Priming effect and its regulating factors for fast and slow soil organic carbon pools:A meta-analysis
2022-03-02ChangfuHUOJunyiLIANGWeidongZHANGPengWANGandWeixinCHENG
Changfu HUO,Junyi LIANG,Weidong ZHANG,Peng WANGand Weixin CHENG
1KeyLaboratoryof Forest Ecologyand Management,Institute of Applied Ecology,Chinese Academyof Sciences,Shenyang 110016(China)
2Department of Grassland Resources and Ecology,College of Grassland Science and Technology,China Agricultural University,Beijing 100083(China)
3Environmental Studies Department,Universityof California,Santa CruzCA 95064(USA)
ABSTRACT The priming effect(PE)plays a critical role in the control of soil carbon(C)cycling and influences the alteration of soil organic C(SOC)decomposition by fresh C input.However,drivers of PE for the fast and slow SOC pools remain unclear because of the varying results from individual studies.Using meta-analysis in combination with boosted regression tree(BRT)analysis,we evaluated the relative contribution of multiple drivers of PE with substrate and their patterns across each driver gradient.The results showed that the variability of PE was larger for the fast SOC pool than for the slow SOC pool.Based on the BRT analysis,67%and 34%of the variation in PE were explained for the fast and slow SOC pools,respectively.There were seven determinants of PE for the fast SOC pool,with soil total nitrogen(N)content being the most important,followed by,in a descending order,substrate C:N ratio,soil moisture,soil clay content,soil pH,substrate addition rate,and SOC content.The directions of PE were negative when soil total N content and substrate C:N ratio were below 2 g kg−1 and 20,respectively,but the directions changed from negative to positive with increasing levels of this two factors.Soils with optimal water content(50%—70%of the water-holding capacity)or moderately low pH(5—6)were prone to producing a greater PE.For the slow SOC pool,soil pH and soil total N content substantially explained the variation in PE.The magnitude of PE was likely to decrease with increasing soil pH for the slow SOC pool.In addition,the magnitude of PE slightly fluctuated with soil N content for the slow SOC pool.Overall,this meta-analysis provided new insights into the distinctive PEs for different SOC pools and indicated knowledge gaps between PE and its regulating factors for the slow SOC pool.
KeyWords:boosted regression tree,fresh C input,recalcitrant carbon,soil carbon cycling,soil carbon mineralization,soil moisture,soil nitrogen content,soil organic carbon
INTRODUCTION
The priming effect(PE)plays a key role in regulating soil organic carbon(SOC)cycling(Kuzyakov,2010;Chenget al.,2014;Lianget al.,2018).Extensive studies have investigated native SOC decomposition after fresh carbon(C)(typically biochar,litter,and sugar)input,and results from individual studies have shown great diversity(Luoet al.,2016).This could be attributed to the fact that PE is dependent on multiple factors,such as soil properties,substrate(fresh C)characteristics,and experimental conditions(Kuzyakov,2010).To date,the relative contributions of these factors to PE are unknown.
The varying responses of PE among studies are likely a result of many factors.First,soils with lower nutrient availability tend to produce greater PE than those with higher nutrient availability(Fontaineet al.,2004;Dimassiet al.,2014).However,similar magnitudes of PE have been detected in two soils with different total C and nitrogen(N)contents(Qiaoet al.,2014).Moreover,soils of neutral pH(6—8)were thought to show high PE(Blagodatskaya and Kuzyakov,2008),and yet a strong PE was reported by several studies on acidic soils(Luoet al.,2011;Ayeet al.,2018).Other soil properties,such as SOC and clay content,may also affect PE(Chenet al.,2019;Cotrufoet al.,2019).Second,the addition of high-quality substrates(indicated by low C:N ratio)has been found to induce greater(Pascaultet al.,2013;Bernalet al.,2016),lower(Zhang and Wang,2012;Liuet al.,2020),or similar(Chenet al.,2014b)PE compared to that of low-quality substrates.In addition to substrate quality,the magnitude of PE increases with the addition rates of substrate(Wuet al.,1993;Tianet al.,2016;Liuet al.,2017).Finally,experimental conditions(incubation temperature and soil moisture)can not only influence SOC decomposition directly(Dashet al.,2019),but also indirectly by influencing PE(Chenet al.,2014a;Wanget al.,2016).To date,the relationships between PE and its drivers have not yet been elucidated.
Soil total C consists of heterogeneous SOC pools with intrinsic stability and mean residence time(Trumbore and Czimczik,2008).Reports published in the literature have indicated that PE regulates decomposition of not only fast(labile)SOC pool,but also slow(recalcitrant)SOC pool(Fontaineet al.,2007;Bernalet al.,2016).Blagodatskayaet al.(2014)used a three-source partitioning approach,addition of14C labeled organics to soil after C3and C4vegetation,and reported that the primed C originated simultaneously from SOC younger than 12 years(C4)and older than 12 years(C3).In addition,a modeling study suggested that fresh C input stimulated decomposition of SOC,particularly the slow pool(Luoet al.,2017).At present,limited information is available for different SOC pool priming,especially the relationships between different SOC pools and their driving factors.Thus,it is necessary to distinguish the SOC pools(at least two pools)involved in priming.
Meta-analysis provides a quantitative statistical approach for synthesizing the results of multiple independent studies.Although several meta-analyses on PE were conducted(Zhanget al.,2013;Luoet al.,2015;Huoet al.,2017;Dinget al.,2018),none of them have distinguished the slow and fast SOC pools responsible for PE.This meta-analysis aimed:i)to evaluate the relative contributions of investigated factors(e.g.,soil N content,substrate C:N ratio,and soil moisture)for explaining the variation in PE and ii)to identify the relationships between PE and its driving factors for the two SOC pools.
DATA COLLECTION AND ANALYSES
We searched the ISI Web of Science for articles that had the terms“priming effect”and“soil organic carbon”in the topics(Luoet al.,2015).More than 800 articles were screened,and articles that met the following criteria were selected:i)the cumulative efflux(or release rate)of native SOC-derived CO2-C separately from substratederived CO2-C by the13C or14C isotopic signature was reported;ii)soils without substrate(i.e.,controls)under the same experimental conditions as those with substrate(i.e.,treatments)were included;iii)for two-pool model data assimilation(see details below),more than five temporal measurements of soil CO2efflux were used during the entire experimental period;and iv)the experimental duration was more than one week because one week is too short to provide reliable estimate of the decay rate of the slow SOC pool(Schädelet al.,2013;Blagodatskayaet al.,2014).A total of 147 individual experimental comparisons(control without substrate and treatment with substrate)from 46published articles were identified as suitable for our database(Table SI,see Supplementary Material for Table SI)for further analysis.All of the experiments were incubation studies,and no experiments were conducted under field conditions because of the methodological limitations.
For each study,native SOC-derived CO2-C efflux rates in the treatments with substrate and controls without substrate were tabulated.We also unified the units(e.g.,mg CO2-C g−1for SOC)among studies prior to statistical analysis.Factors were collected for analysis based on their potential role in regulating PE,availability in published articles,and independence of each other.A total of 10 factors were identified as suitable for further analysis:soil properties(e.g.,total N content,SOC content,clay content,pH,and microbial biomass C content),experimental conditions(incubation temperature and soil moisture),and substrate characteristics(C:N ratio,input rate,and type).Substrate types were grouped into biochar,litter,and simple(sugars,amino acids,and other small-molecule substrates)on the basis of C quality.Our database covered a wide range,such as soil total N content from 0.4 to 17.0 g kg−1,soil pH from 3.5 to 10,and substrate C:N ratio from 2.1 to 280.0(Table SI).The data presented in the figures in the original articles were extracted using the GetData Graph Digitizer(v2.20)software.
The first-order two-pool model was employed to fit the soil C decay rate to explore the responses of different SOC pools to fresh C input(Collinset al.,2000):
wheretis the incubation time(d),Ccum,tis the proportion of cumulative SOC decayed at timet,a0is the initial proportion of the fast SOC pool(0<a0<0.2),andk1(0<k1<1)andk2(0<k2<1)are the decomposition rate constants(k)for the fast and slow SOC pools(d−1),respectively.The fast and slow SOC pools are two conceptual C pools.The SOC with largerkwas classified as the fast SOC,and the SOC with smallerkwas classified as the slow SOC.For each SOC dynamic dataset(≥5 time series points)of the controls without substrate and treatments with substrate,the parametersa0,k1,andk2were estimated on the basis of fitting Eq.1 using nonlinear least squares(performed using the OriginPro(v8.5)software).The two-pool model could fit the dataset of SOC dynamics well,and coefficients of determination(R2)>0.95 accounted for 94%and 95%of the treatment and control datasets,respectively.Notably,a small proportion(<5%)of time series data did not converge and was discarded.The estimateda0,k1,andk2of individual experiments ranged from 0.2‰to 99.1‰,0.005 to 0.944 d−1,and 3.775×10−6to 1.930×10−3d−1,respectively(Fig.S1,see Supplementary Material for Fig.S1).
It has been argued that SOC decomposition follows a reactivity continuum.To validate our results on the basis of the two-pool model,we repeated the analysis of the datasets by using the reactivity continuum model(Koehler and Tranvik,2015),and the results are similar(Fig.S2,see Supplementary Material for Fig.S2).Therefore,only the two-pool model results are shown in the main text.
The effect size(ES)was quantified as a standardized measure of SOC decomposition with substrate across studies.For each experimental comparison,we calculated the natural logarithm of response ratio(RR)as ES(Hedgeset al.,1999):

wherekTandkCare the SOCkfor the treatment with substrate and the control without substrate,respectively.ES=0 indicates no PE,ES>0 means positive PE,and ES<0 represents negative PE.To help interpretation,ES was converted to a percentage,(expES−1)×100%,which equals to the percentage change of SOCkin the treatment with substrate relative to the control without substrate.
The boosted regression tree(BRT)modeling was used to partition the independent influences of soil properties,experimental conditions,and predictors of substrate characteristics on the ES.The BRT modeling method is a powerful tool used to evaluate the relationships between ecological processes(e.g.,forest productivity)and predictors(Zhanget al.,2012).This method has at least three main advantages(De’ath,2007).First,the BRT modeling method can handle predictor variables with different types(categorical and continuous types)and distributional functions.Second,the independence of predictors is not required because the interactions of predictors are modeled automatically by the hierarchical structure of a tree.Third,the model can handle missing values in predictors.We performed BRT modeling in R(v2.15.2,R Development Core Team,2012)using the gbm package(Elithet al.,2008).The Gaussian function was chosen as the error structure for the loss function because of the attribution of our response variable.The fitting of a BRT model is also constrained by four parameters:i)the learning rate,which determines the contribution of each tree to the growing model;ii)the tree complexity,which controls the level of interactions in the BRT;iii)the bagging fraction,which sets the proportion of observations used in selecting variables;and iv)the cross-validation,which specifies the number of times to randomly divide the data for model fitting and validation(De’ath,2007).The parameter setting was based on the empirical rules recommended for BRT modeling to select the optimal model(Elithet al.,2008;Zhanget al.,2012,2015).Twenty-seven models were fitted with the following parameter settings:learning rates of 0.01,0.005,and 0.001;bag fractions of 0.6,0.5,and 0.4;cross-validations of ten-,eight-,and five-fold;and a tree complexity of 4 to account for potentially large numbers of interactions between predictor variables.Through trial and error analysis for 27 models,we found that the optimal BRT model had cross-validation deviances of 0.681±0.181 and 0.236±0.054(mean±standard error)from learning rates of 0.005 and 0.001,bag fractions of 0.6and 0.6,and cross-validations of five-fold and ten-fold for the fast and slow SOC pools,respectively.
The BRT model could give out the total percentage of the explained variation in the ES,which was calculated on the basis of test sample residuals(Zhanget al.,2015).The relative influence of each predictor was based on the number of times that a variable was used in the model for splitting,weighted by the squared improvement to the model as a result of each split,and averaged over the entire model(De’ath,2007).The relative influence of each variable(i.e.,contribution of each variable to the explained variation in ES)was scaled such that the sum was 100%.The higher the value of the relative influence,the stronger the influence on PE.Considering that the BRT model cannot directly provide confidence intervals(CIs),we estimated the 95%CI for each variable using a bootstrap technique as described by Carslaw and Taylor(2009).The BRT model outputs the relative influence of each predictor rather than the absolute influence.The relative influence above the value(e.g.,5%)was identified as a potential important factor in previous ecological studies that used the BRT method(Zhanget al.,2012;Dinget al.,2018).In accordance with these empirical rules,the same criteria were used in the current metaanalysis for the fast SOC pool.Notably,the total percentages(67% for the fast SOC pool and 34% for the slow SOC pool)of the explained variation in ES were different for the two SOC pools.To obtain the relative influences(i.e.,the contributions of the variables to the explained variation in ES)comparable between the two SOC pools,the adjusted value,10%(0.67/0.34×5%),was used to identify potential important factors for the slow SOC pool.
RESULTS
Across studies,the ES values for the fast and slow SOC pools were 0.13±0.91(mean±standard deviation(SD))and 0.17±0.49(mean±SD),respectively(Fig.1).This indicated that substrate treatments(fresh C addition)accelerated the decomposition of the fast and slow SOC pools by 13.9%((e0.13−1)×100%)and 18.4%((e0.17−1)×100%),respectively,as compared with the controls without substrate.The ranges of ES were wide,resulting in considerable variances in the mean values.The final BRT model explained 67%of the variation in the ES from observation for the fast SOC pool(R2=0.668),whereas it only explained 34%for the slow SOC pool(R2=0.336)(Fig.2).In addition,the fast and slow pools on average accounted for 1.1% and 98.9% of SOC in the controls without substrate,respectively,and 1.7%and 98.3%in the treatments with substrate,respectively(Fig.S1).
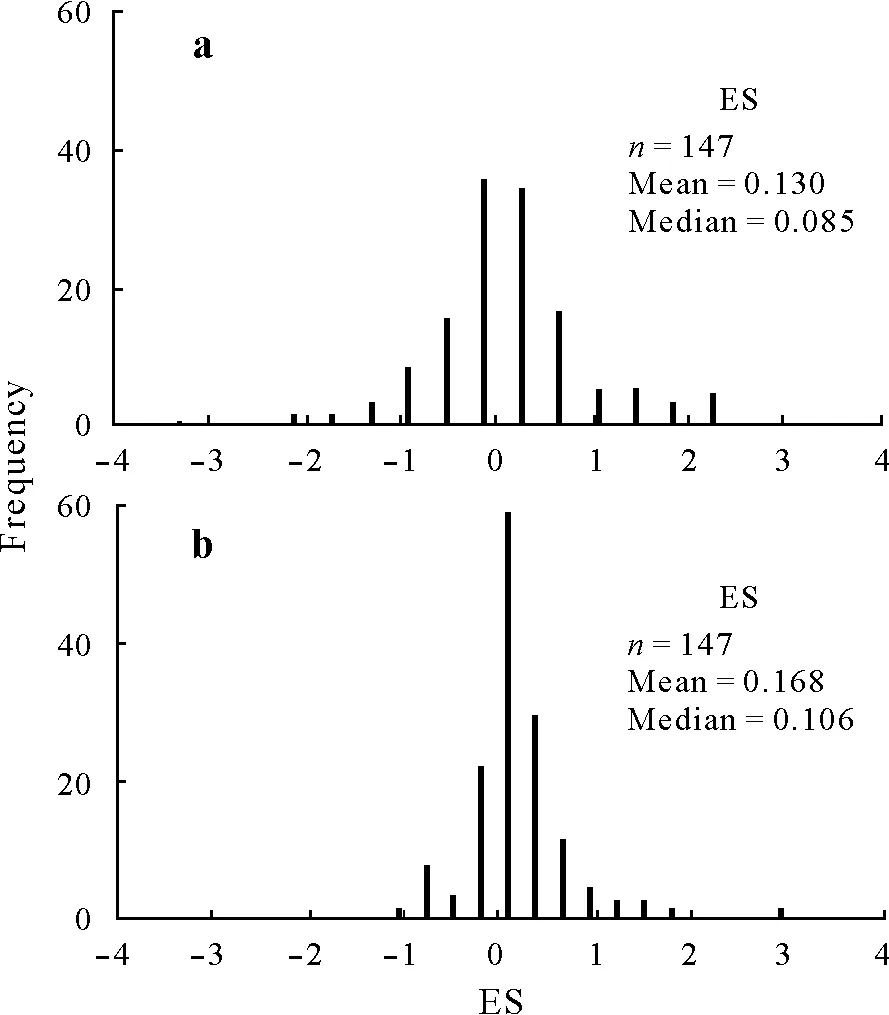
Fig.1 Frequencies of effect size(ES,the natural logarithm of response ratio calculated using Eq.2)for the fast soil organic C(SOC)pool(a)and slow SOC pool(b).A total of 147 comparison sets(treatment with substrate and control without substrate)of observations were independently calculated.
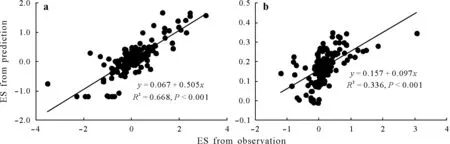
Fig.2 Scatter plots showing the linear regressions between the effect size(ES,the natural logarithm of response ratio calculated using Eq.2)values from prediction using the boosted regression tree(BST)model and those from observation for the fast soil organic C(SOC)pool(a)and slow SOC pool(b).
Fast soil C pool
Soil total N content was the first important factor that contributed 23%of the explained variation in ES for the fast SOC pool(Fig.3).The ES values from prediction intensively increased from negative to positive with increasing soil total N content from 0.4 to 2.0 g kg−1and then slightly decreased with increasing soil total N content from 2.0 to 5.0 g kg−1before plateauing with increasing soil total N content from 5.0 to 17.0 g kg−1(Fig.4a).Furthermore,the plateau line may be because of the rare observations of high soil total N content(Fig.4a).The second factor,namely,the substrate C:N ratio,contributed 16%of the explained variation in ES(Fig.3).The ES values increased with increasing substrate C:N ratio from 2 to 50 and then plateaued(Fig.4b).It is noteworthy that the directions of PE were negative when the substrate C:N ratio was below 20.The third factor,namely,the soil moisture,contributed 13%of the explained variation in ES(Fig.3).The ES values showed a parabolic shape with increasing soil water content from 40% to 100% of water-holding capacity(Fig.4c),indicating the optimal range of soil moisture for priming.The fourth factor,namely,the soil clay content,contributed 12%of the explained variation in ES(Fig.3).The ES values decreased with increasing soil clay content(Fig.4d).The fifth factor,namely,the soil pH,contributed 11%of the explained variation in ES(Fig.3).The ES values were high within the optimal range(pH 5—6)of soil pH(Fig.4e).The sixth and seventh factors,namely,the substrate addition rate and SOC content,contributed 10%and 9%of the explained variation in ES,respectively(Fig.3).The ES values slightly fluctuated around zero with the two variables(Fig.4f,g).In addition,other investigated factors,such as soil microbial biomass C content,incubation temperature,and substrate types,had little effect on the variation in ES(Fig.3).
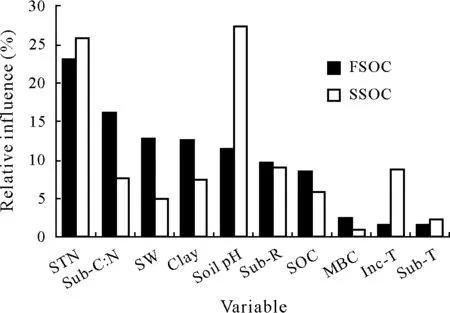
Fig.3 Relative influence values of the variables on the effect size(ES,the natural logarithm of response ratio calculated using Eq.2)(i.e.,the contributions of the variables to the explained variation in ES)using the boosted regression tree(BRT)model for the fast and slow soil organic C pools(FSOC and SSOC,respectively):soil total N content(STN),substrate C:N ratio(Sub-C:N),soil moisture(soil water content,SW),soil clay content(Clay),soil pH,substrate addition rate(Sub-R),soil organic C content(SOC),soil microbial biomass C content(MBC),incubation temperature(Inc-T),and substrate type(Sub-T).
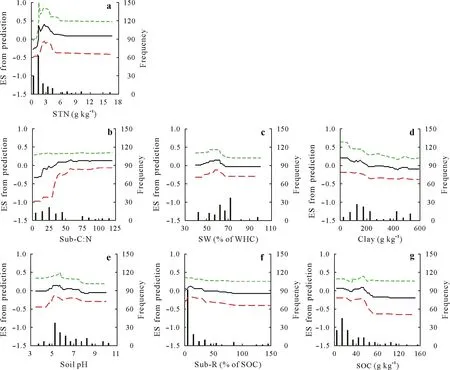
Fig.4 Partial dependence plots showing the variation in the effect size(ES,the natural logarithm of response ratio calculated using Eq.2)of the variables from prediction using the boosted regression tree(BRT)model for the fast soil organic C pool:soil total N content(STN,a),substrate C:N ratio(Sub-C:N,b),soil moisture(soil water content,SW,c),soil clay content(Clay,d),soil pH(e),substrate addition rate(Sub-R,f),and soil organic C content(SOC,g).Soil microbial biomass C content,incubation temperature,and substrate type are not shown because of their little effects.The fitted functions(black solid lines)show the relationships between the ES from prediction and explanatory variables,whereas all other explanatory variables are kept constant at their mean levels.The green and red dashed lines show the 95%confidence intervals estimated from 500 bootstrap samples of the dataset.The vertical gray solid lines show the frequency of observations across the explanatory variable range of the dataset.WHC=water-holding capacity.
Slow soil C pool
Among the ten investigated factors,the soil pH and soil total N content contributed 27%and 26%of the explained variation in ES for the slow SOC pool,respectively(Fig.3).The predicted ES values decreased with increasing soil pH(Fig.5a),suggesting that acidic soils were likely to produce strong positive PE.The predicted ES values slightly fluctuated around 0.2 with the soil total N content(Fig.5b).The relationships between the soil total N and ES for the slow SOC pool showed different patterns from those for the fast SOC pool.Other factors(e.g.,substrate C:N ratio,soil moisture,soil clay content,substrate addition rate,SOC content,soil microbial biomass C content,incubation temperature,and substrate types)had little effects on the variation in ES for the slow SOC pool(Fig.3).
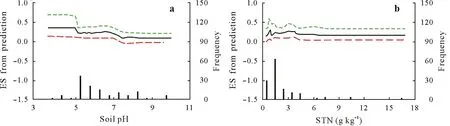
Fig.5 Partial dependence plots showing the variation in the effect size(ES,the natural logarithm of response ratio calculated using Eq.2)of the variables from prediction using the boosted regression tree(BRT)model for the slow soil organic C pool:soil pH(a)and soil total N(STN,b).Substrate C:N ratio,soil moisture,soil clay content,substrate addition rate,soil organic C content,soil microbial biomass C content,incubation temperature,and substrate type are not shown because of their little(or no)effects.The fitted functions(black solid lines)show the relationships between the predicted ES and explanatory variables,whereas all other explanatory variables are kept constant at their mean levels.The green and red dashed lines show the 95%confidence intervals estimated from 500 bootstrap samples of the dataset.The vertical gray solid lines show the frequency of observations across the explanatory variable range of the dataset.
DISCUSSION
General patterns of PEwithin the fast and slow SOC pools
Our results demonstrated that PE impacted not only the fast SOC pool,but also the slow SOC pool.Previous studies generally thought that PE mainly affected the fast(labile)SOC pool(Kuzyakovet al.,2000).However,increasing evidence has shown that the slow(recalcitrant)SOC pool can be primed by adding fresh C(Fontaineet al.,2007;Kuzyakovet al.,2009;Bernalet al.,2016;Vestergårdet al.,2016).For example,the decomposition of SOC with a mean residence time of>2 000 years(slow SOC pool)in deep soil was stimulated by adding fresh C(Fontaineet al.,2007).Our meta-analysis provided new evidence for PE simultaneously impacting different SOC pools.Large variances in PE were observed among individual studies for the fast and slow SOC pools(Fig.1),with mean magnitudes of PE of 13.9%and 18.4%,respectively.The wide range reflected the large uncertainties associated with the direction and magnitude of measured PE by extensive individual studies(Zhanget al.,2013).In fact,PE appeared to be sensitive to almost all investigated factors within the experiments,and these factors could lead to variation in PE(Kuzyakov,2010).In addition,our results showed that the slow pool,rather than the fast pool,dominated the SOC stock(Fig.S1).This finding indicated that under the same magnitude of PE,the majority of the primed C was derived from the slow pool rather than the fast pool.Thus,PE for the slow SOC pool would play a key role in soil C cycling.Overall,our meta-analysis suggested that the response sensitivities of the fast and slow native SOC pools to external fresh C input might differ.
The BRT model explained different levels of variation in PE for the two SOC pools.For the fast SOC pool,the relatively high explained levels(67%)of the variation in PE indicated that the current knowledge of factors regulating PE was considerable.By contrast,the BRT model only accounted for 34%of the variation in PE for the slow SOC pool.The lack of explanatory power could be explained by three reasons.First,the uncertainty of the decay rate estimates of the slow pool might result in the low explained level.The decay rates of the slow SOC pool described an extremely long-term process.Moreover,insufficient information was provided by the dataset because the incubation studies did not last very long(Schädelet al.,2013).Despite the fact that extremely short-term(less than one week)studies were excluded from the database(Table SI),we acknowledged that the uncertainty still existed.Second,variability in ES was found to be explained less for the slow SOC pool than for the fast SOC pool,thereby causing the BRT model to perform poorly for the slow pool(Fig.2).Finally,factors other than the ten investigated factors in this meta-analysis,such as soil microbial community,soil extracellular enzymes,and liberation of SOC,might control PE for the slow SOC pool(Fontaineet al.,2007;Blagodatskayaet al.,2014;Keiluweitet al.,2015).Nevertheless,our meta-analysis results pointed out different knowledge gaps for the two SOC pools within priming studies.
Effects of factors on priming for the fast SOC pool
Soil total N content was identified as the most important factor controlling the variation in PE for the fast SOC pool.To the best of our knowledge,this is the first report of the relationship between PE and soil N content with a wide range(from 0.4 to 17.0 g kg−1)on the basis of metaanalysis.Individual studies that explore the relationship between soil N content and PE are difficult because the ranges of soil N content are insufficient within a limited number of utilized soils(Nottinghamet al.,2015;Perveenet al.,2019).Our results showed that soils with extremely low N contents(<2.0 g kg−1)could have negative PE(Fig.4a).This finding could be explained by the microbial N immobilization hypothesis(Luet al.,2018).Fresh C input will intensify N immobilization when soil N availability is extremely limited,thereby suppressing microbial activity and soil organic matter(SOM)decomposition,causing a negative PE(Luet al.,2018).Our results also showed that the magnitude of positive PE decreased with increasing soil total N content(Fig.4a),suggesting the microbial N mining hypothesis.This hypothesis states that microbes use external fresh C as an energy source to mine SOM for N(Craineet al.,2007),thereby commonly inducing a positive PE(Perveenet al.,2019).Therefore,when soil N is abundant,the microbial mining of SOM could be weakening(Fontaineet al.,2011).Overall,the current meta-analysis pointed out a nonlinear relationship between PE and soil N content.
Substrate quality(C:N ratio)was the second important factor accounting for the variation in PE.Our results indicated that PE increased with an increasing substrate C:N ratio from 2 to 50(Fig.4b).Certain studies have reported that stronger positive PE is induced by substrates with higher C:N ratio than by substrates with lower C:N ratio(Guenetet al.,2010;Wanget al.,2015),which is consistent with our results.Moreover,numerous studies have reported that substrates with additional N input induce lower PE than those without additional N input(Bernalet al.,2016;Ayeet al.,2018).Notably,a negative PE was likely to occur when substrates had relatively low(<20)C:N ratio(Fig.4b).This finding could be explained by the microbial preferential substrate utilization hypothesis.Microbes may prefer using labile substrates to recalcitrant SOM when N is abundant within substrates,thereby causing a negative PE(Cheng,1999).Our results confirmed that the direction and magnitude of PE may depend on the substrate quality.
Soil moisture played an important role in explaining the variation in PE.Thus far,only a few studies have investigated the effect of soil moisture on PE(Chenet al.,2014a;Toosiet al.,2017).The study of Wanget al.(2016)showed that the medium soil moisture(69%of the water-holding capacity)led to a stronger PE than the low(44%)and high(95%)soil moisture,indicating the optimal range of soil moisture for PE.The current meta-analysis provided new evidence supporting the idea of optimal soil moisture.Considering the current climate change scenarios,the effect of soil water stress on PE calls for further studies.Soil clay content also played an important role in regulating PE.To date,the effect of soil clay content on PE has rarely been reported.Our results showed a negative relationship between PE and soil clay content(Fig.4d),thereby supporting the idea that the mineral protection of SOC is against decomposition by microbes(Schmidtet al.,2011;Chenet al.,2019).Under high soil clay content,SOC and microbial decomposing enzymes are tightly bonded to the mineral surface,thereby decreasing the SOC decay rate(Allison and Jastrow,2006;Luoet al.,2016).However,another study(Zhanget al.,2017)reported that soils with high clay content produced high PE,thereby contradicting our results.Accompanying clay content changes,other soil properties(such as C and N)may account for these inconsistent results(Zhanget al.,2017).
Soil pH explained substantial variation in PE.Our results suggested that soils with moderately low pH levels(5—6)were prone to producing greater PE(Fig.4e).This finding is in accordance with that of an experimental study(Nottinghamet al.,2015)reporting that PE was the greatest in moderately acidic soils(pH 5.3)among a series of tropical forest soils with initial pH levels ranging from 3.9 to 6.8.This phenomenon could have several potential causes.First,mineral-associated C tends to be liberated by oxalic acid added into soil,which was consumed by decomposer organisms to produce high PE(Keiluweitet al.,2015).Second,acidic soils are favorable for fungal growth(Rousket al.,2009).Fungi are thought to act as major decomposers to produce long-lasting PE(Kuzyakov,2010).Third,PE of alkaline soils is underestimated because of the considerable amount of primed CO2-C dissolved in alkaline soil solution(Blagodatskaya and Kuzyakov,2008).However,the mechanisms may be elucidated by experimental studies using soil matrices with different pH values.
Substrate addition rate and SOC content were identified as two potential factors shaping PE(Fig.3).First,no obvious relationship was found between substrate addition rate and PE in the current meta-analysis(Fig.4f).Although a positive linear relationship between substrate addition rate and PE was reported by several studies(Liuet al.,2017;Shahzadet al.,2019),negative and nonlinear relationships were also found(Blagodatskaya and Kuzyakov,2008;Guenetet al.,2010),thereby partially supporting our results.Second,no obvious relationship was found between SOC content and PE(Fig.4g).Thus,we expected that SOC fractions(e.g.,mineral-associated and particulate organic C pools),rather than SOC content,may be responsible for PE(Cotrufoet al.,2019).Overall,our results suggested that the effects of substrate addition rate and SOC on PE should not be ignored in experimental studies.
Effects of factors on priming for the slow SOC pool
Soil pH was identified as a dominant factor regulating PE for the slow SOC pool(Fig.3).The magnitude of PE tended to decrease with increasing soil pH values(Fig.5a).This finding is consistent with previous studies reporting that soils with low pH values produce high PE(Luoet al.,2011;Sheng and Zhu,2018).The pattern of the relationship between soil pH and PE for the slow SOC pool was similar to that for the fast SOC pool.Our results indicated that soil pH could simultaneously affect the decomposition of different SOC fractions.Increasing evidence has shown that soil pH is a dominant factor governing microbial community and activity(Rousket al.,2009;Sheng and Zhu,2018),thereby influencing the rate of SOC decomposition(Kemmittet al.,2006;Jiaet al.,2017).Although the effect of soil pH on different SOC fractions has been rarely studied,soil pH should be recognized for its important role in regulating PE.
Soil total N content was identified as another dominant factor regulating PE for the slow SOC pool(Fig.3).The magnitude of PE fluctuated with the change in soil total N content(Fig.5b),thereby indicating the complicated and volatile mechanisms underlying this phenomenon.For the descent phases of the curve,the microbial N mining hypothesis could explain the relationship between PE and soil N content(Craineet al.,2007;Fontaineet al.,2011).This finding is the same as that for the fast SOC pool.In contrast,for the rising phases of the curve,the relationship could be explained by another hypothesis,namely,the microbial stoichiometric hypothesis,which assumes that the microbial activity is high when soil N status matches microbial demand(Hessenet al.,2004).Therefore,our results suggested that the two hypotheses can coexist in PE of the slow SOC pool.The different microbial community compositions may be responsible for the two competing hypotheses(Chenet al.,2014b;Fanget al.,2018).The mechanisms underlying the relationship between soil N content and PE for the slow SOC pool are likely different from those for the fast SOC pool.Notably,PE was negative for the fast SOC pool when the soil total N content was extremely low(<2 g kg−1),whereas positive PE occurred for the slow SOC pool.Further studies are required to identify the diverse mechanisms for different SOC fractions.
CONCLUSIONS
Our quantitative analysis of 147 groups of data from 46studies revealed that the range of PE was wider for the fast SOC pool than for the slow SOC pool.We critically evaluated the factors influencing PE for the two SOC pools using BRT modeling.The final BRT model explained most of the variation in PE for the fast SOC pool.In contrast,the largely unexplained variation in PE for the slow SOC pool indicated the uncertainty or unknown factors influencing PE for the slow SOC pool.Furthermore,soil total N content,substrate C:N ratio,soil moisture,soil clay content,soil pH,substrate addition rate,and SOC content were identified as factors affecting PE for the fast SOC pool.Meanwhile,soil pH and soil total N content substantially explained the variation in PE for the slow SOC pool.Moreover,several relationships between the factors and PE emerged,and these relationships might differ for different SOC pools.These insights can help to better predict the effect of fresh C amendment on soil C dynamics.
ACKNOWLEDGEMENTS
We acknowledge the work done by the researchers whose published data were included in this meta-analysis.We thank two anonymous reviewers for their valuable comments on the early version of this manuscript.This work was financially supported by the National Natural Science Foundation of China(Nos.31830015,31870429,and 31570620).
SUPPLEMENTARY MATERIAL
Supplementary material for this article can be found in the online version.
杂志排行
Pedosphere的其它文章
- Elevated carbon dioxide stimulates nitrous oxide emission in agricultural soils:A global meta-analysis
- Hydrogen cyanide production by soil bacteria:Biological control of pests and promotion of plant growth in sustainable agriculture
- Effects of different continuous fertilizer managements on soil total nitrogen stocks in China:A meta-analysis
- Microplastics in soil:Impacts and microbial diversity and degradation
- Rhizosphere microbiomes can regulate plant drought tolerance
- Difficult-to-culture bacteria in the rhizosphere:The underexplored signature microbial groups
