Actinobacteria-enhanced plant growth,nutrient acquisition,and crop protection:Advances in soil,plant,and microbial multifactorial interactions
2022-03-02DebasisMITRARittickMONDALBahmanKHOSHRUAnsumanSENAPATITRADHABhaswatimayeeMAHAKURNavendraUNIYALEiMonMYOHananeBOUTAJBeatrizElenaGUERRASIERRA0PeriyasamyPANNEERSELVAMArakalagudNanjundaiahGANESHAMURTHYSneanaANELKOVITanjaVASIAnjuRANISubhad
Debasis MITRARittick MONDALBahman KHOSHRUAnsuman SENAPATIT.K.RADHABhaswatimayee MAHAKURNavendra UNIYALEi Mon MYOHanane BOUTAJBeatriz Elena GUERRA SIERRA0Periyasamy PANNEERSELVAMArakalagud Nanjundaiah GANESHAMURTHYSnežana ANÐELKOVIĆTanja VASIĆAnju RANISubhadeep DUTTA and Pradeep K.DAS MOHAPATRA∗
1Department of Microbiology,Raiganj University,Raiganj,West Bengal 733134(India)
2Department of Sericulture,Raiganj University,Raiganj,West Bengal 733134(India)
3Department of Soil Science,Facultyof Agriculture,Universityof Tabriz,Tabriz 51664(Iran)
4Crop Production Division,Indian Council of Agricultural Research(ICAR)National Rice Research Institute,Cuttack,Odisha 753006(India)
5Division of Soil Science and Agricultural Chemistry,ICAR Indian Institute of Horticultural Research,Bengaluru,Karnataka 560089(India)
6Department of Botany and Biotechnology,Ravenshaw University,Cuttack,Odisha 753003(India)
7Genetics and Tree Propagation Division,Forest Research Institute,Uttarakh and 248003(India)
8Biotechnology Research Department,Department of Research and Innovation,Ministry of Education,Kyaukse 05151(Myanmar)
9Laboratory of Biotechnology and Molecular Bioengineering,Faculty of Sciences and Technology,Cadi Ayyad University,Marrakesh 40000(Morocco)
10Universidad de Santander,Facultad de Ciencias Exactas Naturales y Agropecuarias,GrupoMicrobiota,Campus Universitario Lagos del Cacique,Bucaramanga 680002(Colombia)
11Institute for Forage Crops,Kruševac 37251(Republic of Serbia)
12Plant Protection,Facultyof Agriculture,Universityof Niš,Kosančićeva 4,Kruševac 37000(Republic of Serbia)
13Department of Life Sciences,Graphic Era(Deemed to be University),Dehradun,Uttarakhand 248002(India)
14Professor A.K.Bothra Environment Conservation Centre,Raiganj University,Raiganj,West Bengal 733134(India)
ABSTRACT Agricultural areas of land are deteriorating every day owing to population increase,rapid urbanization,and industrialization.To feed today’s huge populations,increased crop production is required from smaller areas,which warrants the continuous application of high doses of inorganic fertilizers to agricultural land.These cause damage to soil health and,therefore,nutrient imbalance conditions in arable soils.Under these conditions,the benefits of microbial inoculants(such as Actinobacteria)as replacements for harmful chemicals and promoting ecofriendly sustainable farming practices have been made clear through recent technological advances.There are multifunctional traits involved in the production of different types of bioactive compounds responsible for plant growth promotion,and the biocontrol of phytopathogens has reduced the use of chemical fertilizers and pesticides.There are some well-known groups of nitrogen-fixing Actinobacteria,such as Frankia,which undergo mutualism with plants and offer enhanced symbiotic trade-offs.In addition to nitrogen fixation,increasing availability of major plant nutrients in soil due to the solubilization of immobilized forms of phosphorus and potassium compounds,production of phytohormones,such as indole-3-acetic acid,indole-3-pyruvic acid,gibberellins,and cytokinins,improving organic matter decomposition by releasing cellulases,xylanase,glucanases,lipases,and proteases,and suppression of soil-borne pathogens by the production of siderophores,ammonia,hydrogen cyanide,and chitinase are important features of Actinobacteria useful for combating biotic and abiotic stresses in plants.The positive influence of Actinobacteria on soil fertility and plant health has motivated us to compile this review of important findings associated with sustaining plant productivity in the long run.
KeyWords:biocontrol agents,microbial inoculant,metabolites,mitrogen fixation,plant growth promoters,sustainable agriculture
INTRODUCTION
Owing to the increasing global population,the demand for agricultural productivity is also increasing.To cope with the rising food demand,large-scale applications of chemically synthesized pesticides and fertilizers have been practiced to boost agricultural productivity(Zhanget al.,2018).With the recent advances in agricultural equipment and innovative methods of application,traditional methods have reached their limits of efficiency(Pivotoet al.,2018).For high-yielding varieties,chemical fertilizers are conventionally used in higher doses to increase productivity.These chemicals not only accumulate in crop plants and seeds,but also pollute the soil and water,including groundwater(Kumaret al.,2007;Heidarpouret al.,2019).In this context,scientists have found several sustainable methods of switching to ecofriendly farming activities(Mishra,2013;Khoshruet al.,2020a).Several studies have reported different solutions to the problematic situation of chemical usage in agriculture.Among the proposed solutions,microorganisms with multifunctional traits have been found to reduce the use of chemical fertilizers and pesticides by producing or releasing different types of bioactive compounds(Janardhanet al.,2014),enzymes(Turanet al.,2016;Verbon and Liberman,2016;Khoshruet al.,2020b),and antimicrobial substances or biocontrol compounds(Dhanasekaranet al.,2005;Liuet al.,2018).In addition,others are plant growth promoters(Pérez-Montañoet al.,2014;Sarikhaniet al.,2016,2020;Tanget al.,2016;Gange and Gadhave,2018;Khoshruet al.,2020c).Considering this point,plant growth-promoting rhizobacteria(PGPR)are a good alternative,and they have demonstrated mutualistic relationships with plants(Tanget al.,2016;Zhanget al.,2016;Rosieret al.,2018;Raklamiet al.,2019;Khoshruet al.,2020a;Sarikhaniet al.,2020).The PGPR are naturally occurring(Rosieret al.,2018),freeliving,rhizosphere-colonizing bacteria that enhance plant yield,growth,and soil fertility,decrease pathogens,and help to tolerate or resist biotic or abiotic stresses(Vessey,2003;Kumaret al.,2014;Sarikhaniet al.,2019;Khoshmanzaret al.,2020).Actinobacteria are among the PGPR equipped with multifunctional plant growth-promoting(PGP)traits and many properties beneficial to plant growth(El-Tarabily and Alkhajeh,2016;Monteiroet al.,2017)(Fig.1).
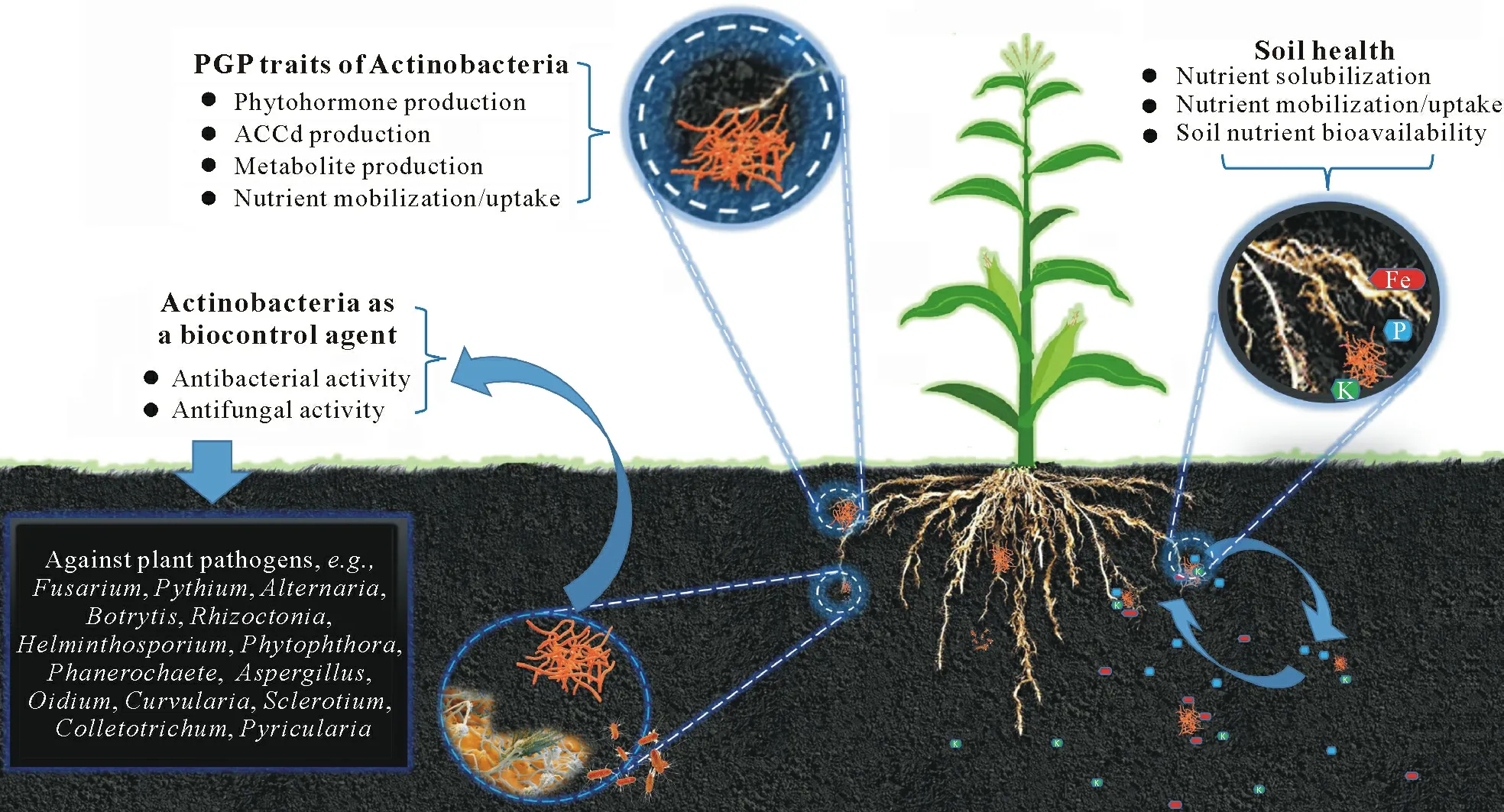
Fig.1 Beneficial impacts and interaction of Actinobacteria with plant and rhizosphere.PGP=plant growth-promoting;ACCd=1-aminocyclopropane-1-carboxylate deaminase.
Today’s world requires ecofriendly methods to achieve high output yield,enhanced crop production,and better soil fertility(Yasariet al.,2009).Actinobacteria as bio-inoculants and bio-pesticides are an alternative to chemical fertilizers.They can improve crop production under multiple stress conditions,such as temperature,pH,salinity,and drought(Chenget al.,2018).Streptomycesis the most abundantly occurring Actinobacteria genus in the soil(Panneerselvamet al.,2021).First,owing to their high growth rate,Streptomyces.SPP.efficiently colonize plant root systems and can withstand adverse growth circumstances through the formation of spores.They produce many enzymes and organic compounds that are beneficial to plant growth(Polak and Provasi,1992;Vonothiniet al.,2008;Syedet al.,2009).Soil Actinobacteria are known to produce active compounds in the rhizosphere,many of which are important in agriculture(Suzukiet al.,2000).Khanet al.(2010)reported that phosphorus(P)could be implicated in several metabolic processes of plant hosts,such as energy transfer,photosynthesis,macromolecular biosynthesis,signal transduction,and respiration.Phosphorus availability to plants is facilitated through the soil P cycle.Richardson and Simpson(2011)noted that Actinobacteria directly solubilize and mineralize inorganic P or mediate organic P availability through microorganism turnover and root system increase.Actinobacteria lower soil pH through the secretion of different types of organic acids,improving P availability to plants(Kauret al.,2016)and in turn plant yield through the establishment and development of the entire root systemshoots(Khanet al.,2010).Sahuet al.(2007)demonstrated the phosphate-solubilizing potential of Actinobacteria in an estuarine environment.The positive influence of pure Actinobacterial strains on plants through multifunctional attributes,such as rock phosphate solubilization,has been briefly described by Hamdaliet al.(2008a,b).Several reports on PGP Actinobacteria with a vast number of phosphate-solubilizing microbes have been represented in several studies(Dastageret al.,2010;Vermaet al.,2013;Singh Pet al.,2014;Anwaret al.,2016).According to Karlidaget al.(2007),plant growth is also improved by potassium(K)-solubilizing Actinobacteria.Similarly,iron(Fe)is an essential nutrient and a necessary co-factor for numerous enzymatic reactions in virtually all organisms.Plant growth is direct or indirect improved by Fe-chelating compound-producing Actinobacteria,includingStreptomyces,Micrococcus,Microbacterium,Kocuria,Corynebacterium,andArthrobacter(Tiwariet al.,2011).There are numerous reports of Actinobacteria involvement in nitrogen(N)fixation,such as members of the genusFrankia,which are widespread endophytic Actinobacteria symbiotically associated with plant roots and fix atmospheric N for host plants.Symbiotic associations with Actinobacteria lead legume crops to undergo biological N fixation and meet their own needs without depending on external sources.Actinobacteria strains have been proven to be effective in a multidimensional way.They are involved in numerous PGP activities,such as siderophore and indole-3-acetic acid(IAA)production,complementing mycorrhizal fungi and maintaining the ecological balance in the soil system.The ability to produce phytohormones is extensively distributed among IAA-producing Actinobacteria and may potentially be employed to improve plant growth(Sharma and Mehta,2016;Tomilovaet al.,2016).Merckxet al.(1987)and Khamnaet al.(2010)reported that the involvement of Actinobacteria is crucial in plant growth promotion through siderophore and IAA production,which results in enhanced nutrient uptake.Moreover,metabolites produced by Actinobacteria restrict fungal phytopathogens likeColletotrichum gloeosporioides(potato dry rot),Alternaria brassicicola(rose apple anthracnose),Fusarium oxysporum(Chinese cabbage leaf spot),Sclerotium rolfsii(damping-offof balsam),andPenicillium digitatum(orange-green mold),thereby reducing disease symptoms.There is much evidence to suggest that Actinobacteria are potential bio-inoculants and biocontrol agents for plant growth improvement.All of the special qualities of this group make them a crucial tool in current agricultural practices(Poovarasanet al.,2016;Panneerselvamet al.,2017,2021).Here,we concentrate on Actinobacteria as an alternative tool for sustainable farming practices and for reducing harmful chemical usage to promote ecofriendly sustainable agriculture,thus reducing environmental damage and transforming chemical farming into organic farming.This review focuses on the interactions and current situation of Actinobacteria in relation to plant growth development,secondary metabolite production,biocontrol activity,and soil nutrient management for sustaining agricultural productivity.
TAXONOMY AND PHYSIOLOGY OF ACTINOBACTERIA AND THEIR ROLES IN SUSTAINABLE AGRICULTURE
The term Actinobacteria(formerly known as Actinomycetes)is derived from the Greek term aktis or aktin,and means“ray fungi”(Wiliams,1990).These bacteria are Gram positive,aerobic,and spore-forming,with a high percentage of guanine and cytosine in their DNA(>55%by mole)(Krogius-Kurikkaet al.,2009).Members of Actinomycetales are commonly referred to as Actinobacteria(Ludwiget al.,2012).Actinobacteria possess both bacterial(the cell wall shows a peptidoglycan structure)and fungal properties(filamentous appendages).The Actinobacteria phylum is the largest in bacteria and is classified into six classes:Coriobacteriia,Actinobacteria,Thermoleophilia,Rubrobacteria,Acidimicrobiia,and Nitriliruptoria(Ludwiget al.,2012).Actinobacteria are categorized with bacteria in the same class asSchizomycetesin a strict taxonomic context,but limited to theActinomycetalesorder(Ludwiget al.,2012).The class Actinobacteria is further divided into 16 orders:Actinopolysporales,Glycomycetales,Actinomycetales,Jiangellales,Streptosporangiales,Micrococcales,Bifidobacteriales,Catenulisporales,Frankiales,Kineosporiales,Micromonosporales,Pseudonocardiales,Propionibacteriales,Streptomycetales,Corynebacteriales,and incertae sedis(Zhiet al.,2009;Ludwiget al.,2012).The recent development of new sequencing technology has allowed a better grading of higher taxa unknown to the vast majority of researchers and has introduced new methods for phylogeny reconstruction.Further,Nouiouiet al.(2018)reported 20 orders of Actinobacteria(Actinomycetales,Acidothermales,Micrococcales,Corynebacteriales,Bifidobacteriales,Micromonosporales,Pseudonocardiales,Cryptosporangiales,Sporichthyales,Nitriliruptorales,Streptosporangiales,Catenulisporales,Frankiales,Geodermatophilales,Glycomycetales,Propionibacteriales,Jiangellales,Kineosporiales,Nakamurellales,and Streptomycetales)based on their whole-genome sequences in the latest reclassification.Different types of soil Actinobacteria,e.g.,Streptomyces,Actinoplanes,Nocardia,Micromonospora,andStreptosporangium,have been extensively used to improve the properties of soil and increase crop yield(Wahyudiet al.,2019).Particularly,the members ofStreptomycessp.have been found to play a major role because of the greater production of different plant-beneficial enzymes and metabolites(Kekudaet al.,2014).Actinobacteria can promote plant growthviavarious enzymes,such as cellulose,protease,pectinase,αamylase,xylanase,lipase,and chitinase(Nascimentoet al.,2002;Vonothiniet al.,2008;Syedet al.,2009;Sreevidyaet al.,2016;Zanget al.,2018;Siddharth and Rai,2019;Sharma and Thakur,2020),siderophore production(Leeet al.,2012),N fixation(Prakash and Cummings,1988),phosphate solubilization(Farhatet al.,2015;Anwaret al.,2016),IAA production(Myoet al.,2019),and plant growth regulator(e.g.,hormones)production(Passariet al.,2016).Actinobacteria play an important role in the management of phytopathogens by suppressing the growth of several types of plant pathogens,e.g.,Fusarium,Pythium,Alternaria,Botrytis,Rhizoctonia,Helminthosporium,Phytophthora,Phanerochaete,Aspergillus,Oidium,Curvularia,Sclerotium,Colletotrichum,andPyricularia,by secreting antimicrobial compounds(Hamdaliet al.,2008b;Luet al.,2008;Prapagdeeet al.,2008;Gopalakrishnanet al.,2013;Goudjalet al.,2014;Sreevidyaet al.,2016).Actinobacteria has a major role in the decomposition of organic materials through which the nutrient recycling process in soil gets fastened by virtue of lignocellulotic enzymes released by this group of bacteria(Daset al.,2007).Actinobacteria can adapt more easily to harsh environments than other microorganisms.They can grow in both alkaline and acidic soils and maintain soil properties under balanced conditions(Phoebeet al.,2001).Hence,Actinobacteria are an excellent choice for sustainable agriculture because they can both promote plant growth and present effective biocontrol activity(Coombset al.,2004;Meguroet al.,2006).
A study on the structural communities of Actinobacteria in soil using next generation sequencing of 16S amplicons from soil DNA found a total abundance of 16.68%±5.93%Actinobacteria in rice rhizosphere soil(Imchenet al.,2019).A related study also found that the population of Actinobacteria was 45%higher in rhizosphere than in nonrhizospheric soil(Janget al.,2020).DNA-stable isotope probing(DNA-SIP)techniques have found that biochar application increased the population of Actinobacteria in an oxisol(Yuet al.,2020).With a total abundance of 42.22%,Actinobacteria was the dominating phylum among other bacterial phylum in millets rhizosphere(Prabhaet al.,2019).Several uncommon Actinobacteria species have been found to be associated with the rhizosphere of alpine plants,among whichKitasatosporaand certain clades ofStreptomyces,includingStreptomyces subrutilus,Streptomyces avidinii,Streptomyces chinensis,Streptomyces mirabilis,Streptomyces olivochromogenes,Streptomyces brevispora,Streptomyces spororaveus,Streptomyces anulatus,Streptomyces camponoticapitis,Streptomyces erringtonii,andStreptomyces scabrisporus,have been identified(Oberhoferet al.,2019).Furthermore,in banana endophytes,Actinobacteria constituted 9.30%of the total bacteria identified,with the population of endophytic microbial community remaining unaffected by phytopathogenic fungal infection(Kaushalet al.,2020).These findings clearly demonstrate that the structural composition of Actinobacteria shifts in accordance with the plant type,soil amendments,and environment and that Actinobacteria have preferences for certain soil and plant types and are commensal microorganisms in plant ecosystem.
ACTINOBACTERIA AS BIOCONTROL AGENTS FOR PLANT PROTECTION
There are several studies and trials based on the usage of pesticides from biological sources instead of chemical sources.A good alternative to chemical sources is microbial sources,which can have multifunctionality and beneficial effects on plants and ecosystems.Actinobacteria are usually regarded as potent natural biocontrol agents in the soil(Gopalakrishnanet al.,2013).They compete with pathogens through the production of different secondary metabolites and enzymes,parasitism,and other biocontroling activities(Hamdaliet al.,2008b;Goudjalet al.,2014;Sreevidyaet al.,2016).Studies have reported that different types of plant pathogens,e.g.,Alternariaspp.,Fusarium oxysporum,Colletotrichum higginsianum,Pythium aphanidermatum,Phytophthora capsici,andFusarium oxysporumf.sp.lactucum,are very sensitive to the different bioactive compounds produced byStreptomycesspp.(Hamdaliet al.,2008b;Luet al.,2008;Goudjalet al.,2014;Sreevidyaet al.,2016).Recently,Wuet al.(2019)reported thatStreptomycesspp.produce a novel compound,antifungalmycin N2,which was found to be effective againstRhizoctonia solani.De Oliveiraet al.(2014)and Kaniniet al.(2013)reported thatStreptomycesspp.demonstrated antimicrobial activity againstXylella fastidiosaandRhizoctonia solani.Similarly,Streptomyces vinaceusdrappusshowed activity against rice fungal pathogens,andStreptomyces violaruswas effective against leaf blight disease pathogens(e.g.,Alternaria alternata).Furthermore,studies have discovered a metabolite,namely A-factor,which induces the production of other secondary metabolites in Actinobacteria(Fiebiget al.,2018).Different kinds of antibacterial agents,such as lankacidin C(Luet al.,2018),lankamycin(Luet al.,2018),actinorhodin(Čiháket al.,2017),aureomycin,and avermectin(Chenget al.,2018),are synthesized byStreptomycesspp.Table I shows the list of Actinobacteria known to suppress plant pathogens and their biocontrol efficiency and activity.
PGP TRAITS AND PLANT PROTECTION ABILITIES OFACTINOBACTERIA
Beneficial effects on rhizosphere
Plant growth-promoting microorganisms are mainly co-lonizers of the rhizoplane and rhizosphere region(Gopalakrishnanet al.,2013,2015b;Sarikhaniet al.,2020).Within the phylum Actinobacteria,the genusStreptomycesis predominant and ubiquitous in soil and water,existing both as rhizosphere-colonizing bacteria and plant endophytes.As someStreptomycesare endophytic,they can easily grow in the host cell without harming the host’s internal appearance(Kumaret al.,2014;Marella,2014).They assist in plant growth by providing various nutrients(e.g.,soluble P)and increasing the water uptake and retention capacityof the plant(Schützeet al.,2014).In return,Actinobacteria receives organic nutrients,such as sugars,from the plant,which help them to proliferate in soil.Streptomycesspp.also produce some catalytic enzymes and provide them to the host systems,wherein these enzymes easily break down complex biomolecular compounds into simpler chemical units(Nascimentoet al.,2002;Syedet al.,2009).Actinobacteria,mainlyStreptomycesand some other beneficial species,possess various plant PGP attributes(Gopalakrishnanet al.,2013;Anwaret al.,2016).Thus,Streptomycesare termed as PGPStreptomyces,with the ability to increase plant growth through direct or indirect biosynthetic pathways(Vurukondaet al.,2018).Common PGP attributes are shown in Fig.2(Saitoet al.,2003;El-Tarabilyet al.,2008;Khamnaet al.,2010).Actinobacteria directly facilitate plant growth by releasing microbial metabolites and providing biological nutrients such as P and N,which are preferred over agrochemicals,which cause serious environmental damage and are also expensive(Tanviret al.,2019).Actinobacteria also indirectly influence plant growth by minimizing the deleterious effects of pathogenic microbes through the production of antagonistic compounds.Table II lists Actinobacteria that have been reported as having PGP attributes.
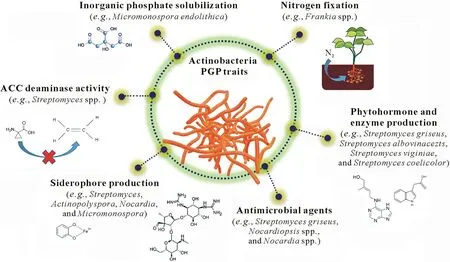
Fig.2 Major plant growth-promoting(PGP)traits of Actinobacteria.ACC=1-aminocyclopropane-1-carboxylate.
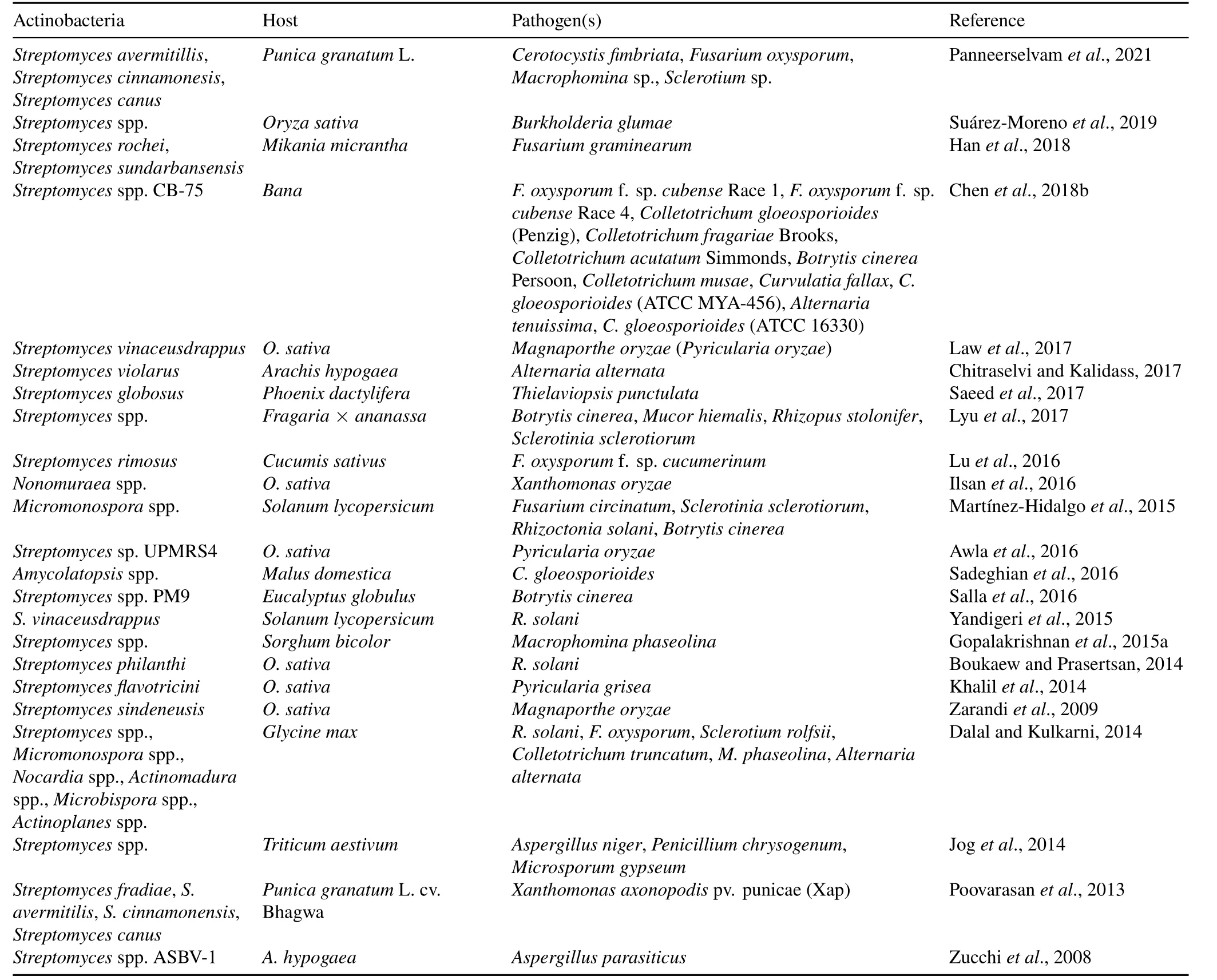
TABLE I Some Actinobacteria and the pathogens that they are against for plant protection and disease management
Nitrogen fixation
Nitrogen is one of the most important elements for plant growth and accounts for about 78%of the atmosphere.Owing to the presence of a stable triple bond,N remains unavailable for most organisms(Callahamet al.,1978).Nitrogen-fixing bacteria convert atmospheric dinitrogen(N2)into the ammonical form(),which is available for plants(Dudejaet al.,2012).Plants undergoing symbiosis with N-fixing bacteria generally do not experience N deficiency,and their coexistence in the soil decreases the rate of N application(Hureket al.,2002).Two groups of N-fixing bacteria,rhizobia andFrankia,form nodules,which are specialized organs required for the coexistence of microorganisms in the roots of the host plant.Diazotrophy in Actinobacteria has been long thought to be restricted to the genusFrankia(Villegaset al.,1997;Buckleyet al.,2007;Trujilloet al.,2010;Dahalet al.,2017).Molecular studies have revealed the existence of thenifHgene(encoding the nitrogenase reductase subunit)in numerous species ofFrankia.This observation has provided deep insights into the origin and emergence of bacterial diazotrophs among the Actinobacteria phyla(Buckleyet al.,2007;Trujilloet al.,2010).These findings have stimulated further research into the basis of diazotrophy andnifHgene transfer in Actinobacteria.Studies have found thatFrankiaspp.establish symbiosis with more than 200 non-legume plant species belonging to 23 genera from eight families,called actinorhizal plants(Normand and Lalonde,1982).Additionally,several studies have been performed on non-FrankiaN-fixing Actinobacteria and discover new plant-Actinobacteria associations among whichMicromonosporabased N fixation is the most prevalent one.Various species belonging to the genusMicromonosporahave been obtained in Italy and Brazil from the root surface of plants such as maize(Carroet al.,2012;Martnez-Hidalgoet al.,2015).In recent years,Micromonosporaespp.have been reported as major symbionts involved in N fixation in the root nodules of both leguminous and actinorhizal plants(Garciaet al.,2010;Carroet al.,2012).
Phosphate solubilization
Phosphorus is one of the key plant nutrients responsible for plant growth promotion.In soil,P is mainly present in unavailable(i.e.,insoluble,precipitated,or immobilized)forms,constituting 30%—65%of the total organic P present in the soil(Shenet al.,2011).Thus,it is very difficult for plants to utilize this type of P because of their association with different cations present in soil.The availability of P directly affects crop production.Plant uptake of P is only possible in the form of soluble monobasic(and dibasicions(Naranget al.,2000).Phosphate-solubilizing soil microbes have the ability to release free phosphate through mechanisms including production of organic acids,such as succinic acid,gluconic acid,oxalic acid,and citric acid,which participate in dissociation of chemical bonds of bound phosphates in soil(Rajputet al.,2013;Verma,2019).Therefore,converting the unavailable forms of P to the available forms by solubilization and mineralization is one of the basic criteria involved in the selection and introduction of P-solubilizing microorganisms(PSMs),among which Actinobacteria have been found to be crucial(Lunggani and Suprihadi,2019).These bacteria are used to secrete different types of organic acids(e.g.,gluconic acid and ketogluconicacid)that decrease the rhizosphere pH,thereby releasing bound forms of P,such as Ca3(PO4)2,in calcareous soils(Farhatet al.,2015).
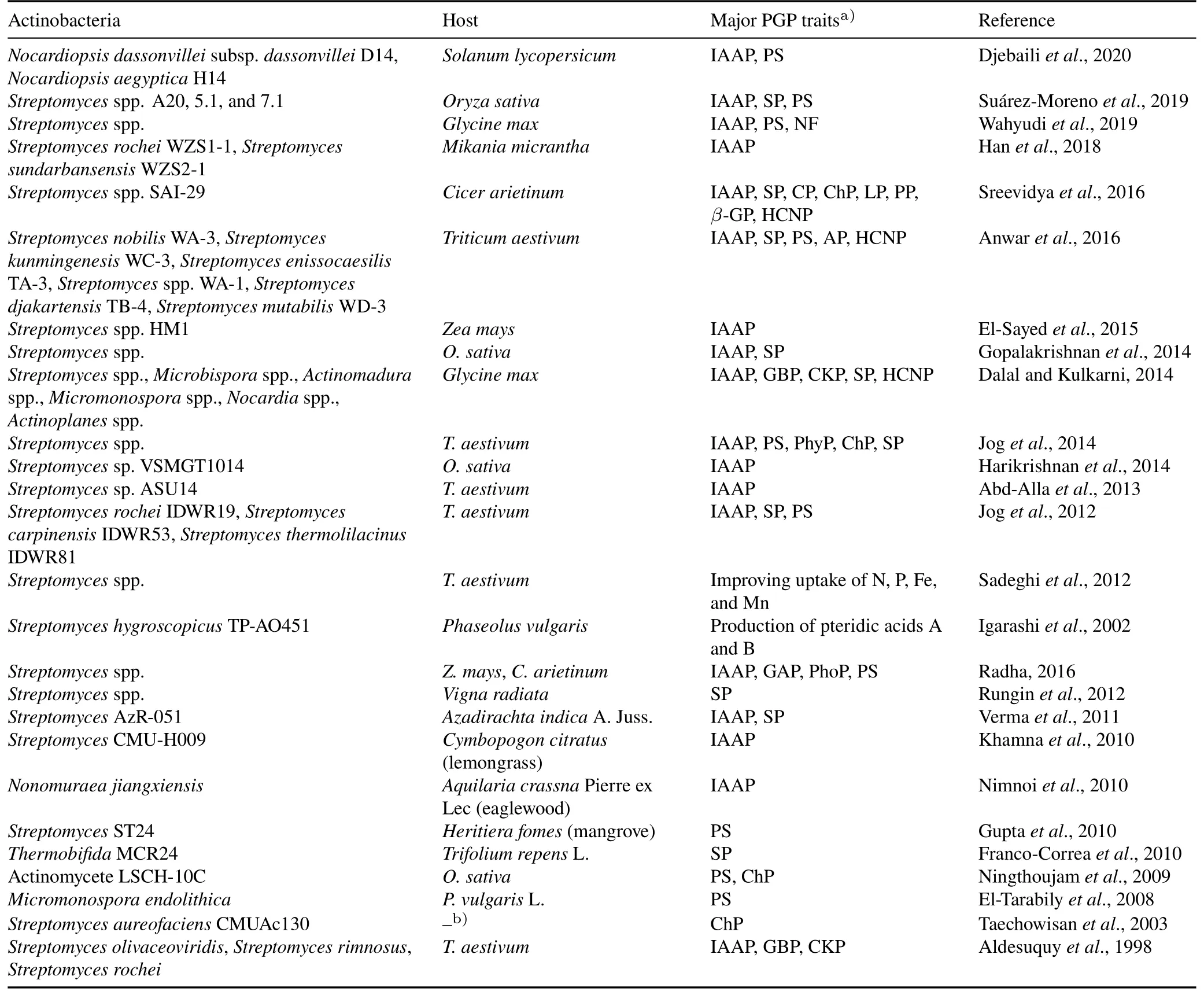
TABLE II Some Actinobacteria and their plant growth-promoting(PGP)traits
Recent research has established that the P-solubilizing activity of Actinobacteria plays a role in sustainable agriculture(Hozzeinet al.,2019).It has been found that about 20%of Actinobacteria,such asStreptomycesandMicromonospora,have high P solubilization potential(Barretoet al.,2008).Joget al.(2014)reported that the highest malate amount was recorded in the P-solubilizing Actinobacteria,Streptomycesmhcr0816.Malate synthesis may be performed by glyoxylate bypass,which was proven among relative expression analysis of isocitrate dehydrogenase,malate synthase,and isocitrate lyase using specifically designed enzymes and primers.Some studies have shown that N-fixing Actinobacteria also present P solubilization activities(Sahuet al.,2007;Gangwaret al.,2012;Salcedoet al.,2014).Actinobacteria with P solubilization potential increase plant growth owing to their ability to produce active metabolites,such as phytohormones,siderophores,and antibiotics,as well as their ability to withstand stressful conditions(Hamdaliet al.,2008c;Tanviret al.,2019).A study found that 44%of all Actinobacteria isolated from the rhizosphere of rice possessed P solubilization activities,and the actinobacteriumS.lavendulaeR22 showed the highest P solubilization of 265 mg L−1(Gangwaret al.,2012).In one experiment,it was reported that among all the Actinobacteria isolated from the rhizosphere ofTrifolium repens,a 20%inorganic P-dissolution activity was observed.In that experiment,all isolates were capable of producing acid phosphatase,and 43%of isolates produced alkaline phosphatase,which increased the potential of Actinobacteria to mineralize organic P(Richardsonet al.,2009).Actinobacteria have been also reported to produce phosphatases(Franco-Correaet al.,2010;Pragyaet al.,2012).
The rate of P solubilization by Actinobacteria depends on:i)the type of insoluble inorganic P sources,ii)the intrinsic P solubilization ability of the Actinobacteria species,and iii)the amounts and types of photosynthetic compounds present in the rhizosphere(Banik and Dey,1982).It has been observed that a single Actinobacteria species can have the capacity to dissolve both inorganic and organic P(Taoet al.,2008;Asaduet al.,2018).Actinobacteria have also been reported to produce the extracellular enzyme phytase in addition to phosphatase.The phytate mineralized through phytase is known as a predominant form of P but unavailable to plant hosts.Phytate is produced by sporulatingStreptomyces,Bacillus,and several other Actinobacteria(Richardson and Simpson,2011;Joget al.,2012).Ghorbani-Nasrabadiet al.(2012)reported the isolation of phytate-degrading Actinobacteria(S.albonigerandS.venezuelae)from arable soil with the highest activity of 46.30%.In another study,P-solubilizing Actinobacteria isolated from Moroccan P mines were tested on wheat crops,and the results suggested an increase in wheat plant biomass.In particular,Actinobacteria strain BH7,showing the best P-solubilizing capacity,stimulated the highest biomass of wheat plants underin vitroconditions.An increase of 70%in plant yield was observed in test tubes and higher than 30%in soil with rock P.Similarly,Joget al.(2014)reported an Actinobacteria strain,Streptomycesmhcr0816,isolated from soil,had a P-solubiling capacity of 1.916 mg L−1and produced auxin,siderophore,and chitinase.The inoculation of wheat with this Actinobacteria not only increased wheat biomass and yield,but also increased the contents of K,Fe,and zinc(Zn).If applied as biofertilizers,efficient strains of Actinobacteria can reduce the use of chemical fertilizers(Sahuet al.,2007;Hozzeinet al.,2019).The PSMs,including heterogeneous and naturally abundant rhizosphere microbes,whose participationin totofulfills plant P demand,have been found to be a viable alternative biotechnology solution for sustainable agriculture(Richardson and Simpson,2011).
Phytohormone and siderophore production
Phytohormones perform an essential role in controlling plant cell physiological processes(Le Bris,2003)and also in plant-microbe interactions(Solanset al.,2011).The effects of phytohormones(e.g.,auxins,abscisic acid,ethylene,cytokines,and gibberellins)on plant growth depend upon their levels(Passariet al.,2016).Some microorganisms(including fungi and bacteria)are capable of synthesizing these phytohormones(Shutsrirunget al.,2013).Thein vitrosynthesis of phytohormones(including gibberellic acid,auxins,and cytokinins)from Actinobacteria,which support plant growth,has been documented by several researchers(Mahadevan and Crawford,1997;Ghodhbane-Gtariet al.,2010;Gopalakrishnanet al.,2014;Anwaret al.,2016;Soláet al.,2019).
The primary plant auxin,IAA,controls many fundamental cellular mechanisms,including cell division,differentiation,and elongation(Le Bris,2003).It also enhances root hair formation,which improves plant nutrient absorption capacity from soil.Auxins also play an integral role in several developmental processes,such as the development of embryos and fruit,vascular tissue differentiation,elongation,organogenesis,root patterning,tropic growth,apical dominance,and apical hook formation(Dobbelaereet al.,1999).Farinaet al.(2012)reported that five isolates of Actinobacteria produced high amounts of IAA when grown on a tryptophan-supplemented medium.Several reports have suggested that the application of Actinobacteria to plants or seeds induces seed germination,seedling growth,rooting,and cell elongation(El-Tarabily,2008;Goudjalet al.,2013).Radha(2016)reported the improved growth and yield parameters of maize and chickpea after inoculation with Actinobacterial strains.Meguroet al.(2006)reported that plants inoculated withStreptomycessp.MBR52(IAA producer)showed improved emergence and elongation of new roots within a few days.Goudjalet al.(2013)reported that theS.rocheistrain PTL2 isolated from Algerian native plants showed an IAA content of 127µg mL−1.Inoculation of this isolate in the tomato plant resulted in significant increases in seed germination and root elongation rates.In a similar experiment,the inoculation of tomato with auxinproducingS.caeruleatusZL2 significantly increased the dry weight and root length of the plant(Zamoumet al.,2017).The inoculation of plants with the auxin and gibberellinproducing-actinobacteriumStreptomycessp.IA1,isolated from field soil,was also found to increase the growth of both tomato(Goudjalet al.,2016)and wheat(Toumatiaet al.,2016).El-Tarabilyet al.(2008)reported that the inoculation of cucumber plants with endophytic Actinobacteria significantly increased the growth of the plant,due to the production of IAA,indole-3-pyruvic acid,and gibberellic acid.Shutsrirunget al.(2013)reported that 64 isolates from mandarin(Citrus reticulataL.)were found to belong to the
Nocardiopsis,Streptomyces,Spirillospora,Microbispora,Nocardia,andMicromonosporagenera,of which 85.3%belonged to theStreptomycesgenus.From these 64 isolates,the top 12 auxin-producing isolates were inoculated into the mandarin plant,which resulted in increased shoot and root fresh weight and height.Cytokinin production in the rhizosphere has been reported in plant-associated Actinobacteria(Khamnaet al.,2010;Patel and Saraf,2017),such asS.flavovirens(Coppola and Giannattasio,1968).Actinobacteria isolated fromOchetophila trinervishave been reported to have the potential for auxin(IAA),cytokinin,and gibberellic acid production(Solanset al.,2011).Based on the reports and reviews,it can be seen that phytohormone-producing Actinobacteria play a superior role in plant-microbe interactions and act as effective plant growth regulators that can be used for rhizospheric engineering purposes(Khamnaet al.,2010;Solanset al.,2011;AbdElgawadet al.,2019).
Actinobacteria are a versatile group of soil bacteria in terms of their metabolic activity.They can produce different types of substances,including siderophores,which are lowmolecular-weight Fe(III)-chelating compounds(Walsh and Marshall,2004;Oves-Costaleset al.,2009).Siderophores are generally produced by microorganisms under Fe-deficient conditions,and they include phenolate hydroxamates,catecholate,salicylates,and carboxylate compounds(Kannahi and Senbagam,2014).Siderophores enhance Fe bioavailability by influencing its mobility and solubility.The Fe is coordinated in a soluble complex through siderophores.Siderophores can supply Fe nutrient during Fe deficiency periods in plant development,which have generally been observed in alkaline soils with pH>7.5(Vansuytet al.,2007).Siderophores have been referred to as micronutrient fertilizers because they improve the nutrient availability of minerals by chelating those which are required in very small amounts(Ahmed and Holmström,2014).Sathyaet al.(2017)suggested that siderophores can also improve bacterial rhizosphere colonization.Actinomadura madurae,Nocardia asteroids,andStreptomyces griseusare known siderophoreproducing Actinobacteria(Kannahi and Senbagam,2014).Actinobacteria mainly produce hydroxymate-and salicylatetype siderophores.Desferrioxamine E,a siderophore,has been reported to be produced byS.griseus(Yamanakaet al.,2005).Similarly,coelichelin(Challis and Ravel,2000;Lautruet al.,2005)and griseobactin(Patzer and Braun,2010)are different types of siderophores produced byStreptomycesspp.A new class of siderophores,heterobactins(hydroxamate and catecholate types),were found to be produced byRhodococcus(Carranet al.,2001).
1-Aminocyclopropane-1-carboxylate(ACC)deaminase activity
When a plant is under stress,ethylene,a phytohormone,evolves through its roots,limiting the growth and development of the roots and consequently the overall growth(Gupta and Pandey,2019).Under stress conditions,the production of the ethylene phytohormone is mediated by the ACC precursor.A wide variety of microbes are able to separate the amine group from the ACC through the enzyme ACC demaminase(ACCd)and convert it into two ammonia molecules(as a source of N)andα-ketobutyrate.Therefore,under stressful conditions,these bacteria promote growth and elongation of the plant root and its development by decreasing ethylene level.Therefore,the plants will have better and more favorable general conditions under stress(Misraet al.,2017).Many bacteria,including the generaEnterobacter(Sarkaret al.,2018)andBacillus(Ghoshet al.,2003),produce ACCd and reduce ACC content in plant tissues.In Actinobacteria,the generaStreptomycesspp.possess ACCd activity(El-Tarabily,2008).
Enzyme and metabolite and antibiotic production
Enzymes play a catalytic role in all biosynthetic and decomposition processes.Actinobacteria play a significant role in decomposition(Tiwariet al.,2019)owing to their saprophytic nature and production of different types of plant cell wall-degrading enzymes(Ramírez and Calzadíaz,2016).These enzymes can help degrade various complex polymers,such as lignin,hemicellulose,laccase,xylanase,and cellulose.Ventorinoet al.(2016)reported that important lignocellulose-degrading enzymes are produced byStreptomycesspp.The degrading enzymes produced by Actinobacteria(Guptaet al.,1995;Fodilet al.,2011)provide soluble nutrients to plants(Fig.3).Apart from the lignocellulolytic enzymes,chitinase produced by Actinobacteria has shown potential for biocontrol activity of several phytopathogens,including fungal and insect pests.Fungal and insect cell walls contain chitin,which can be degraded by chitinasess(Yandigeriet al.,2015).Thus,Actinobacteria(Streptomycesspp.)inhibit the growth of phytopathogens and protect the plant.
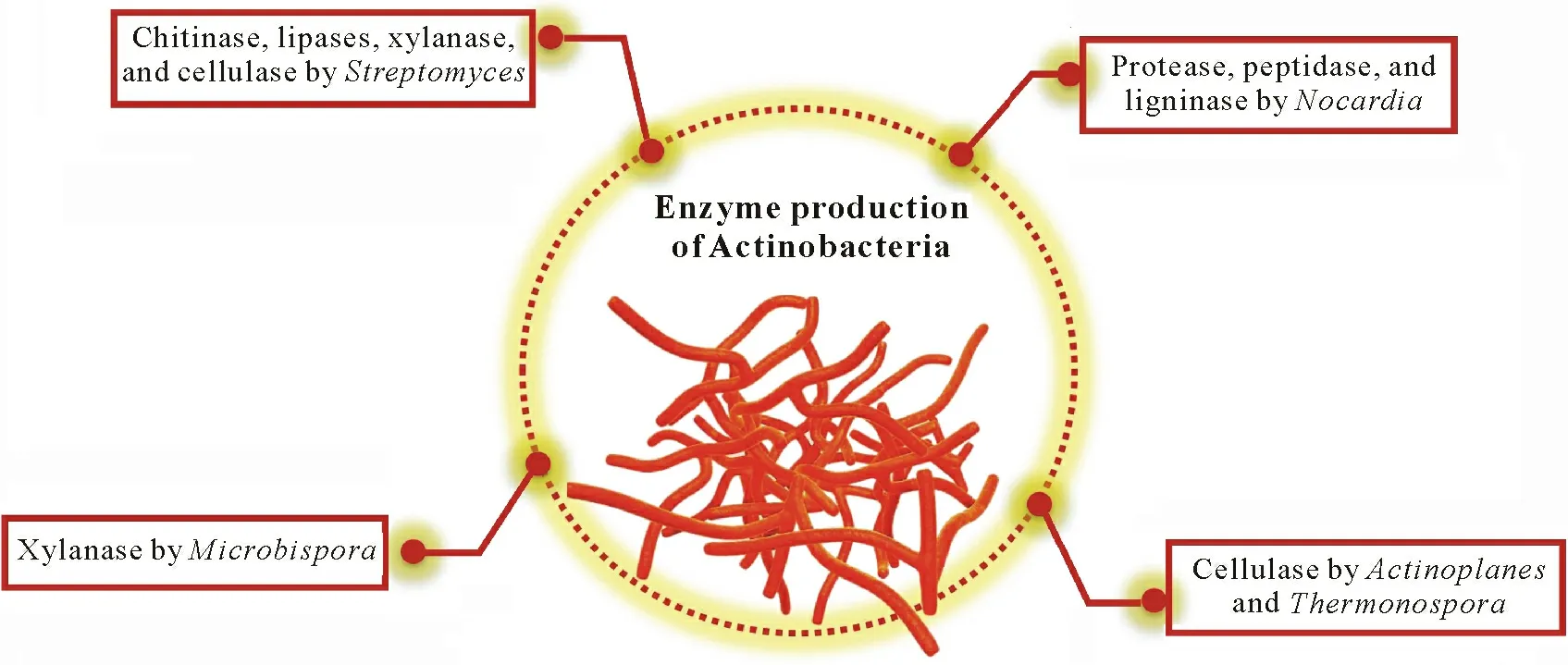
Fig.3 Different enzymes produced by Actinobacteria that can increase organic matter decomposition and crop productivity.
Actinobacteria are known to produce different secondary metabolites,like antibiotics(low-molecular-weight secondary metabolites that can exterminate or inhibit the growth of other organisms),which can indirectly help plant grow by reducing the incidences of phytopathogenic bacteria and fungal species(Table III,Fig.4).A variety of antibiotics,such as polyketides and peptideβ-lactams,in addition to other secondary metabolites,are responsible for antifungal,immunosuppressive,and antitumor activities(B˘ehal,2000).Liuet al.(2012)reported that 45%of all antibiotics were produced from Actinobacteria.Among these,Streptomycesspp.are dominant,as they are capable of producing a wide spectrum of antibiotics(Toumatiaet al.,2015).Streptomycin,produced byS.griseus,was discovered by Selman A.Waksman in 1943(Woodruff,2014)and was the first antibiotic to be effective against tuberculosis.Streptomycesspp.are capable of producing bioactive secondary metabolites with antimicrobial(Singhet al.,2016),antifungal,and antiviral activities(Maldonadoet al.,2010).Molanoet al.(2000)reported that the antibiotic actinomycin,produced byNocardiaspp.,strongly inhibitedF.oxysporum.Similarly,S.avermitilisproduced avermectin,which acts as a potent antimicrobial and antiparasitic agent(Chenget al.,2018).Actinobacteria also produce volatile organic compounds(low-molecular-weight carbon(C)-containing compounds)which are easily evaporated under normal temperature and help reduce pathogenic infection(Wanget al.,2013).In addition,Quecineet al.(2008)reported thatStreptomycesendophytes with chitinolytic activity significantly reduced pathogenic infection as well as promoted plant growth.
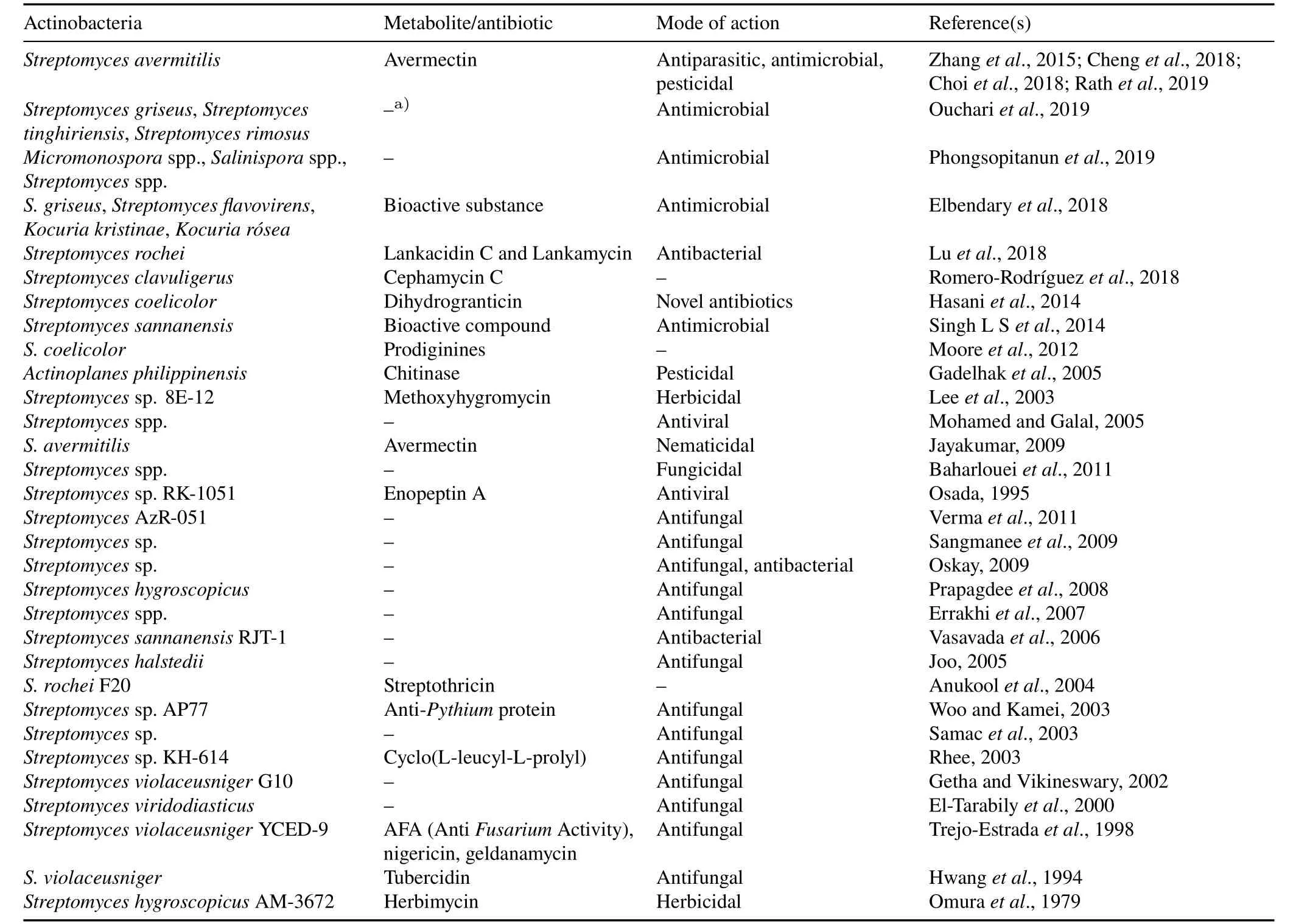
TABLE III Metabolites and antibiotics produced by Actinobacteria and their activities
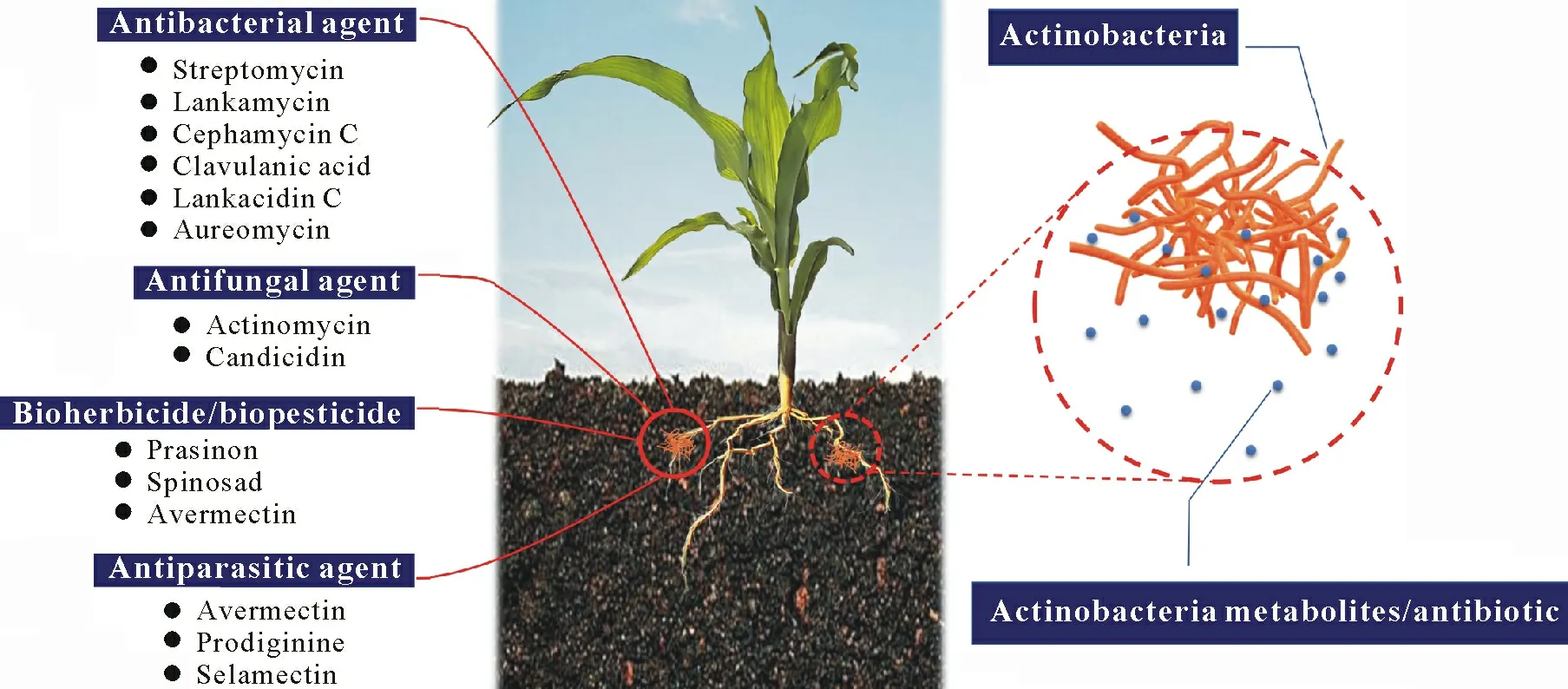
Fig.4 Secondary metabolites and antibiotics produced by Actinobacteria for plant protection and disease control.
Secondary metabolites synthesized by Actinobacteria are not only involved in plant growth promotion and pathogen control,but are also effective against major insect pests of agricultural crops(Table III,Fig.4).The antibiotic avermectin,produced byS.avermitilis,was found to stimulate the gamma amino butyric acid system and disrupt the nicotinic acetylcholine receptors(Adhyaet al.,2018)of parasites.Nikkomycins,a class of nucleoside peptide antibiotics produced byS.tendae,inhibit chitin synthesis in fungi and insects.Similarly,milbemycin,a class of macrocyclic lactone derivatives produced byS.hygroscopicus,showed broad-spectrum activity against agricultural pests,such as aphids,mites,caterpillars,intestinal worms,and other parasites that prey on crops and livestock.Tetranectin,a pesticidal macrotetrolide antibiotic produced byS.aureus,has been used as an agricultural miticide in Japan since 1973.Spinosyn A and Spinosyn D,produced bySaccharopolyspora spinosa,were found to be active against lepidopteran and dipteran pests(Snyderet al.,2007).Chenet al.(2018a)isolated 85 actinomycete strains fromAzadiachta indicaand tested them for their insecticidal action againstMyzus persicae.The results revealed that crude extracts from 24 strains were highly effective againstM.persicae,with eight actinomycetes strains showing insecticidal activity of more than 60%.El-Khawaga and Megahed(2012)screened the antibacterial and insecticidal activities of the crude extract of 20 actinomycete isolates from desert soil samples collected from different locations in Cairo,Egypt.Streptomyces bikiniensisA11 was found to be the most active actinomycete isolate against Gram-positive and negative bacteria,as well as against second-instar larvae of the cotton leaf wormSpodoptera littoralis.Actinobacteria genome sequencing has provided insights into the production of the secondary metabolites and found that the gene clusters responsible for biosynthesis of secondary metabolites are arranged as genomic islands and are dynamic entities transferred from one actinomycete to another through horizontal gene transfer(Pennet al.,2009).The Actinobacterial Database for Evolutionary Studies(ActDES)is a curated database developed using the high-quality genetic sequences of 612 genomes of Actinobacteria covering 80 genera,primarily aimed at elucidating the genetic architecture of Actinobacteria and designing a metabolic engineering framework(Schnieteet al.,2021).Horizontal gene transfer has been mainly found in prokaryotes and contributes to the evolution of new metabolites and pathways of degradation,such as the transfer ofN-succinylamino acid racemization ando-succinylbenzoate synthase from Firmicutes to Actinobacteria,which are required for menaquinone biosynthesis(Bar-Evenet al.,2011;Odokonyeroet al.,2018).
ROLES OFACTINOBACTERIA IN REGULATION OF PLANT STRESS,SOIL HEALTH,AND NUTRIENT MOBILIZATION
Meeting the expanding(about 1.05%annually)global food demand is a massive problem for the agriculture sector(World Population Prospects,2019).Global agricultural productivity and quality are regulated by biotic(infection of the plant due to various pests and pathogens)and abiotic stress(e.g.,salt concentration,water content,and soil nutrient levels)(Atkinson and Urwin,2012;Kumaret al.,2020).Actinobacterial-mediated biotic stress regulation in plants has been fulfilled by the production of different antimicrobial substances.Salinity and drought are the most devastating abiotic stresses to plant productivity.Salinity stress is caused by high concentrations of ions(such as Na+,Ca+,Mg+,K+,Cl−,)in the soil,and results in detrimental effects,i.e.,osmotic stress,oxidative stress,nutrient(e.g.,N,Ca,K,P,Fe,and Zn)deficiency,and ion toxicity,in plants(Groveret al.,2016).Drought stress is an important environmental stress caused by inadequate soil moisture.Under drought stress conditions,plants have limited water and experience nutrient deficiencies(Groveret al.,2016).Actinobacteria play a critical role in stress mitigation by synthesizing phytohormones,siderophores,secondary metabolites,and extracellular polysaccharides,solubilizing phosphate,increasing ACCd activity,and regulating stressresponsive genes in plants(Table IV)(Groveret al.,2016).
IMPROVEMENT OFPLANT RESIDUE DECOMPOSITION
The lignin degradation potential ofStreptomycesspp.is higher than that of any other species of bacteria(Januszet al.,2017).Molecular insights into the role played by Actinobacteria during plant residue decomposition have found the reason for the predominance of Actinobacteria throughout the decomposition process,particularly during the degradation of crop residues(Weiet al.,2018).Lignocellulose decomposition in the soil occurs through an array of oxidative biochemical reactions caused by a series of enzymes secreted by different groups of microorganisms(Kumar and Chandra,2020),which have definite successive orders depending upon the type of plant residue,as found in wheat crop residue(Zhonget al.,2020)and rice residue decomposition(Baoet al.,2020)in the soil.Lignin is an important polymer of lignocellulose present in the plant cell wall.It provides strength to the plant residue and its decomposition requires coordinated actions of different lignin-degrading catabolic enzymes.Kraft lignin decomposition studies using gel permeation chromatography coupled with mass spectrometry revealed that Streptomycessp.S6 produces an array of lignin-degrading enzymes,including laccases,lignin peroxidases,dye-decolorizing peroxidases,and aryl-alcohol oxidases,resulting in a kraft lignin reduction of 2115.7 Da in 7 d,with the release of at least eight types of aromatic compounds(Riyadiet al.,2020).Recent advancements have revealed cryptic pathways involved in the metabolism of aromatic compounds generated as byproducts during microbial lignin degradation.The derivatives of microbial lignin degradation byproducts,vanillate,cumarate and other lignin aromatic hydrocarbons,which are referred to as protocatechuate and phenylacetate,are converted into succinyl-CoA and acetyl-CoA through theβ-ketoadipate pathway by using thepca(encoding protocatechuate)andpaa(encoding phenylacetate)genes.In contrast,the phenol derivatives of homoprotocatechuate enter the meta-cleavage pathway,leading to the production of succinyl-CoA regulated by theGab(encoding succinate semialdehyde dehydrogenase)gene,which enters the trichloroacetic acid(TCA)cycle,completing the oxidation of lignin to CO2and H2O,as found in the soil ActinobacteriaKocuria rhizophila(Takaradaet al.,2008).Genome sequencing has revealed the presence of different catabolic pathways in addition to those discussed above,e.g.,the 2-hydroxypentadienoate pathway,gentisate pathway,homogentisate pathway,and hydroxyquinol pathway,as found in the nocardioform ActinobacteriaRhodococcus ruberChol-4(Guevaraet al.,2019).Further insights into the genome ofArthrobactersp.Rue61a revealed the presence of a meta-cleavage pathway,wherein 4-hydroxyphenylacetate catabolism was found to support the growth of this Gram-positive bacteria,whereas phenylacetate could not,demonstrating the presence of an incomplete set ofpaagenes(Niewerthet al.,2012).Actinobacteria have a different type of Type VII secretionsystem,known as the WXG100 secretion system,for secreting larger protein molecules.This system was found to share homology with similar secretion systems found in some members of other phyla,including Proteobacteria,Firmicutes,Cyanobacteria,and Lentisphaerae(Sutcliffe,2011).The presence of the competent enzymatic machinery and robust secretion system available for the complete catabolism of lignin polymers make Actinobacteria the last sucessors during the decomposition of plant residues.Among the other lignocellulose-degrading enzymes produced by actinomycetes are auxiliary members of glycoside hydrolases,including lytic polysaccharide monooxygenases(LPMOs)as found in the ActinobacteriaJonesia denitrificans(Mekashaet al.,2020)andKitasatospora papulosa(Corrêaet al.,2019),which are capable of enhancing the rate of lignocellulose degradation,particularly affecting the crystalline cellulose polymers.The first representative of bacterial LPMO was found in the cellulose-binding module CBH1 ofS.olivaceoviridis(Schnellmannet al.,1994).Later,AA10-type LPMOs,such as CelS2,were identified inS.coelicolor(Forsberget al.,2011),Streptomycessp.SirexAA-E(Takasukaet al.,2013),suggesting that the AA10 group of LPMOs are predominantly present inStreptomycesspp.(Booket al.,2014).Moreover,the recent genome sequencing of the ActinobacteriaStreptomyces albusCAS922 has provided insights into the vast lignocellulolytic enzyme repertoire,including 232 glycoside hydrolases with 3 belonging to the AA10 family identified as LPMO(Tippeltet al.,2020).Earlier reports found that some members ofStreptomyces,such asS.flavovirens,produce lignin-degrading enzymes and cause the decay of thick vascular tissue walls as in Douglas fir(Sutherlandet al.,1979).Moreover,S.viridosporusT7 A,which has been reported to be a potent lignin depolymerizer owing to its secretion of high amounts of extracellular esterases,was also found to be a potent phytopathogen(Donnelly and Crawford,1988).Presently,several nonpathogenic beneficial Actinobacteria have been described,among which,some lignocellulose-degrading Actinobacteria species(includingActinoplanes,Agromyces,Arthrobacter,Curtobacterium,Frankia,Kocuria,Microbacterium,Microbispora,Micromonospora,Nocardia,Streptomyces,andRhodococcus)have been classified as probiotic entities for plants owing to their different PGPR traits,including,as mentioned above,phytohormone and siderophore production,P solubilization,and antimicrobial and antagonistic activities(Menendez and Carro,2019).
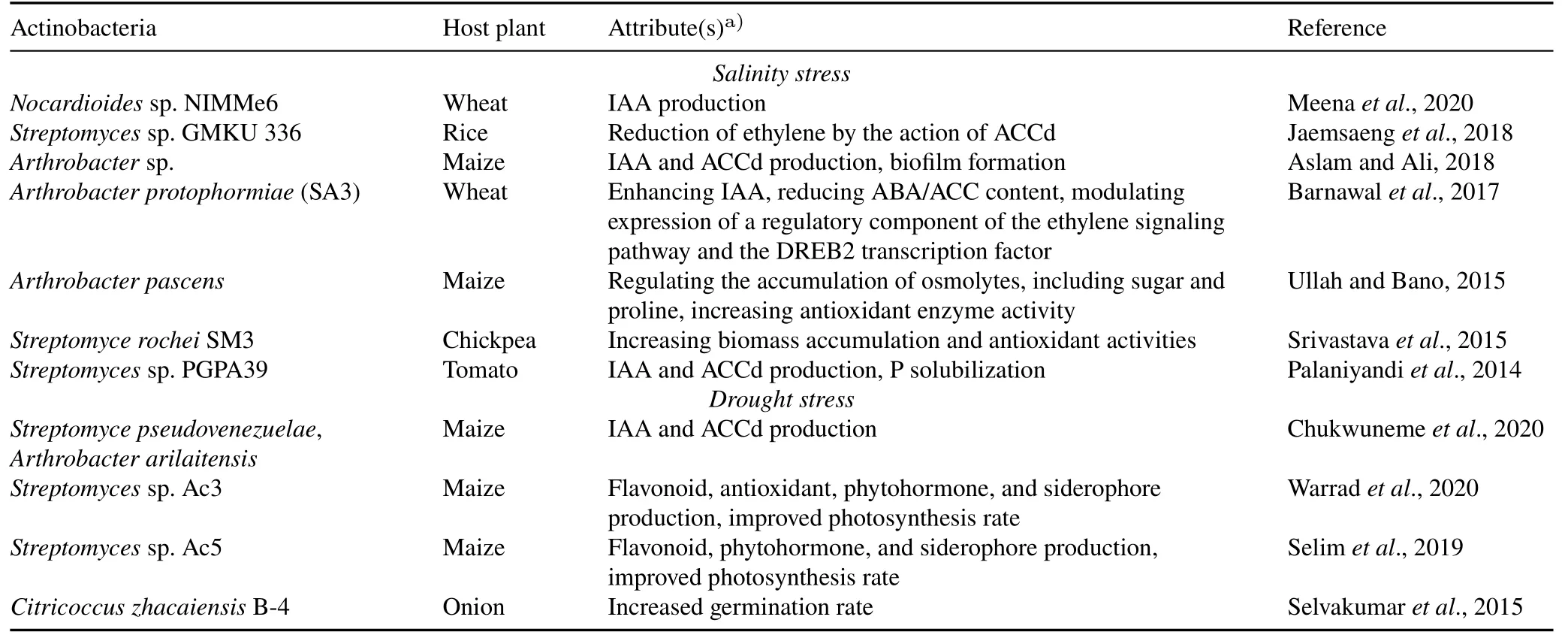
TABLE IV Actinobacterial-mediated alleviation of abiotic stresses in plants
Soils contain a huge population of living microorganisms that derive their energy by oxidizing organic residues,which are generally left behind after harvesting crops or by livestock feeding on these crops(Hoorman,2010).Beneficial soil microorganisms can be classified into four major groups:bacteria,fungi,Actinobacteria,and algae.Bacteria and fungi are the first to act upon organic residues,followed by Actinobacteria because of their slow activity and growth.In soil,Actinobacteria decompose the organic residues that are more resistant and indecomposable and generate numerous darkbrown to black pigments that add to the dark color of soil humus.They are also responsible for the further decomposition of humus.Actinobacteria are an intermediate group between bacteria and fungi,and 70% of soil Actinobacteria have been identified asStreptomyces.In general,Actinobacteria play a positive role in nutrient mobilization,which is associated with the capacity to mineral nutrients,such as Zn,Fe,and selenium(Sathyaet al.,2017).Actinobacteria such asStreptomycesspp.are involved in nutrient management and thus affect soil fertility as nutrient enhancers(Joget al.,2014).They play a critical role in soil health through many mechanisms,such as organic acid production(Rózycki and Strzelczyk,1986),P solubilization(Salcedoet al.,2014;Farhatet al.,2015),K solubilization(Nafiset al.,2019),N fixation(Kuchoet al.,2017),organic matter decomposition(Daset al.,2007),PGP hormone production(Soláet al.,2019),plant growth regulation,siderophore production(Leeet al.,2012),plant protection against biotic stress,biocorrosion,and biodegradation/bioremediation(Limayeet al.,2017).Actinobacteria can also be a source of metabolites that encourage or enhance the growth and development of host plants,as well as decrease the symptoms of disease induced by plant pathogens or environmental stresses.Radha(2016)reported the improvement of soil chemical properties,nutrient mobilization,soil biological activities(i.e.,beneficial microbial populations),and soil enzyme activities(e.g.,dehydrogenase,acid and alkaline phosphatases)with the inoculation of Actinobacteria on maize and chickpea.Viaeneet al.(2016)recently emphasized the contribution ofStreptomycetesto the growth and nutrient management of plants.In plants,Actinobacteria play a major role in shaping the root microbiome by modulating the composition of root exudates(chemotaxis)and nutritional exchanges.Plant root exudates are the source of metabolic signals(such as strigolactones,flavonoids,and terpenoids),which participate in shaping the rhizosphere microbial communities.The signals that attractStreptomycetesto the rhizosphere of plants are not clearly understood.Streptomycetescolonize the root tissues by entering the root from the rhizosphere(Coombs and Franco,2003).Actinobacteria influence soil fertility through the involvement of many components and serve as nutrient enhancers.They play a critical role in maintaining soil fertility and converting complex nutrients into simple mineral forms by secreting hydrolytic enzymes,including amylase,chitinase,cellulase,invertase,lipase,keratinase,peroxidase,pectinase,protease,phytase,and xylanase(Ramírez and Calzadíaz,2016).
IMPACTS OF FARMING PRACTICES ON ACTINOBACTERIA DIVERSITY IN SOIL
A culture-independent study revealed the population of Actinobacteria to be higher in non-cultivated soil than in cultivated soil(Wolińskaet al.,2019).The finding suggested that Actinobacteria are sensitive to physical disturbances in the soil,which break the actinomycetal hyphae that are difficult to repair.The cell diameter ofS.coelicolor,although less than 1µm,can reach 100µm through hyphal extension occurring at the tips through the membrane vesicle transporter,which can become perturbed by tangential forces in the soil acting on the hyphae,resulting in mechanical disruption(Goriely and Tabor,2003).Therefore,soil texture plays an important role in determining the dominance of soil bacterial species.Related studies have found conservation tillage practices to improve the abundance of functional bacteria populations in the soil(Wanget al.,2016).Actinobacteria play an important role in the C biogeochemical cycle,wherein the cellulose catabolism rate seems to be controlled by the C/N ratio of the soil(de Menezeset al.,2015).Although some studies have found the application of inorganic fertilizers to impact the spatial community structure of Actinobacteria(Piaoet al.,2008),there is evidence to suggest the improvement of the relative abundances of Actinobacteria populations under long-term doses of only N fertilization compared to those fertilized with a combination of N,P,and K(Daiet al.,2018).Research has also found that a certain Actinobacteria consortium,comprising fourStreptomycessp.strains(A2,A5,A11,and M7),demonstrated the capability to recover 70.3% of the recalcitrant insecticide lindane from a contaminated soil.This shows that Actinobacteria are promising tools for restoring soils contaminated with agrochemicals and could thereby help in the reclamation of soil arability(Raimondoet al.,2019).Moreover,metagenomic studies have confirmed that Actinobacteria have higher diversity in organic soil than in conventional modern agricultural soil(Sharmaet al.,2019).The association of Actinobacteria with arbuscular mycorrhizae fungi(AMF)is intriguing since the close association ofArthrobacterwith the AMFRhizophagus intraradicesabsorbed cadmium from the soil,making it unavailable to plants and reducing the cadmium toxicity in rice(Chenet al.,2019).Owing to the high price of chemical fertilizers and the widening gap between supply and demand,the solubilization of nutrients by microorganisms has been increasingly seen to be useful and economical(Fig.5).Microbial inoculants are ecofriendly and environmentally secure and only require low-cost technology,providing a solution for enhancing productivity and decreasing environmental problems.
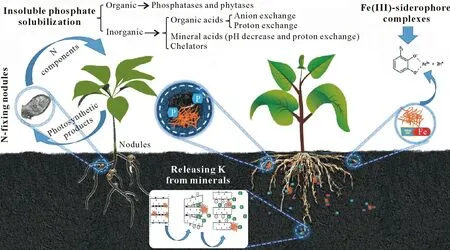
Fig.5 Actinobacteria help to manage the availability of nutrients in plants and rhizospheres.
CONCLUSIONS AND PROSPECTS AND CHALLENGES FOR THE FUTURE
In this highly demanding era,the scarcity of food and scientific ability to face new challenges have led to the introduction of novel agricultural tools and chemicals.The indiscriminate use of various chemicals has decreased soil quality,including soil fertility,texture,etc.An alternative way to sustain crop yield is to focus on biological sources.Actinobacteria are a special and proven group of bacteria that can easily troubleshoot several soil-related problems.These bacteria participate in different PGP activities,such as IAA,enzyme,hormone,and siderophore production,P solubilization,N fixation,and the secretion of other useful metabolites.Moreover,their strong biocontrol activities,such as competition with other microrganisms,parasitism,antibiosis,and volatile organic compound production,provide additional traits for Actinobacteria as well as their PGP activities,which make these bacteria a good alternative to chemical fertilizers and pesticides.Taking into consideration all of these factors,we can affirm that Actinobacteria are a very promising group of soil microbes with a proven record in agricultural practices,which could help to improve ecofriendly crop production.
The large and diverse group of Actinobacteria has become widely known primarily for its contribution to the production of different types of metabolites,which have a tremendous impact on agriculture.However,continuing research will allow us to discover more properties of Actinobacteria that will be useful for improving agricultural productivity and maintaining agricultural sustainability.The discoveries of new members of Actinobacteria will generate new challenges and changes,not only in terms of taxonomic reclassification but also in the potential discoveries of many more plant-beneficial properties that could have important biotechnological applications to meet future needs.
CONTRIBUTION OFAUTHORS
Debasis MITRA,Rittick MONDAL and Bahman KHOSHRU contributed equally as the first authors.
ACKNOWLEDGEMENTS
The authors are thankful to Raiganj University,Indian Council of Agricultural Research(ICAR)National Rice Research Institute,ICAR Indian Institute of Horticultural Research,Ravenshaw University,Forest Research Institute,and Graphic Era(Deemed to be University)of India;University of Tabriz,Iran;Ministry of Education,Myanmar;Cadi Ayyad University,Morocco;Universidad de Santander,Colombia;and Institute for Forage Crops and University of Niš,Republic of Serbia for the support.Debasis MITRA is grateful to the Government of West Bengal,India for the Swami Vivekananda Merit Cum Means Ph.D.Scholarship.Rittick MONDAL would like to acknowledge Department of Science and Technology(DST),India for Inspire Fellowship(No.IF190457).The authors are grateful to editors and reviewers for their valuable suggestions to improve the scientific quality of the manuscript.
杂志排行
Pedosphere的其它文章
- Elevated carbon dioxide stimulates nitrous oxide emission in agricultural soils:A global meta-analysis
- Hydrogen cyanide production by soil bacteria:Biological control of pests and promotion of plant growth in sustainable agriculture
- Effects of different continuous fertilizer managements on soil total nitrogen stocks in China:A meta-analysis
- Microplastics in soil:Impacts and microbial diversity and degradation
- Rhizosphere microbiomes can regulate plant drought tolerance
- Difficult-to-culture bacteria in the rhizosphere:The underexplored signature microbial groups
