Soil protists:An untapped microbial resource of agriculture and environmental importance
2022-03-02KomalCHANDARANAandNatarajanAMARESAN
Komal A.CHANDARANA and Natarajan AMARESAN
C.G.Bhakta Institute of Biotechnology,Uka Tarsadia University,Maliba Campus,Bardoli,Surat 394 350(India)
ABSTRACT Protists are essential components of soil biodiversity and ecosystem functioning.They play a vital role in the microbial food web as consumers of bacteria,fungi,and other small eukaryotes and are also involved in maintaining soil fertility and plant productivity.Protists also contribute to regulating and shaping the bacterial community in terrestrial ecosystems via specific prey spectra.They play a role in plant growth promotion and plant health improvement,mostly via nutrient cycling,grazing,and the activation of bacterial genes required for plant growth and phytopathogen suppression.Thus,protists may prove to be a useful inoculant as biofertilizer and biocontrol agent.They can also be applied as model organisms as bioindicators of soil health.Despite their usefulness and essentiality,they are often forgotten and under-researched components of the soil microbiome,as most of our research focuses on bacteria and fungi.In this review,we provide an overview of the role of protists in plant productivity and plant health management and in shifts in soil bacterial community composition,as well as their roles as bioindicator.We also discuss the perspectives of knowledge gaps and future prospects to further improve soil biology.More research in soil protistology will provide insights into sustainable agriculture and environmental health alongside the study of bacteria and fungi.
KeyWords:bacterial community,bioindicator,microbial food web,plant health,plant productivity,soil biodiversity,soil health,soil microbiome
INTRODUCTION
The highly heterogeneous soil environment is a factor for the microbial diversity encompassing approximately 25%of global biodiversity(Coleman and Wall,2015;Trapet al.,2016).Besides bacteria and fungi,protists constitute a highly abundant group of soil organisms(Geisenet al.,2014,2015).Recent molecular approaches,including environmental DNA isolation and high-throughput sequencing,have revealed the immense diversity of protists(Mahéet al.,2017;Öztopraket al.,2020).They are unicellular and span the entire phylogenetic tree of eukaryotic life,especially in green plants,animals,and fungi(Adlet al.,2012).Protists play a significant role in soil ecosystem functioning by acting as consumers of bacteria,fungal feeders,primary producers,predators of other protists and small metazoans,and parasites of plants,protists,and metazoans(Geisenet al.,2018).They may be photoautotrophs,heterotrophs,or mixotrophs(Geisen and Bonkowski,2018).Based on morphological characteristics,they were divided into four groups:naked amoebae,testate amoebae,ciliates,and flagellates,which are traditionally known as protozoa(Gaoet al.,2019).The phagotrophic soil protists are key components of the soil food web for maintaining soil fertility and health.Bacterivorous soil protists play a role in plant growth promotion(PGP)vianutrient mineralization,which was first observed by Clarholm(1985),who put forth the often-cited hypothesis of“the microbial loop in soil”.In a series of experiments,Bonkowski and Brandt(2002)demonstrated the role of protists not only in the microbial loop,and proposed the concept of an auxiliary microbial loop in soil,in which the number of plant growth-promoting rhizospheric bacterial species increases during grazing and plays a role in PGP and nutrient release.
Thus,protists provide essential services to plants through nutrient mineralization,beneficial shifts in bacterial community composition,and pathogen suppression(Clarholm,1985;Rønnet al.,2002;Bonkowski,2004;Jousset and Bonkowski,2010;Xionget al.,2020).All these services take place in the plant rhizosphere and are responsible for increased plant growth,enhanced nutrition,reduced phytopathogens,and biotic or abiotic stress mitigation.Thus,they may offer immense potential to improve plant yields,of increasing importance for feeding our growing populations.Most current research and field approaches rely on introducing new bacteria and fungi as biofertilizers and biocontrol agents to fight against phytopathogens and environmental stresses and to meet the increasing demands of our populations.The often-forgotten aspects of the soil microbiome(i.e.,protists)remain overlooked,despite the fact that the soil microbiome contains a diverse group of microorganisms that influence plant health and productivity.This may be due to the methodological constraints for cultivation and mass production,but the current molecular methods can provide a deep insight into protistology(Geisen and Bonkowski,2018).
In this review,we provided an overview of the functional importance of soil phagotrophic protists in plant productivity and plant health.We also discussed the mechanisms through which they imparted their roles and provided services to ecosystem functioning and their interactions with bacteria and fungi.Finally,we highlighted their roles as bioindicator.We also pointed out some research gaps in this field of research and future prospects to develop a new bioengineered product for sustainable agricultural development.
PROTIST EFFECTS ON SOIL FOOD WEB AND MICROBIOME
The soil food web consists of complex relationships between a diverse group of macro-and microorganisms.It is fueled by primary producers,including aboveground plants and belowground soil organisms(i.e.,bacteria,fungi,protists,nematodes,microarthropods,and other small burrowing animals).Energy flows from plant litter,root exudates,other organisms,and organic waste by-products to decomposers and predators.As a result,nutrients are converted from one form to another and made available to the plant.In contrast to this conventional food web,the findings in a new conceptual model of the food web emphasize carbon(C)inputs from plant roots instead of nutrient cycling back through leaf litter(Alberset al.,2006).Special attention was given to the base of the nutrient cycling channel,that is,decomposers such as bacteria and fungi(Moore and Hunt,1988;Holtkampet al.,2011),and consumers such as heterotrophic protists(Crottyet al.,2011).These consumers determine nutrient cycling and availability to plants(Clarholm,1985;Kuikmanet al.,1990;Ekelund and Rønn,1994;Bonkowski,2004;Bonkowski and Clarholm,2012)and impart their role in soil food web.It has been estimated that protists are responsible for approximately 14.2%—66.0%C assimilation(Ekelund and Rønn,1994),20.0%—40.0%of nitrogen(N)mineralization(Griffiths,1994),and 70.0%of soil animal respiration(Foissner,1987).Although the precise contributions are still under research,it is clear that protists are the key component in enhancing nutrient flow for the benefits of other microorganisms and plants.It has been proved in many studies that protist and bacterial interaction enhances plant root architecture,the number of lateral roots,and the improvement of N and phosphorus(P)uptake in microcosm(Herdleret al.,2008;Asilogluet al.,2020;Zhaoet al.,2020).
PROTIST EFFECTS ON PLANT GROWTH
Microbial loop in soil and nutrient mineralization
The microbial loop concept was originally described for aquatic ecosystems,with the term coined by Azamet al.(1983),but Clarholm(1981)observed the same concept for nutrient mineralization involving protists and plant roots in terrestrial ecosystems.The concept was based on the assumption that growing plant root tips allocate the photosynthetically fixed C in the form of exudates,to lubricate their root tips and to penetrate the belowground(Joneset al.,2009).Free-living C-limiting microorganisms,especially bacteria,are attracted to these C sources,which in turn results in increased microbial biomass and bacterial activity(Wardle,1992;Alpheiet al.,1996).In addition,bacteria mineralize N from soil organic matter for their own growth(Clarholm,1985).Subsequently,these rhizobacteria are topdown regulated by bacteria-feeding microfaunal predators,especially phagotrophic protists and nematodes;otherwise,plant-available C that strongly sequesters during microbial growth would remain locked up in the form of bacterial biomass.Because of relatively low assimilation efficiency and small difference in the predator-prey C/N ratio,excess N is released in the form of ammonium,which is readily available to other organisms and hosts(Griffiths,1994;Bonkowskiet al.,2001b;Bonkowski,2004;Bonkowski and Clarholm,2012).
Chemical characterization of N-containing organic matter in soil is challenging.However,it has been claimed that most N in the soil is locked up in microbes(Jansson and Persson,1982).Approximately 20.0%—40.0% of the N is bound as amino acids and 5%—10%as amino sugars;both are the constituents of the cell wall of microorganisms(Campbell,1978).Plants cannot directly take up this organic N unless the decomposition of microbes releases mineral N.Microfauna plays a crucial role in N mineralization by consuming and subsequently releasing bacterial biomass,thereby releasing a portion of N into the soil inorganic N pool,which increases the amount of N available for plant uptake in the form of ammonia.Several experiments have been conducted to prove this theory of N mineralization by protists(Clarholm,1985;Kuikmanet al.,1990;Rønnet al.,2002;Muraseet al.,2006;Bonkowskiet al.,2009;Ekelundet al.,2009;Kolleret al.,2013a).For example,when protists were added to a wheat plant in a microcosm study,the N taken up by plants increased by 75%compared to bacterial inoculation alone(Clarholm,1985).A similar result was observed by Ekelundet al.(2009),who found that the presence of protists significantly increased N mineralization,plant N uptake from organic N sources,plant growth,and plant N content inHolcus lanatusgrass.Similarly,Muraseet al.(2006)found greaterconcentrations(0.7—1.1µmol g−1soil)in the soil with protist-treated microcosms compared to the soil from control microcosms(0.01µmol g−1soil),which indicates stimulated N mineralization in the presence of protists.Furthermore,they also demonstrated the role of protists in the sulfur cycle by detecting the reduction ofIt remains unclear how protists affectreduction,but the authors suggested it could beviadirect predation onby reducing bacterial species or stimulation of overall microbial activities by protists,which indirectly creates a more reduced soil environment.
Microbial C-use efficiency has been shown to decrease in the presence of protists(Manzoniet al.,2012),leading to a lower microbial C/N ratio.This could be due to the protists feeding on senescent bacterial cells,allowing younger cell populations to maintain a higher metabolic activity(Bonkowski,2004).Additionally,protists release undigested food particles and labile C,which are readily available for bacterial regrowth(Pussardet al.,1994)and contribute to decreased microbial C-use efficiency.The C/N ratio ultimately determines microbial respiration and turnover rates(Kuikmanet al.,1990;Alpheiet al.,1996;Crottyet al.,2013),leading to higher N release by bacterivores.It has also been observed that protist predation promotes microbial C turnover and decomposition rates(Bonkowskiet al.,2000;Hünninghauset al.,2017).C mineralization increased by a factor of 1.15 when protists were inoculated in microcosms containing maize litter residues.Moreover,different protist predators influenced the C mineralization rate during the microbial decomposition process,indicating that a rapid succession of protists is responsible for the enhanced bacterial C mineralization of maize plant detritus(Hünninghauset al.,2017).This type of predator-induced C mineralization is also affected by abiotic factors such as soil structure and temperature.For example,Zahnet al.(2016)observed a disaggregation effect on protist-induced mineralization in no-till soils,which was significantly greater for C mineralization and moderately greater for N mineralization under high temperatures,suggesting a coupling of C and N mineralization dynamics.However,Griffithset al.(2012)suggested that soil microbes maintain strict homeostasis with respect to nutrient availability.Soil microbes maintain their stoichiometry by regulating their N-use efficiency by releasing excess N elements,depending on their C-use efficiency(Mooshammeret al.,2014);which will then be available to plant roots and protists(Kuzyakov and Xu,2013).Compared to their resources,soil microbes maintain their stoichiometry,and soil microbial biomass C/N/P ratio is relatively stable(Xuet al.,2013).Changes in the C/N/P ratio can occur after changes in the composition of the bacterial community(Faninet al.,2013),because P-rich soil has been shown to be saturated with microorganisms(Griffiths,2012).Therefore,in the presence of protists,the shift in microbial biomass stoichiometry is reflected in the N and P availability to the plant.Moreover,most experiments show that protist-induced N gain is allocated to plant shoots,whereas P gain is allocated to roots(Kolleret al.,2013c);although this is not always true for each experiment—it depends on the environmental conditions,soil,protist species,and soil microbial community.To date,some experimental conditions are known to increase nutrient content without affecting plant biomass,and some are known to increase plant biomass with N and P in the plant tissue.Overall,researchers suggest that bacterivorous protists play crucial roles in improving plant growth and ecosystem functioningvianutrient cycling and maintaining the C/N/P ratio of microbial biomass and nutrient availability to plants.
Plant growth promotion via altered root archite ctures
Plant growth promotionviathe nutritional effect of protists has been well documented(Clarholm,1985;Kuikman and van Veen,1989;Ekelundet al.,2009;Kromeet al.,2009,2010;Asilogluet al.,2021b).However,increased N uptake is not the only trait attributed to increased plant biomass.In a series of experiments,some researchers found a non-nutritional effect of PGPviaroot branching and root elongation in the presence of protists(Jentschkeet al.,1995;Bonkowski and Brandt,2002;Kreuzeret al.,2006;Kromeet al.,2009).Increased root growth is a fundamental aspect promoting plant productivity,as the ability of plants to forage for water and nutrients relies on lateral root formation and root branching(Malamy,2005).Kreuzeret al.(2006)demonstrated that the N content of rice shoots increased by 45% in the presence ofAcanthamoebae,and that this improved uptake was likely due to the increased root volume.
Similarly,the increased numbers of fine roots and the length of lateral roots in spruce seedlings were observed by Bonkowskiet al.(2001b)in the presence ofAcanthamoebasp.Moreover,an increased root tip sinks more root exudates(Farrar,2003)into the soil,releasing more C sources and promoting bacterial and protist growth.The subsequent feedback of increased protist growth is ultimately reflected in the increased N content in plants.The impact of protists modify root architecture,leading to the development of a higher number of lateral root tips and induce the elongation of lateral roots,thereby increasing shoot N content.This could be due to the availability of more nutrients that are released during the predation of bacterial species in the rhizospheric regions(Muraseet al.,2006;Hünninghauset al.,2017;Jousset,2017).
Although the development of root system depends on natural biotic and abiotic factors(Malamy,2005),the root morphology is also influenced by genetic guidelines.Moreover,the plant itself exerts different effects on the composition of rhizospheric root-colonizing bacteria(Stephanet al.,2000;Wielandet al.,2001),and grazing differs due to the difference in the quality of root exudates.For example,grasses and cereals,which develop highly branched root systems,respond more strongly than forbs with larger root cortices.Plant cultivars also respond differently to bacterivores.For instance,rice plants respond toAcanthamoebaeby developing strong root branching(Kreuzeret al.,2006;Herdleret al.,2008);however,Somasundaramet al.(2008)suggested that not all rice cultivars respond to protists in the same manner.Japonica rice cultivars of lowland significantly differed in their response to protists,possibly due to the loss of some chemical signaling or essential genes(Bonkowskiet al.,2009).These findings appear to be crucial for narrowing down the research area with respect to plant cultivars and rhizospheric microbial communities.
Plant growth promotion via hormonal effect
Lateral root primordia and root elongation in plants occur mainly due to an auxin,indole-3-acetic acid(IAA)(Celenzaet al.,1995;Aloniet al.,2006).As a master regulator,any internal changes in auxin synthesis,conjugation,or degradation are directly reflected in the root architecture.Moreover,it is well documented that root architecture is also regulated by external signals released by different plant growth-promoting rhizobacteria(PGPR)strains in the rhizosphere,which is a common mechanism regulating plant growth.However,whether protists can produce such plant growth-promoting hormones remains an under-researched area.The biomass of pea seedlings grown in the culture fluid of amoeba was 48%higher than that of pea seedlings grown in the culture fluid ofAzotobacter,which increased by only 3%—4%(Bonkowskiet al.,2009).The number and length of first-order lateral roots of watercress seedlings increased by a factor of 3.9,and 5.2,respectively,in the presence of amoebae(Bonkowski and Brandt,2002);however,by growing plants in the presence of amoebae and non-IAA-producing bacterial species,it was suggested thatAcanthamoebaspp.were not able to increase lateral root formation while feeding on the non-IAA-producing strain.It was found that root growth increased when amoebae and IAA-producing strains were co-inoculated,suggesting that the actual factor responsible for root branching was IAA-producing bacterial species(Bonkowski and Brandt,2002).These reports revealed stimulated hormone production by bacteria-amoebae interaction,instead of direct hormone production by amoebae.However,the genes for auxin biosynthesis as well as for free-auxin deactivationviaformation of IAA conjugates were recently discovered in the genome ofAcanthamoeba castellanii(Clarkeet al.,2013).It was suggested thatA.castellaniimay directly increase root growth through the production of hormones;however,to date,there is no experimental evidence for such hormone production byAcanthamoebasp.
In addition,the activity of IAA-producing soil bacteria was stimulated in the presence of amoebae,but internal plant hormone levels also changed when exposed to bacterivorous amoebae(Kromeet al.,2010).Studies have reported thatArabidopsis thalianaplants transformed by the cytokinininducibleARR5-promoter-GUSconstruct responded similarly in the presence ofAcanthamoeba,with increased root branching.Increased root branching suggests substantial hormone availability in plants,which presumably had to be downregulated in the presence of cytokinin.However,this was not the case in the presence of amoebae,which showed internal hormonal changes in a plant in the presence of protists.
Effects on plant reproduction and metabolome
The positive effects of protists on plant biomass and root architecture can be reflected in increased plant reproduction.However,research on the plant growth-promoting effect of protists remains scant,likely due to a lack of mass production methods for protistan species applied in field research.Some studies on this topic were conducted by Bonkowskiet al.(2001a)and Kromeet al.(2009),who reported increased seed production(by 24%)and individual seed weight(by 32%)in barley plants,and a 3-to 7-fold increase,respectively,inA.thalianain the presence of amoebae compared to bacteria alone.Concurrently,the metabolomic study of plants using protists as a bioinoculant is also an underresearched area.Recently,Kuppardtet al.(2018)studied the metabolome ofZea maysL.plants in the presence of a mixture of three protists with different filter-feeding modes:Cercomonas longicauda(flagellum mediated),Tetrahymena pyriformis(cilium-mediated),andAcanthamoeba polyphaga(surface gliding).Gas chromatography-mass spectrometry profiling of the plants treated with the selected protist species and native soil bacterial community showed decreased plant stress-related metabolites,such as polyols,several carbohydrates,and metabolites connected to phenolic metabolism.Moreover,terminal restriction fragment length polymorphism community profiles revealed overall changes in bacterial community composition,richness,and evenness in the presence of predators in the microcosms.These results indicate a correlation between shifts in the composition of bacterial communities and decreased stress levels in plants by the protistan effect,which could ultimately result in plant growth promotion.
INTERACTION BETWEEN PROTISTS AND MYCORRHIZAE
Mycorrhizae are one of the essential belowground symbionts on Earth.Approximately 80% of the herbaceous and woody plants in the terrestrial ecosystem are colonized by arbuscular mycorrhizae or ectomycorrhizae symbionts(Allen and Read,1991;Smith and Read,2008).It has been confirmed that this symbiont is essential for plants to acquire nutrients such as N(Hodgeet al.,2000;Leighet al.,2009)and P(Smith and Read,2008;Smith and Smith,2011).
In this symbiotic relationship,plants invest C sources and,in turn,benefit from enhanced plant growth(Bagoet al.,2000)by improving access to growth-limiting soil nutrients such as N(Hodgeet al.,2000;Leighet al.,2009)and P(Smith and Read,2008).It has been estimated that approximately 30%of the C allocation by plant roots is directly taken up by root-colonizing fungal symbionts(Joneset al.,2009).Thus,the growth of bacteria and bacterivores,which depends on plant C,should be indirectly affected by mycorrhizae,but this is not evident actually.The above hypothesis indirectly suggests that mycorrhizal fungi affect protist growth and,thereby,the plant growth-promoting effect of protists.Again,researchers did not find this hypothesis to be true in their studies and no net negative impact was found for protists in the presence of mycorrhizae.However,the simultaneous presence of both organisms had a profound effect on N and P uptake by plants,as shown in Table I.Protists increase N availabilityviagrazing and liberating bacterial biomass for mycorrhizal uptake,according to the microbial loop hypothesis(Clarholm,1985),and may foster the transportation of mineralized Nviafungal hyphae to plant roots(Kolleret al.,2013c).Additionally,the effect of protists on plant growthviaramified root architecture can be altered by mycorrhizal fungi(Bonkowski,2001b),because the fungal symbiont is well known to be able to modify plant root biomass and architecture(Brownet al.,2013).With amoeba alone,the root system developed more complex root architectures,whereas with mycorrhiza and amoeba,root length,length of fine roots,and the number of root tips were reduced by 47%,47%,and 40%,respectively.Despite this reduction in root growth,the combination of protists and mycorrhizae positively affects plant shoot biomass and nutritional value in terms of N and P contents(Bonkowskiet al.,2001b).Assessment of plant N uptake,C fixation,and C partitioning using stable isotope(15N and13C)techniques revealed a 2-fold increase in concentration in the presence of protists(Kolleret al.,2013a,b,c).Overall,the root architecture of plant is alternately affected by the mutualisms of plantmycorrhiza and bacteria-mediated plant-protists;however,the net effect of protist-mycorrhiza interaction appears to be complementary.Mycorrhizae modify the root system by capturing nutrients far from the roots and transporting them to plants,whereas protists increase nutrient availability through nutrient mineralization in bacterial biomass(Henkeset al.,2018).
The effects of both mutualisms on plants remain present in terms of enhanced nutrient uptake,because they complement each other.Despite their importance in plant nutrition acquisition,only a few studies(Table I)have been conducted on mycorrhiza-protist interaction.Further studies are needed to discover the interactions of both ecto-and arbuscular fungi with bacterivores,as these fungi are physiologically different in colonizing the plants in different soil habitats.
PROTIST EFFECTS ON SOIL MICROBIAL COMMUNITY
Effects on microbial biomass
Soil bacterial biomass and diversity are regulated by soil physicochemical characteristics such as soil texture,moisture content,pH,and C/N ratio.However,predators such as protists play significant roles in soil microbial community composition and ecosystem functioning.Bacterivorous protists feed on microbial cells,which implies that the presence of protists results in decreased microbial biomass(Andersonet al.,1977;Darbyshireet al.,1994;Ekelund and Rønn,1994;Ekelundet al.,2009).It has also been confirmed that the presence of bacterivorous nematodes could increase the abundance of certain microbial species(Inghamet al.,1985;Sundinet al.,1990),and that losses in specific bacterial genera are compensated for by other genera(Flueset al.,2017).Closely related species of protists may have different capacities or strategies to feed on microbial cells,therebyincreasing or decreasing certain bacterial taxa in terms of biomass(Swallowet al.,2013).Therefore,predation helps to maintain bacterial diversity,evenness,complementarity,and richness(Saleemet al.,2012;Kuppardtet al.,2018).This is probably due to the varying capacity of the bacterivorous species to feed on certain taxa,the predation-resistant effect of certain bacterial species(Matz and Kjelleberg,2005;Jousset,2012),mode of ingestion(Rønnet al.,2012),the effect of soil texture(Rutherford and Juma,1992),and the supply of nutrients(Elliottet al.,1980).
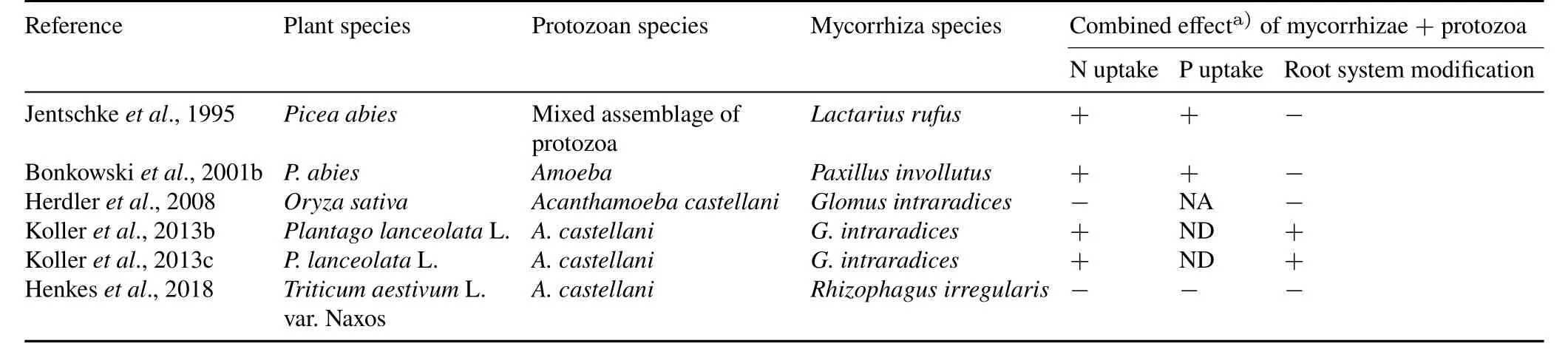
TABLE I Combined effects of mycorrhizae and protists(traditionally known as protozoa)on plant growth parameters compared to control treatments
Selective feeding on bacterial community
Several factors and mechanisms may contribute to the grazing effect on bacterial communities,as described earlier.In addition,protists can discriminate between edible and nonedible bacterial sources,depending on their size and surface characteristics(Montagneset al.,2008;Jousset,2017).For a planktonic environment,size-selective predation by protists exerts an influence on the planktonic bacterial community(Matz and Jürgens,2005).Medium-sized bacterial cells are more susceptible to predation by ciliates and flagellates than smaller and filamentous forms(Hahn and Höfle,2001).The groundwater flagellateSpumella guttulaingests bacteria with lengths between 0.8 and 1.5µm at a high rate(Matzet al.,2002;Kinneret al.,1998),whereas grazing by ciliates and flagellates allows the growth of starlike and filamentous bacteria in seawater(Bianchi,1989).This type of size-selective feeding is common in ciliates and flagellates.In contrast,surface-associated naked amoebae engulf bacteria by phagocytosis,feed on large prey,and are less constrained in size selection(Ekelund and Rønn,1994).Owing to their high biomass and feeding modes,these soil amoebae can access most soil bacteria attached to root surfaces and soil particles,and crawl,wobble,or attach to surfaces to feed on the bacteria(Rønnet al.,2012).
Shifts in microbial communitycomposition
Predation-induced shifts in the bacterial community structure can directly affect selective feeding on size-specific bacteria,which may be due to changes in the balance between different bacterial populations.Modern molecular fingerprinting techniques now permit direct studies on the genetic structure and changes in the taxonomic composition of soil bacterial communities during grazing.Fluorescentin situhybridization,microautoradiography(Wagneret al.,2003),stable isotope probing and metagenomics(Wellingtonet al.,2003),denaturing gradient gel electrophoresis(DGGE),community-level physiological profiling using Biolog plates(Rønnet al.,2002),phospholipid fatty acid(PLFA)(Henkeset al.,2018),and 16S rRNA amplicon sequencing analyses(Asilogluet al.,2021b)are some of the advanced tools that enable the user to directly visualize changes in the composition of the bacterial community during predation.The PLFA analysis indicates that the Gram-positive bacterial population increases in response to predation,compared to Gram-negative bacteria.High-intensity DGGE bands indicate that high G+C Gram-positive bacteria related toArthrobactersp.increased in response to protist grazing,whereas Gram-negative bacteria decreased,showing lowintensity bands(Rønnet al.,2002;Hünninghauset al.,2017;Xionget al.,2020).This indicates that Gram-positive bacteria are,in general,less suitable food for protists than Gram-negative bacteria.Mycobacterium chlorophenolicum,a Gram-positive bacterium,appears to be completely unaffected by grazing(Rønnet al.,2002).Although the lower edibility may be due to the more complex cell-wall structure of the Gram-positive bacteria(Muraseet al.,2006),it is not always the absolute factor for grazing resistance,as many Gram-positive bacteria are edible(Singh,1941).This does not mean that all Gram-negative bacteria are always preferred and suitable food for protists.For example,Pseudomonasspp.are not affected by grazing(Rønnet al.,2002).Nonetheless,many non-pigmented Gram-negative bacteria from the Enterobacteriaceae family have positive effects on the growth of free-living soil amoebae such asAcanthamoebasp.(Weekerset al.,1993).In laboratory experiments,a highly recommended bacterial food source for protists isEscherichia coli,a Gram-negative species from the Enterobacteriaceae family.Furthermore,it has been repeatedly demonstrated that predation changes the soil bacterial community,specifically at the class level,from Alphaproteobacteria,Betaproteobacteria,and Sphingobacteria towards Gammaproteobacteria and Opitutae,implying that Alpha-and Betaproteobacteria are less resistant to grazing(Muraseet al.,2006;Rosenberget al.,2009;Flueset al.,2017;Kuppardtet al.,2018).This observation could be linked to high soil fertility,which could be due to predation by protists because many PGPR species belong to Proteobacteria and Bacteroidetes(Asilogluet al.,2021a).Furthermore,it was recently revealed by Asilogluet al.(2020)that inoculation of a mixed culture of heterotrophic protists enhances the survival of plant growth-promotingAzospirilliumsp.B510 and its positive effects on early rice plant growth.The authors reported a high abundance ofAzospirillliumsp.B510 in the rhizosphere in the presence ofNaegleriasp.andHeteromita globose,using quantitative real-time polymerase chain reaction and amplicon sequencing methods.In addition to inoculated PGPR species,they also found an increased abundance of other potential plant hormoneproducing bacterial species of the generaArthobacterandClostridiumin protist-inoculated treatments.In addition,in their next experiment with poultry litter biochar and rice husk biochar-amended paddy field soil,Asilogluet al.(2021b)observed higher N uptake by plants in the presence of protists,compared to control and bacteria-alone treatments.They predicted a high abundance of putative bacterial genes involved in N mineralization,dissimilatory nitrate reduction to ammonium,andassimilation in the presence of protists.This suggests that PGP traits by protists may be due to the regulation of the bacterial community by predation,which increases the bacterial mineralization of organic matter toand preventsloss by dissimilatory nitrate reduction to ammonium andassimilation.In addition to shifting to beneficial bacterial communities,protists also play a significant role in shaping the bacterial community after flooding and fertilization,as soil water content is a crucial factor for protist activity(Zhanet al.,2019;Asilogluet al.,2021a).
Stimulation of bacterial activity
Selective grazing-induced shifts in the microbial community composition may result in changes in plant growthstimulating bacterial communities(Bonkowski,2004).It was stated in preceding sections that protistan grazing favors IAAproducing bacterial strains(Bonkowski and Brandt,2002).In addition to this,protists have also been shown to enhance the nitrification process and stimulate nitrifying bacteria(Verhagen and Laanbroek,1992;Alpheiet al.,1996;Bonkowski,2004),indicating that grazing facilitates certain bacterial activities.Increases in carbon dioxide and pigment production were reported for bacteria after exposure to protists,especially to amoebae and ciliates.These metabolites were investigated for their biocontrol activity against soil-borne plant pathogens.Furthermore,the activity of some PGPR strains against plant pathogens is upregulated in the presence of protists,such asstimulated production of siderophores byPseudomonas fluorescencein the presence of amoebae(Levratet al.,1992)and upregulation of cyclic lipopeptides(Mazzolaet al.,2009)or 2,4-diacetylphloroglucinol(2,4-DAPG)(Jousset and Bonkowski,2010)by bacteria in the presence of amoebae.Although the exact mechanisms and chemical signals involved in the interaction and upregulation of secondary metabolites are not yet known,these findings suggest the stimulation of PGP and plant-pathogen suppressing activities of bacterial species in the presence of protists.However,this is an indirect effect on PGP by protists,because not all potential PGPR species always benefit.Agrobacteriumsp.significantly increases in the presence ofVermamoeba vermiformisandH.globosa,but slightly decreases in the presence ofNaegleriaspp.In addition,Herbaspirillumsp.was found to be relatively reduced in the protist treatments(Asilogluet al.,2020).Further studies are required to identify the protist species responsible for enhancing soil PGPR and indigenous PGPR species functioning for better plant performance in the natural soil environment.
PROTISTS AS BIOCONTROL AGENT
As shown in Table II,there are several ways through which protists can influence phytopathogenic activity.Protists may protect the plant by directly feeding on pathogens(Dumacket al.,2016b;Geisenet al.,2016;Xionget al.,2020)or by shifting the functional bacterial community(Joussetet al.,2006;Mazzolaet al.,2009).Recently,Xionget al.(2020)observed that the abundance of predators was negatively correlated with pathogen(Ralstonia solanacearum)abundance and suggested that the interaction between predators and prey influences the pathogen performance.Moreover,mycophagous protists can directly feed on a range of fungal spores and yeast cells,including plant pathogenic fungi(Geisenet al.,2016;Dumacket al.,2019).Food choice experiments by Dumacket al.(2016b)showed that terrestrialFiscullaspp.(Cercozoan species)can feed onFusarium culmorum.Moreover,a recent environmental sequencing survey on protist diversity revealed a highly abundant and diverse group of omnivorous thecate amoebae,Rhogostomidae(Öztopraket al.,2020).The species usually feed on algae,fungi,and even bacteria(Dumacket al.,2016a,b,2017,2019;Seppeyet al.,2017).Together,the investigation of these protists may help develop an efficient inoculum for sustaining soil health.Furthermore,the widespread mycophagous amoebae(vampyrellid)and ciliates(grossglockneriid)in soil(Geisenet al.,2016)may also contribute to the development of potential biocontrol agents.
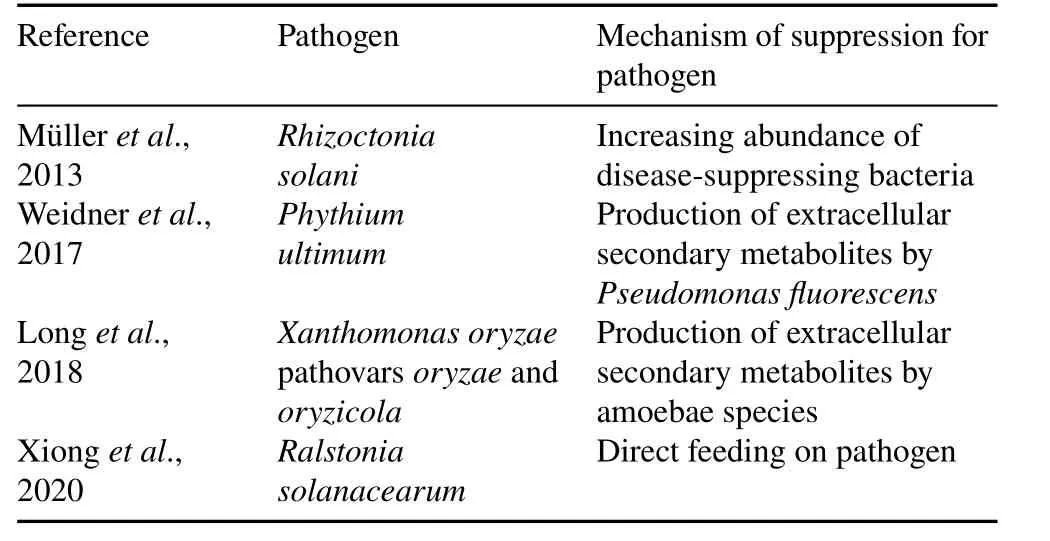
TABLE II Mechanisms of phytopathogenic suppression by protists
In addition,the production of extracellular toxic metabolites in response to predation by bacteria is of paramount importance for the bacteria-protist interaction in the terrestrial ecosystem.As these toxins inhibit predation,act antagonistically against phytopathogens,and promote plant growth(Dubuiset al.,2007),successful manipulation of such interactions may facilitate biocontrol of soil-borne phytopathogens.For instance,the application ofCercomonasspp.andNaegleriaspp.supports the growth ofPseudomonasspp.This interaction suggests that the linkage between the predation-resistant traits of bacteria enhances pathogen inhibition and the direct PGP effect of bacteria under predation pressure(Amackeret al.,2020).Incubation of theP.fluorescensstrain CHA0 with culture supernatant ofA.castellaniitriggered expression of thephlAandprnAgenes(DAPG and pyrrolnitrin toxin-producing genes)in bacteria and a 2.0—2.2-fold increase in toxin production in response to protists.A contrasting effect on gene expression was observed upon co-inoculation of both organisms.Incubation of amoebae and live bacterial cells increases the density of amoebae and reduces the expression of toxin-producing genesphlAandpltAby up to 66%and 55%,respectively,suggesting that protists can repress bacterial activity(Jousset and Bonkowski,2010).This intrinsic chemical warfare between protists and bacteria indicates mutual perception.
Müller(2013)investigated the effect of predation pressure on soil bacterial communities carrying genes responsible for producing antifungal compounds 2,4-DAPG,pyrrolnitrin,and hydrogen cyanide in the presence ofA.castellanii.The presence of amoeba did not affect the density of cultivable bacteria and pseudomonads.Instead,it increased the abundance and frequency ofphlDandhcnABgenes in the bacterial community,mostly pseudomonads;thereby,it was effective againstRhizoctonia solani,which causes sugar beetroot disease.The production of secondary metabolites byP.fluorescencesuppresses the plant pathogenPythium ultimum,which is upregulated after exposure toA.castellanii(Weidneret al.,2017).Significant upregulation of the type VI protein secretion system(T6SS)was observed in the protist treatment,suggesting that the bacteria defend themselves by upregulating the T6SS(Flueset al.,2017),which was found to play a role in defense against other competing bacteria and protists(Coulthurst,2013).Flueset al.(2017)also investigated the downregulation of the type VIII protein secretion system(T8SS),which might lead to reduced surface colonization of bacteria in response to predation,because T8SS is responsible for the secretion of curli,an amyloid that is required for biofilm formation,colonization of inert surfaces,and host cell adhesion.In addition to direct consumption on bacteria and modulating the regulation of bacterial genes,protists produce extracellular metabolites that directly inhibit pathogen growth.For example,the supernatant of some soil amoebae species can inhibit the growth of the rice plant pathogensXanthomonas oryzaepathovarsoryzaeandoryzicola(Longet al.,2018).The predator can even sense their prey from a long distance in a porous soil environment.For example,volatile organic compounds(VOCs)ofDyella,Paenibacillus,andBurkholderiaspecies reduced the activity ofVermamoebaandSaccamoebaand stimulated the activity ofTetramitus(Schulz-Bohmet al.,2017).Such species-specific responses of protists to bacterial VOCs suggest co-evolutionary dynamics in their interactions.It can be used against potential soil-borne pathogens as an alternative method of biocontrol.
The above studies suggest that predation pressure upregulates multiple toxin-producing genes in the presence of amoeba,which in turn provides broad-spectrum biocontrol against more than one phytopathogen.This property of bacteria might help them to persist longer in soil and allow researchers to develop better biocontrol inoculums.The overlap of bacterial traits such as predation resistance and stimulation of phytopathogen-suppressing metabolite genes may prove to be a stepping stone to enhance bacterial performance against pathogens and be implemented as alternative pesticides.
BACTERIAL RESPONSES TO PROTIST PREDATION
Generally,in an ecosystem,bacteria-feeding protists constrain bacterial growth and survival.In line with this,the topic of defense against predators by prey has received considerable attention in aquatic and terrestrial ecosystems.According to several recent findings,bacterial adaptation to predators plays a significant role in bacterial community persistence,and bacteria have developed many traits to inhibit ingestion or digestion by protists.These include motility speed(Matzet al.,2005),morphological changes(Hahn and Höfle,1998),microcolony formation through the production of an exopolysaccharide layer(Hahn,2000;Matz and Kjelleberg,2005),biofilm formation(Matzet al.,2004a),production of bioactive compounds(Matzet al.,2004a;Songet al.,2015),cell-to-cell communication(Matzet al.,2004a),and the prevalence of natural antibioticresistant genes(Nguyenet al.,2020).Such antipredator bacterial traits affect grazing efficiency and support the view that selective predation is an important determinant of bacterial diversification(Matzet al.,2005).
Bacteria frequently alter their size or shape to inhibit predation.Hahn and Höfle(1998)provided evidence that flagellateOchromonassp.caused a substantial decline in the abundance ofVibriostrain CB5,whileComamonas acidovoransresponded to grazing with a strong expansion in cell length,morphing towards a filamentous form.This pre-ingestion change in cell morphology resulted in a high percentage of the inedible cell population ofC.acidovorans.However,the flagellateOchromonassp.influences theFlactobacillussp.growth rate and bacterial biomass,and indirectly stimulates filament formation.However,no such grazing-resistant ability of filamentous form was observed for soil bacterial communities by Rønnet al.(2002)through advanced molecular tools.Another adaptive trait,high-speed motility(Matzet al.,2005),is generally attributed to the planktonic environment;soil bacteria in the absence of swimming medium do not show such characteristics.
Some soil-dwelling bacterial species produce different types of toxic compounds that resist predation or even kill predators(Joussetet al.,2006,2008;Pedersenet al.,2010,2011).The production of antibiotic compounds or the development of antibiotic-resistant systems by bacteria is one of the traits that allow them to cope with and/or escape predation pressure(Nguyenet al.,2020).Chromobacterium violaceum,a pigmented soil bacterium that produces toxic pigment violacein to evade predation,is regulated by quorum sensing(Matzet al.,2004b).By combining live imaging mass spectroscopy with whole-genome transcriptome analysis,Songet al.(2015)found that soil-dwellingPseudomonas fluorescensproduced several metabolites to avoid predation by the protistNaegleria americana.Lipopeptide biosynthesis and putrescine biosynthesis were upregulated inPseudomonasupon grazing by a predator.Such multifaceted studies provide strain-specific interactions to survive in a highly competitive rhizospheric environment.
PROTISTS AS SOIL HEALTH BIOINDICATOR
The indicator of soil health should be selected and validated according to land use,climate zones,and land management practice differences.Griffithset al.(2016)evaluated and recommended a policy-relevant and cost-effective biological indicator for soil ecosystem functions at the European scale.Bioindicators are living organisms whose presence,abundance,or behavior can provide information about environmental health.Generally,organisms that are sensitive to environmental changes can be used as bioindicators.However,the basic physiological properties of an organism are critical indicators.For example,the properties of protists such as consumption of more bacterial food per mass unit,shorter generation time and life span,faster reproduction rate,and higher respiration rate per mass unit(Foissner,1999),compared to meso-and macro-fauna,make them a suitable organism to be considered as a bioindicator of soil health.Moreover,a faster response to external environmental changes than other macrofauna,distribution across all soil types,low genetic differentiation among the globally distributed species,and similar eukaryotic genomes as metazoans play a key role in the soil food web,nutrient cycling,and plant growth(Foissner,1999;Payne,2013).However,some other aspects of protists restrict their use as bioindicators.These include unknown specific food requirements for different species,time-consuming enumeration,lack of literature,and lack of expertise(Foissner,1999;Griffithset al.,2016).A review by Payne(2013)suggested that most literature studies were conducted in the context of water quality,including wastewater treatment,using ciliates,foraminifera,and diatoms.Studies on soil pollution and reconstruction of water tables in peatlands have mainly included testate amoebae.The production of decay-resistant shells makes testate amoebae sensitive to environmental changes,such as climate change,fire,pollution,and nutrient starvation(Geisenet al.,2018).
Only a few toxicological studies have been conducted on soil ciliates and amoebae.Nowadays,the overuse of pesticides has changed soil health by changing and affecting the beneficial soil microbial diversity.The use of pesticides also influences non-targeted organisms.This was proven by Amaroli(2015),who used protists as a model organism.Dictyostelium discoideum,a slime mold(amoebae),is known to habituate agricultural soil and to enhance soil fertility and resistance against plant pathogens.The effects of pesticides on slime mold were investigated by measuring the fission rate,differentiation ability,ability to eat bacteria,and stress markers such as pseudocholinesterase activity and heat-shock protein 70(Amaroli,2015).Likewise,soil ciliates and colpodids have been studied under heavy metal pollution and found to be resistant to heavy metals(Díazet al.,2006).
The long-term application of N fertilizers is shown to reduce soil microbial diversity such as bacterial and fungal taxa(Wanget al.,2018),and might exert a detrimental effect on agricultural ecosystem and sustainability.Protist communities have been found to be more susceptible to fertilizer application(Zhaoet al.,2020).Recent investigations on the interactions of soil microbiome with N fertilizer have revealed altered key protist taxa in microbiome networks(Zhaoet al.,2019)and reduced protist diversity(Zhaoet al.,2020).Compared to the immense diversity of protists and their roles in the ecosystem,the scope of study should be expanded to examine the effects of pollutants on soil biodiversity and ecosystem functioning.
FUTURE ASPECTS AND GAPS IN RESEARCH
Previous studies on protists,showed that protists can efficiently help sustain agricultural practices and soil and plant health.They play a key role in enhancing nutrient cycling,which may accelerate the mineralization of organic fertilizer or organic residues(Hünninghauset al.,2017),and boost plant growth.Moreover,predation on pathogens or senescent bacteria also improves plant growth and the survival of plant-beneficial microbes(Bonkowski and Brandt,2002;Jousset,2017),making them an efficient co-inoculant.Furthermore,some disease-suppressing bacteria elicit functional gene expression in the presence of protists(Mazzolaet al.,2009;Jousset and Bonkowski,2010;Songet al.,2015),making them a possible alternative to pesticides.In addition to these activities,their ability to form cysts facilitates their survival under harsh soil environmental conditions and proves advantageous in the formulation as a bioinoculant.
Gaps in the current literature on plant performance
To analyze the effects of protists on plant growth,most studies have focused only on increased N mineralization,increased N uptake by plants,increased plant biomass,increased plant-free and-bound auxin,and PGP effectsviabacterial community shifts(Table III).Studies on the solubilization or mobilization of other macro-and micronutrients,as conducted for plant growth-promoting bacteria,have not been considered for protist inoculants.The essential nutrient P is also a less frequently measured parameter(Bonkowskiet al.,2001a),although it is a limiting nutrient in many soil environments.Unfortunately,little work has been done on bacterivores to determine their effects on plant reproduction.Bonkowskiet al.(2001a)and Kromeet al.(2009)conducted experiments that showed enhanced seed production in barley plants andA.thaliana,respectively,in the presence of protists.Therefore,more studies are needed to include various plant growth parameters,nutrient acquisition,and different environmental conditions to elucidate the effect of protists on plant performance.
Lack of literature with regard to species diversity
Most published literature on the interactions between protists and plants have investigated plant performance using a mixed assemblage of protists and native soil bacterial communities.This could be due to methodological constraints in the extraction and cultivation of these taxa.However,the most frequently used bacterivorous species as a model organism isA.castellanii.Most studies have focused on this species becauseAcanthamoebais a globally-occurring soilinhabiting species(Geisenet al.,2014)and it is frequently found in serial dilutions.Moreover,it can be grown axenically(without any bacterial food)on simple laboratory agar media(e.g.,proteose peptone-yeast extract-glucose medium with 10 g L−1peptone,10 g L−1glucose,and 5 g L−1yeast extract)(Mülleret al.,2013),to avoid any transfer of bacteria when performing different experiments.Some recent studies have used a combination of new protist species in their experiments,including ciliates and flagellates such asT.pyriformis,Colpoda steinii(ciliate),C.longicauda(flagellate),andAcanthamoebasp.(Saleemet al.,2013;Hünninghauset al.,2017;Kuppardtet al.,2018;Asilogluet al.,2020,2021a,b).In addition,a recently discovered highly abundant and diverse group ofRhogostomidae(Öztopraket al.,2020)opens the door to introduce an increasing number of species in research,to improve protistology.
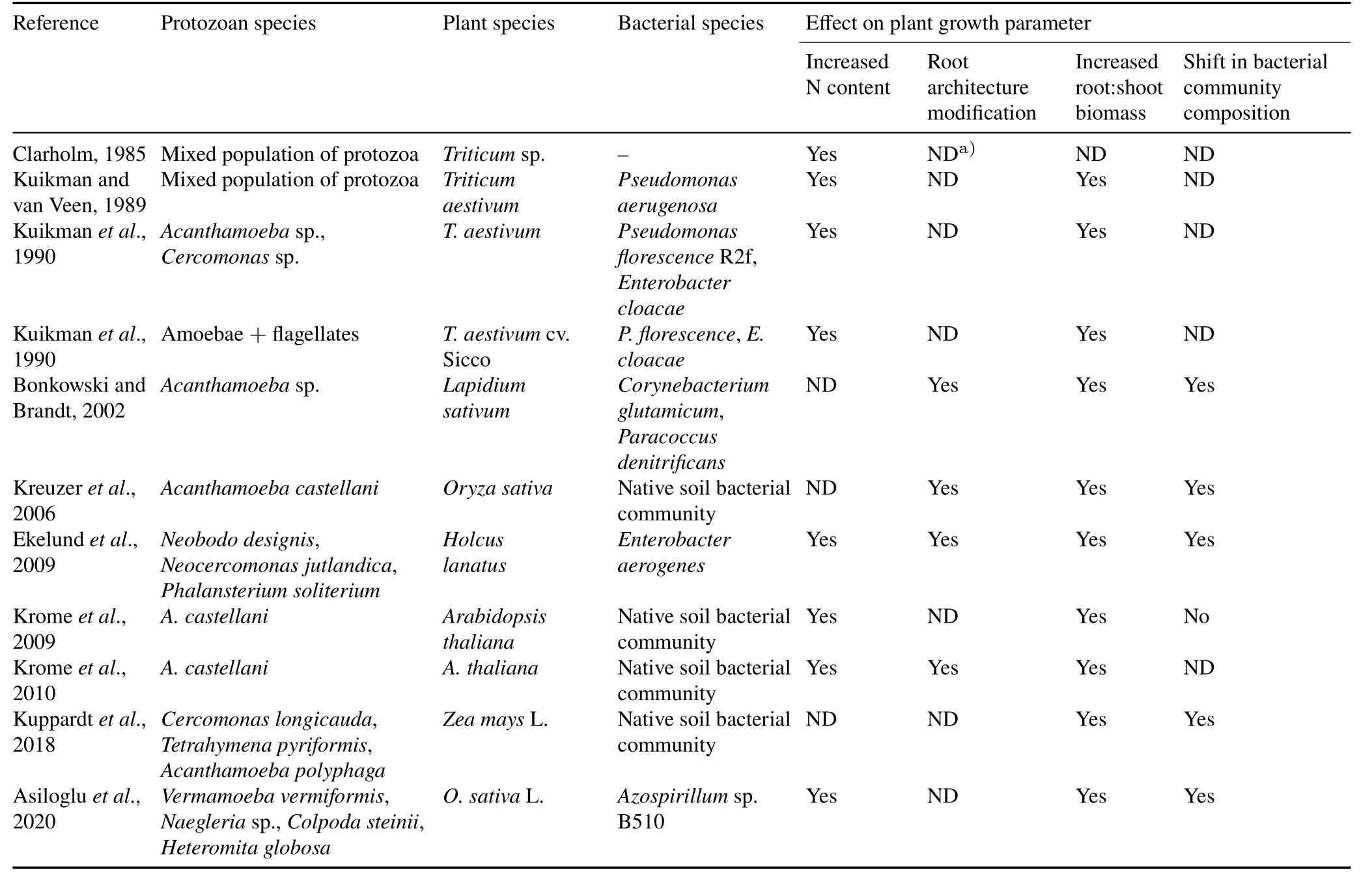
TABLE III Some of the studies revealing positive effects of protists(traditionally known as protozoa)on plant growth
Methodological constraints in protistological studies
There are several methods to study the abundance and diversity of protists.Although recently developed reliable molecular methods and the emergence of high-throughput sequencing methods,such as amplicon sequencing,metagenomics,and metatranscriptomics(Geisen and Bonkowski,2018)provide opportunities to explore the importance of soilborne protists,the traditional method of direct observation of protists(Smirnov and Brown,2004)is still in use to study the specific morphology of protist species.Nonetheless,a lack of comparisons between traditional culture-based methods and recent molecular studies hampers the advancement in identifying the taxa and abundance of protist species.Highthroughput sequencing methods help to discover the diversity of protists,but the whole large clades can be known only from sequences.Moreover,closely related protist species are fundamentally different in their copy numbers of targeted barcode genes.Therefore,much more effort is needed to cultivate and describe the immense diversity of protists.
ACKNOWLEDGEMENT
This work was supported by the Department of Science and Technology,Science and Engineering Research Board(DST-SERB),New Delhi,India under an ECRA grant for researchers to NA(ECR/2017/001977).
杂志排行
Pedosphere的其它文章
- Elevated carbon dioxide stimulates nitrous oxide emission in agricultural soils:A global meta-analysis
- Hydrogen cyanide production by soil bacteria:Biological control of pests and promotion of plant growth in sustainable agriculture
- Effects of different continuous fertilizer managements on soil total nitrogen stocks in China:A meta-analysis
- Microplastics in soil:Impacts and microbial diversity and degradation
- Rhizosphere microbiomes can regulate plant drought tolerance
- Difficult-to-culture bacteria in the rhizosphere:The underexplored signature microbial groups
