their preparation strategies,which need to be further confirmed by scientists in this area.
Until now,significant progress in functionalizing graphene has been made,however,there are still several issues that need to be addressed.The efficient fabrication techniques of functionalized graphene materials,which can precisely control and tune the structures of the products,especially the functionalized graphene composites,should be developed.Besides,efficient characterization techniques should be established as a tremendous amount of functionalized graphene composites have been prepared and many of them have complex structures.Moreover,the correct evaluation on the performance of functionalized graphene materials should be done and the generally accepted criterions should be given.Most importantly,the roles and mechanisms of functionalized graphene materials in most devices are still obscure,which hinders the development in this area.In future,much more effort should be devoted to these issues.Additionally,the unique properties of functionalized graphene materials should be further explored and used efficiently——the reported functionalized graphene materials are not irreplaceable in functional systems because other materials can achieve the same results as well.
Although there are many challenges in functionalized graphene materials,it is believed that functionalized graphene materials have a bright future.
