Reduced graphene oxide modified melamine sponges filling withparaffin for efficient solar-thermal conversion and heat management
2022-03-01LuYueLiuZhuangLiuHanYuPengXiaoTingMuQianZhaoXiaoJieJuWeiWangRuiXieLiangYinChu
Lu-Yue Liu,Zhuang Liu,2,,Han-Yu Peng,Xiao-Ting Mu,Qian Zhao,Xiao-Jie Ju,2,Wei Wang,2,Rui Xie,2,Liang-Yin Chu,2,
1 School of Chemical Engineering,Sichuan University,Chengdu 610065,China
2 State Key Laboratory of Polymer Materials Engineering,Sichuan University,Chengdu 610065,China
Keywords:Reduced graphene oxide sponges Thermo-regulation Phase change Solar-thermal conversion Heat management
ABSTRACT A novel type of reduced graphene oxide (rGO) modified melamine sponges (rGS) filling with paraffin(rGS-pf) is developed for efficient solar-thermal conversion and heat management.The microstructures,filling and holding capacity of paraffin in porous rGS,solar-thermal energy conversion and energy harvesting efficiency of the prepared rGS-pf have been investigated systematically.The content of rGO nanosheets coated on the skeletons of rGS-pf is only 0.11%,while the loading content of paraffin in the rGS-pf is as high as 97.53%.Based on the solar-thermal conversion property of rGO nanosheets in the rGS-pf and the heat storage ability of paraffin in the rGS-pf,the proposed rGS-pf provides excellent performance for heat management.The efficiency of solar-thermal conversion could reach up to 92.5%.The thermo-regulation provided by the proposed rGS-pf is real-time,repeatable and long-term stable.The results in this study provide valuable guidance for developing functional materials for efficient solarthermal conversion and heat management.
1.Introduction
Thermo-regulation is closely linked with human life.For example,in some places,the temperature varies greatly between day and night;therefore,it is important to maintain the room temperature to provide comfortable living environments for human beings.Similarly,the environmental temperature also plays an important role in the growing processes of vegetables,fruits and even animals [1,2].Hence,it is significant to regulate the environmental temperatures in appropriate ranges to provide suitable local environments for humans,animals and plants.To achieve such purposes,energy storage technologies have been developed to regulate temperatures by using phase change materials(PCMs).In such technologies,the PCMs-equipped materials,which can store the extra heat in the daytime and release the heat at night,play key roles in heat management to buffer the environment temperature variation[3–6].Therefore,the development of functional materials for efficient heat management and thermo-regulation is highly desired.
Up to now,the primary method to achieve functional materials for heat management and thermo-regulation in buildings is to introduce PCMs or their capsules directly into the building materials[7–9].However,in such cases,the contents of the PCMs are accordingly low for the safety consideration of constructing the buildings.Furthermore,the PCMs introduced in the building materials can only store heat generated by temperature difference and part of solar energy.Solar energy cannot be transferred to thermal energy quickly and in time,leading to the delayed response to temperature variation and poor performances of heat management[10].According to the quantum theory,solar-thermal conversion materials can convert solar energy to intense heat[11,12].Therefore,the combination of the solar-thermal conversion materials with the PCMs is an effective method for heat management [13–16],and is promising to store solar energy efficiently in the daytime and release heat at night.So far,there are two main approaches to combine solarthermal conversion materials with PCMs as follows:(1)Directly dispersing carbon nanomaterials powders into organic PCMs [13,15–18].However,this method is limited by leakage problems and the nonuniform distribution of the carbon nanomaterials.Besides,this method may lead to the deposition of carbon nanomaterials after repeated thermal cycles because of the density difference between carbon nanomaterials and PCMs.Meanwhile,the carbon nanomaterials in PCMs do not construct a continuous pathway for heat transfer,which is inefficient and unsuitable for long-term heat management.(2) Filling PCMs into porous carbon materials such as graphene aerogels.Although it solves the problems of leakage and deposition,it is still restricted by the complex fabrication process.In this method,only the solar-thermal materials on the surfaces work for the solar-thermal conversion.This method cannot be easily scaled up and is barely used in heat management and in-time thermo-regulation[19–24].Hence,it is still challenging to fabricate functional materials efficient for light-harvesting and solar-thermal conversion and easy to scale up for efficient heat management and in-time thermo-regulation.
Herein,we report on the development of reduced graphene oxide modified melamine sponges filling with paraffin (rGS-pf)for efficient solar-thermal conversion and heat management.It has been reported that reduced graphene oxide (rGO) materials possessing sp2electronic configuration structure and low Fresnel reflection are promising for efficient solar-thermal conversion applications [17–20].With increasing the surface roughness of rGO materials,the optical path of the photons is increased due to Lambertian scattering [21].By coating rGO nanosheets onto the skeletons of three-dimensional porous melamine sponges (MS)with rough surfaces and long optical paths for light trapping,the light-harvesting efficiency can be improved.The rGO materials with high thermal conductivity can facilitate the rate of energy storage [22,23].Paraffin,which is one of the most widely used PCMs with high heat storage capacity,variable phase change temperatures and low price [24,25],is used in this study as the model PCM.In this work,the fabrication process,which simply involves the synthesis of rGO-coated melamine sponges (rGS) and filling with paraffin,can be easily scaled up.The microstructures,filling and holding capacity of paraffin in porous rGS,solar-thermal energy conversion and energy harvesting efficiency of rGS-pf have been investigated systematically.The results in this study provide valuable guidance for developing functional materials for efficient solar-thermal conversion and heat management.
2.Experimental
2.1.Materials
Melamine sponge (MS) was purchased from Hubei Xijie Daily Necessities Co.,Ltd.(China).Paraffin (pf,OP28E) purchased from Hangzhou Ruhr New Material Technology was used as a phase change material with melting/crystallization point at 27–29 °C and latent heat ca.(245 ± 18) J·g-1.Graphite was purchased from Nanjing XFnano Technology (China).Potassium persulfate(K2S2O8),concentrated sulfuric acid(H2SO4),phosphorus pentoxide(P2O5),potassium permanganate (KMnO4),hydrogen peroxide(H2O2),hydrochloric acid(HCl),hydrogen iodide(HI,≥45.0%(mass))and ethanol were purchased from Chengdu Kelong Chemicals(China).All the chemicals were used without further purification.
2.2.Fabrication of GO nanosheets
Graphene oxide(GO)nanosheets were synthesized according to the modified Hummers method [26,27],and dialyzed in deionized water for a week to remove ions from the synthetic process.The GO nanosheets were exfoliated by ultrasonic for 30 min and centrifuged at 4000 r·min-1for 10 min before use.
2.3.Fabrication of rGS-pf
The fabrication process involved the synthesis of rGS and filling with paraffin,as shown in Fig.1.Firstly,the GO nanosheets wrapped up the skeleton of a commercial porous MS (Fig.1a) by repeating the “squeezing-adsorbing” processes.The porous structure of MS was fully filled with the GO solution by capillary force and pressure difference.After vacuum drying process,the obtained GO-nanosheets-coated MS is coded as GS (Fig.1b).The GS is then reduced to form rGS (Fig.1c).It has been reported that rGO nanosheets are a blackbody that could trap sunlight over the whole UV–vis–NIR range [10,28,29],which can realize efficient solarthermal conversion.The lamellar structures of the rGO nanosheets benefit phonon vibration and heat transfer [30].Besides,heat can flow immediately through a 3D continuous pathway provided by rGS.Secondly,the melting paraffin is infused into the porous rGS(Fig.1d).
Typically,the commercial porous MS with dimensions of 20 mm × 20 mm × 20 mm was immersed into 50 ml GO solution with a concentration of 4.8 mg·ml-1after squeezing out the gas inside the porous MS.The porous structure of MS was fully filled with the GO solution by capillary force and pressure difference.After vacuum drying,the GO nanosheets adhered to the MS skeletons to form GS.Because the GO nanosheets with only oneatom thickness were soft,the GO nanosheets tended to wrap up the skeletons of porous MS via van der Waals’ force.Subsequently,GS was reduced in the 20 ml HI solution (≥45.0%(mass)) at 90 °C for 60 seconds to form rGS [31,32].Next,the rGS sample was washed with ethanol and DI water alternately at least 10 times to remove all the HI residue.After dried in a vacuum at 200 °C for 2 h,the rGS sponge was obtained for further use.
Then,50 ml paraffin was melted at 60 °C until it was fully melted.By repeatedly squeezing out the gas inside the sponges and sucking the melting paraffin solution into the sponges,the melting paraffin solution was completely filled into porous blank MS and rGS sponges.Next,the MS-pf and rGS-pf were stored in a refrigerator at 4 °C until the paraffin inside MS and rGS sponges were solidified completely.Subsequently,the solidified MS-pf and rGS-pf sponges were cut into pre-determined sizes for further characterizations and applications.
2.4.Morphological and chemical characterizations of MS and rGS
The digital camera (E-PL5,Olympus) was used to take digital photographs of MS and rGS.The morphologies and microstructures of corresponding samples were investigated by a Scanning Electronic Microscope(SEM,G2 Pro,Phenom).The Raman spectra were obtained from a Raman microscope (Horiba Jobin Yvon LabRAM HR) with an excitation wavelength of 532.1 nm.Paraffin contact angles of rGO film and rGS sponge were measured by a contact angle measuring system (IL4201,KRÜSS GmbH Germany) with a droplet volume of 4 μl at room temperature.
2.5.Thermal enthalpy of rGS-pf and filling ratio of paraffin
The phase transition temperatures and enthalpies of MS-pf and rGS-pf during the heating and crystallization processes were measured by a Differential Scanning Calorimeter (DSC,Q2000,TA Instruments) at a nitrogen atmosphere,with pure paraffin as the control group.During the heating process,the temperature increased from 0 to 50 °C with a rate of 2 °C·min-1,which was the same as that for the cooling process from 50 to 0 °C.
The filling ratio of paraffin is calculated as the enthalpy ratio(R),which shows the thermal property of rGS-pf compared with that of pure paraffin,as follows [7,33–35]:

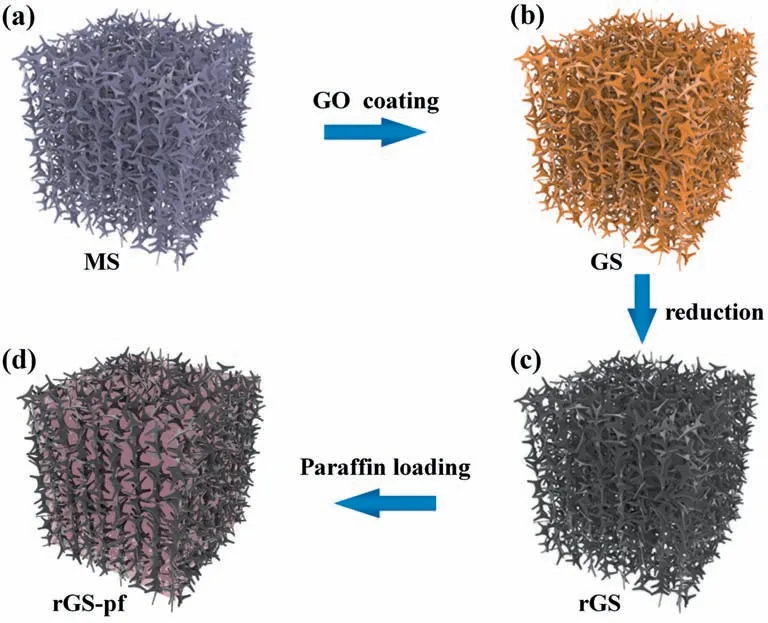
Fig.1.Schematic illustrations of the fabrication process of rGS-pf used to regulate room temperature.(a,b) A commercial porous melamine sponge (MS) with porous structure(a) is submerged in GO solution by the repeated “squeezing-adsorbing” processes to obtain the GS (b).(c) The rGS is produced by reducing the pre-dried GS in HI solution.(d) The melting paraffin solution is loaded in the porous rGS to form rGS-pf.
where,ΔHm,x—pfand ΔHm,paraffinare the melting enthalpies (J·g-1) of the x-pf and pure paraffin,respectively,in which the subscript x refers to either MS or rGS.
2.6.Tests of the solar-heat conversion and leakage of paraffin
The MS-pf and rGS-pf were cut into cubes with dimensions of 10 mm × 10 mm × 10 mm,and then exposed under simulated sunlight generated via a high-voltage xenon short-arc lamp(250 W,Napu Photoelectricity) at an intensity of 1000 W·m-2for 6 min.The initial temperature of the surroundings was set at 18 °C.The surface temperatures of the MS-pf and rGS-pf samples were recorded via an infrared thermal imaging camera (E40,FLIR system).The surface average temperatures of the MS-pf and rGSpf samples were obtained from a matched software (FLIR tool+).The leakage test was performed by putting solid paraffin,MS-pf and rGS-pf with the same size (2 cm × 2 cm) on the hot stage at 80 °C and then observing the effect of melting on the leakage.DSC and a digital camera were used to assist in studying the shape-stability of the samples.
The efficiency of solar-thermal conversion (η) is calculated as follows [36]:

where,m is the mass of the samples (g),ΔH is the melting enthalpies (J·g-1) of the samples calculated by DSC,ρ is the intensity of simulated sunlight (W·m-2),A is the surface area (m2) of the samples exposed in simulated sunlight,and tsand teare respectively the start time (s) and end time (s) of phase change process.
2.7.Thermo-regulating performances of rGS-pf
3D-printed model houses (length=30 mm,width=20 mm,height=25 mm) made of resin (Somos Imagine 10000,WeNext Co.,Ltd.) were fabricated with a thickness of 0.7 mm for the walls and roof.Then,the walls and roof of a model house were equipped with the as-prepared rGS-pf.Next,two model houses with and without rGS-pf were exposed under the same simulated sunlight.The surface temperature distributions of the model houses were recorded via an infrared thermal imaging camera (E40,FLIR system) for 20 min.At the same time,the temperatures inside the model houses were measured every 30 seconds by a thermocouple thermometer with dual channels(UT320D,UNI-T).After turned off the light,the surface and inside temperatures of the two model houses were recorded until the inside temperature reached a constant level.The heating and cooling processes were operated several times to test the stability and repeatability of the rGS-pf.
3.Results and Discussion
3.1.Morphology,composition and solar-thermal property of rGS

Fig.2.(a-f)Optical images(a,d)and SEM images(b-c,e-f)of original MS(a)and rGS(d).(c,f)The magnified SEM images of the skeletons of MS and rGS.The red circles in e and f show the differences in morphology between MS and rGS.(g) Raman spectra of MS,GS and rGS.(h) The temperature–time curves of surface average temperatures labeled by red point frames of MS(i,j)and rGS in(k,l).The surface average temperatures are measured and calculated every 1 min by a matched software(FLIR tool+).(i-l)Infrared thermal images of surface temperature distributions of MS (i,j) and rGS (k,l) at 0 min (i,k) and 6 min (j,l) when applying simulated sunlight at an intensity of 1000 W·m-2.Scale bars are 1 cm in (a,d),200 μm in (b,e),10 μm in (c,f),and 0.5 cm in (i-l).
The commercial MS is white (Fig.2a) with a porous structure(Fig.2b).After coating with rGO nanosheets,the color becomes black(Fig.2d),but the structure of obtained rGS still remains porous(Fig.2e).The SEM images show that the rGO nanosheets do not block the interconnected pores of the MS sponge.The magnified SEM images show that the skeletons of MS are smooth (Fig.2c).While after the reduction process,some wrinkles appear on the surface of the skeletons (marked with red circles in Fig.2f).These wrinkles indicate that the rGO nanosheets have been coated on the skeletons of MS to form rGS.In the Raman spectra of MS,GS and rGS (Fig.2g),the peaks at 1356 cm-1(D peak) and 1594 cm-1(G peak) are characteristic peaks of GO and rGO,which appear in the Raman spectra of GS and rGS.Compared the ratio of D/G intensity (ID/IG) in GS with that in rGS,the ID/IGvalue remarkably increases from 1.15 in GS to 1.96 in rGS,which confirms that the GO nanosheets coated on the skeletons of sponges have been converted into rGO nanosheets [37].According to the previous model to quantify the density of defects (σ),i.e.,the average distance between defects is LD=1/,ID/IG=A/(LD)2,where A=188 or 145 nm2depended on different excitation wavelengths has been found [38,39].Therefore,the values of the density of defects of GS and rGS can be calculated as 0.006 nm-2and 0.010 nm-2,respectively.The increased defects in rGS do not affect the thermal conductivity too much.When the concentration of graphene is low,the defects become the center of heat flow scattering.This center weakens the heat dissipation potential of graphene[40–42].The solar-thermal conversion properties of MS and rGS are illustrated in Fig.2h–l.Upon exposing the MS and rGS to the simulated sunlight for 6 min,the surface temperature of rGS increases sharply.The surface temperature of rGS reaches over 120°C within 1 min(Fig.2h,k and l).The rGS skeletons could absorb solar energy and convert to heat,resulting in the increase of temperature.In contrast,the surface temperature of the commercial MS nearly does not change (Fig.2h,i and j).The results confirm the efficient and fast solar-thermal conversion property of the prepared rGS.
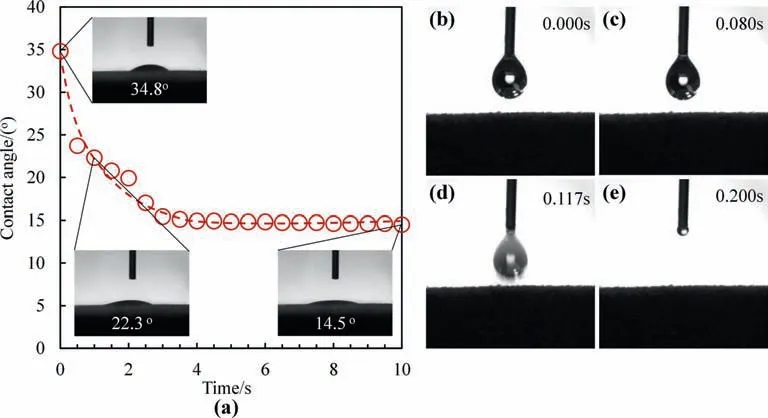
Fig.3.(a) Paraffin contact angle as a function of time on the rGO membrane.(b-e) Permeating behavior of paraffin droplet on the surface of rGS.
The paraffin contact angles of rGO film and rGS sponge are illustrated in Fig.3.The intrinsic contact angles of paraffin on the rGO coatings are measured by dropping 4 μl melting paraffin on the rGO film prepared with the same reduced condition as that of rGS sponge.The contact angle of the melting paraffin on the rGO film is about 14.5° (Fig.3a),showing the oleophilic property of the rGO nanosheets.In the process of dynamic contact angle variation of melting paraffin on the rGS sponge surface(Fig.3b–e),the melting paraffin droplet is sucked into the rGS sponge immediately as soon as the paraffin droplet contacts the rGS sponge.The whole sucking process only takes less than 0.2 s,because the porous structure and rough surface of rGS provide a capillary force and super-oleophilic property for the fast paraffin filling.
3.2.Thermal property and stability of rGS-pf
As shown in Fig.4a,MS and rGS with a diameter of 20 mm and a height of 2 mm are both placed on the top of solid paraffin.When the simulated sunlight turns on,MS stays in the original position during the whole process,and the paraffin beneath it doesn’t melt because of the absence of the solar-thermal conversion.However,the rGS could absorb the solar energy and convert it to heat,which causes the melting of the solid paraffin.The melting liquid paraffin fills in the rGS sponge (Fig.4b–c),and finally the solid paraffin completely melts and the rGS sinks to the bottom of the beaker (Fig.4d).
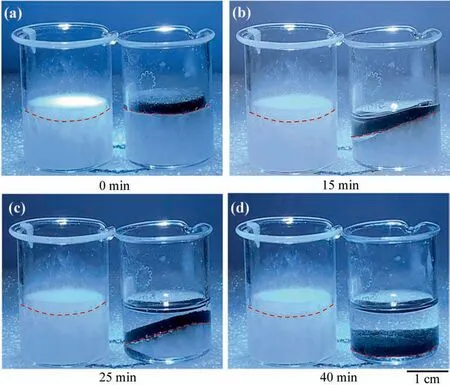
Fig.4.The optical photographs of MS and rGS sinking with the melting of paraffin when applied simulated sunlight at 0 min (a),15 min (b),25 min (c) and 40 min (d).
The rGS and MS filled with liquid paraffin,labeled as rGS-pf and MS-pf,are taken out from the liquid paraffin solution to cool down.After complete solidification at 4 °C,the MS-pf and rGS-pf are cut into 20 mm × 20 mm × 2 mm.The SEM images show that the paraffin is fully filled in the rGS networks(Fig.5a and b).The capillary force provided by the rGS and strong affinity between paraffin and rGO coatings is conducive to the full filling of paraffin in the porous rGS.To confirm the shape stability,the paraffin cube,MS-pf and rGS-pf are heated to 60°C by a hot stage,while all the samples are solid at first (Fig.5c).The paraffin cube melts completely at 6 min,but MS-pf and rGS-pf remain intact without noticeable liquid leakage during the entire heating process (Fig.5d).By repeatedly heating and cooling the rGS-pf for 20 cycles,the mass loss of rGS-pf is completely negligible (Fig.5e).The above-mentioned results confirm the shape stability and repeatability of rGS-pf without paraffin leakage.
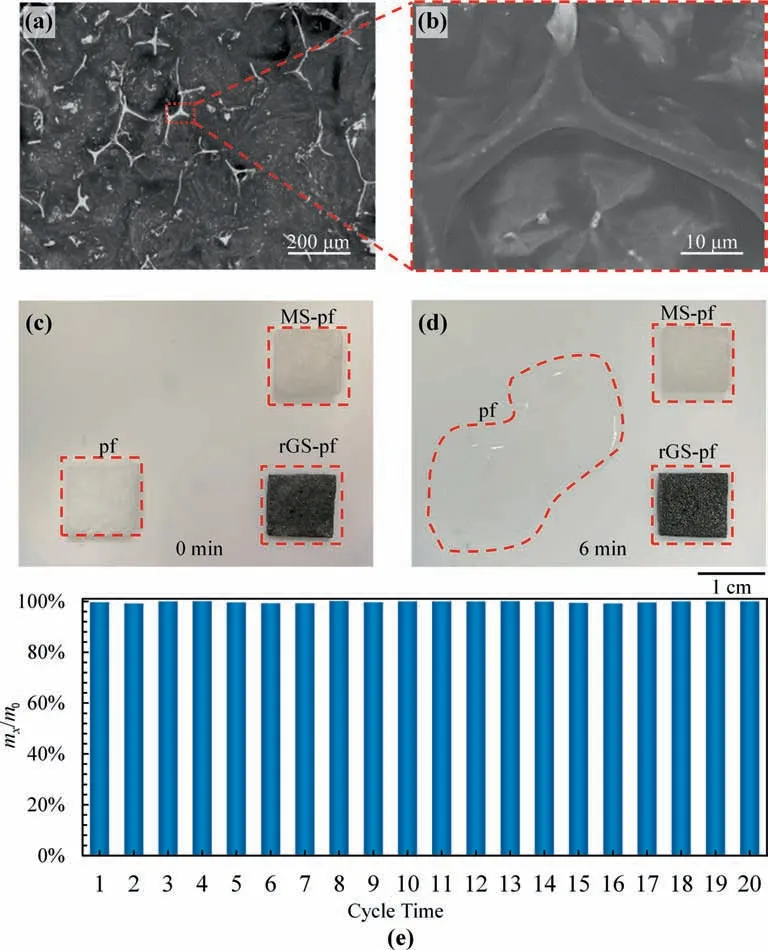
Fig.5.(a,b)The cross-section SEM image(a)and the high magnification image(b)of rGS-pf.The paraffin covers the rGS network uniformly.(c,d)The shape-stabilization of pf,MS-pf,rGS-pf at 0 min(c)and 6 min(d).The rGS-pf shows no obvious leakage during the whole heating process.(e)The mass change of rGS-pf for 20 cycles in the repeated heating and cooling processes,in which m0 is the initial mass of rGS-pf and mx is the mass of the rGS-pf after repeatedly heating and cooling for x cycles.
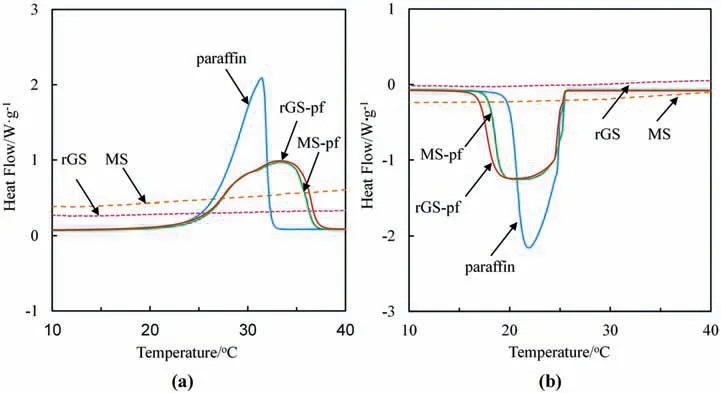
Fig.6.(a) DSC curves of MS,rGS,MS-pf,rGS-pf and pure paraffin during melting and (b) cooling processes.
The pristine MS and rGS show no thermal storage property,which is confirmed by the flat DSC curves during the melting(Fig.6a) and crystallization (Fig.6b) processes.The melting curve of pure paraffin displays an onset at 26.1 °C and a peak position at 31.5 °C.Comparing with the sharp melting peak and crystallization peak,the DSC curve of rGS-pf shows a lower onset and delayed melting peak temperature (Tm,p).The different shapes of curves between rGS-pf and paraffin are mainly resulted from the different purities of the samples.Paraffin is a pure material,while rGS-pf is a compound which is consists of paraffin,rGO nanosheets and the polymer melamine sponge.The increase of impurity contents may lead to the broader shape of peaks.Besides,the shape of the sample during the heating process is another factor that could affect the shape of DSC curve.The pure paraffin could not maintain its shape during the heating process,while rGS-pf remains blocky shape;thus,the heat transfer is different in these two cases.Therefore,the curves of rGS-pf and pure paraffin are different in shape(Fig.6).However,the integral areas are not influenced by the shapes of the curves.The integral areas that stand for the heat enthalpy are almost the same (Table 1).The melting enthalpies of paraffin,MS-pf and rGS-pf are 251.5,241.4 and 245.3 J·g-1,respectively,while the crystallization enthalpies of them are-249,-242.4 and -243.6 J·g-1,respectively.Because the latent heat of the rGS-pf depends on the amount of the paraffin filled in the rGS,the calculated filling ratio of paraffin in rGS-pf could reach up to 97.53% according to the Eq.(1),which is almost the same as the filling ratio obtained by weighting method (98.15%).Besides,the experimental results show that the rGO coated on the sponge skeletons in the rGS-pf is less than 0.11%.Compared with the previous works [13,16,28,43–46],the rGS-pf proposed in this work is thus proven to be a better strategy for immobilizing PCM.In the proposed rGS-pf,a tiny amount of rGO is consumed to incorporate a large amount of PCM,which guarantees a sufficiently high energy storage density of PCM-immobilized materials in per unit mass.
The as-prepared rGS-pf exhibits excellent thermal repeatability for long time use.The peak areas of the DSC curves of rGS-pf are almost the same after 50 or 100 heating/cooling cycles(Fig.7a).Both the melting enthalpy and the crystallization enthalpy nearly do not change after 50 or 100 heating/cooling cycles (Fig.7b).The results indicate that the filling of paraffin in the rGS is very stable,and no obvious leakage of paraffin occurs even after 50 or 100 heating/cooling cycles,which are consistent with the results in Fig.5.
3.3.Solar-thermal energy conversion and thermo-regulation characteristics of rGS-pf
To investigate the solar-thermal energy conversion characteristics of rGS-pf,simulated sunlight of 1000 W·m-2is applied to both MS-pf(Fig.8a)and rGS-pf(Fig.8c)for 6 min,and the surface average temperatures (labeled by red point frames) calculated by the matched software are shown in Fig.8e.The surface temperature of rGS-pf reaches the melting onset temperature of paraffin within 1 min,and paraffin begins to store the energy.After 2 min,the paraffin in rGS-pf has melted completely,leading to the increased surface temperature of rGS-pf.After turning on the simulated sunlight for 6 min,the rGS absorbs solar energy and its surface temperature continually rises(Fig.8d),while the surface temperature of MS-pf only increases a little(Fig.8b).The results are in agreement with those in the UV–vis–NIR absorption spectra.As shown in Fig.S1(see Supplementary Material),pure paraffin and MS exhibits weak absorption,especially in the visible light range.While rGS exhibits extreme broad absorption over the whole UV–vis–NIR range,because the rGO nanosheets are blackbody that could trap light convert effectively.The same goes for the rGS-pf.The existence of rGO nanosheets in rGS enhances the light absorption of rGS-pf and converts solar energy to heat efficiently,and the energy is stored in paraffin via phase change.The efficiency of solar-thermal conversion in this work could reach up to 92.5% according to Eq.(2),while the mass fraction of solar-thermal conversion materials in the rGS-pf is only 0.11%.Compared with the previous results reported in published literatures [28,43,47–50],the proposed rGS-pf in this study could achieve the highest efficiency of solar-thermal conversion with the lowest mass fraction of solar-thermal conversion materials(Table S1).The results show that the strategy proposed in this worknot only reduces the consumption of rGO,but also makes full use of the solar-thermal conversion ability of rGO nanosheets on the MS skeletons.

Table 1 Thermal properties of pure paraffin (pf),MS-pf and rGS-pf
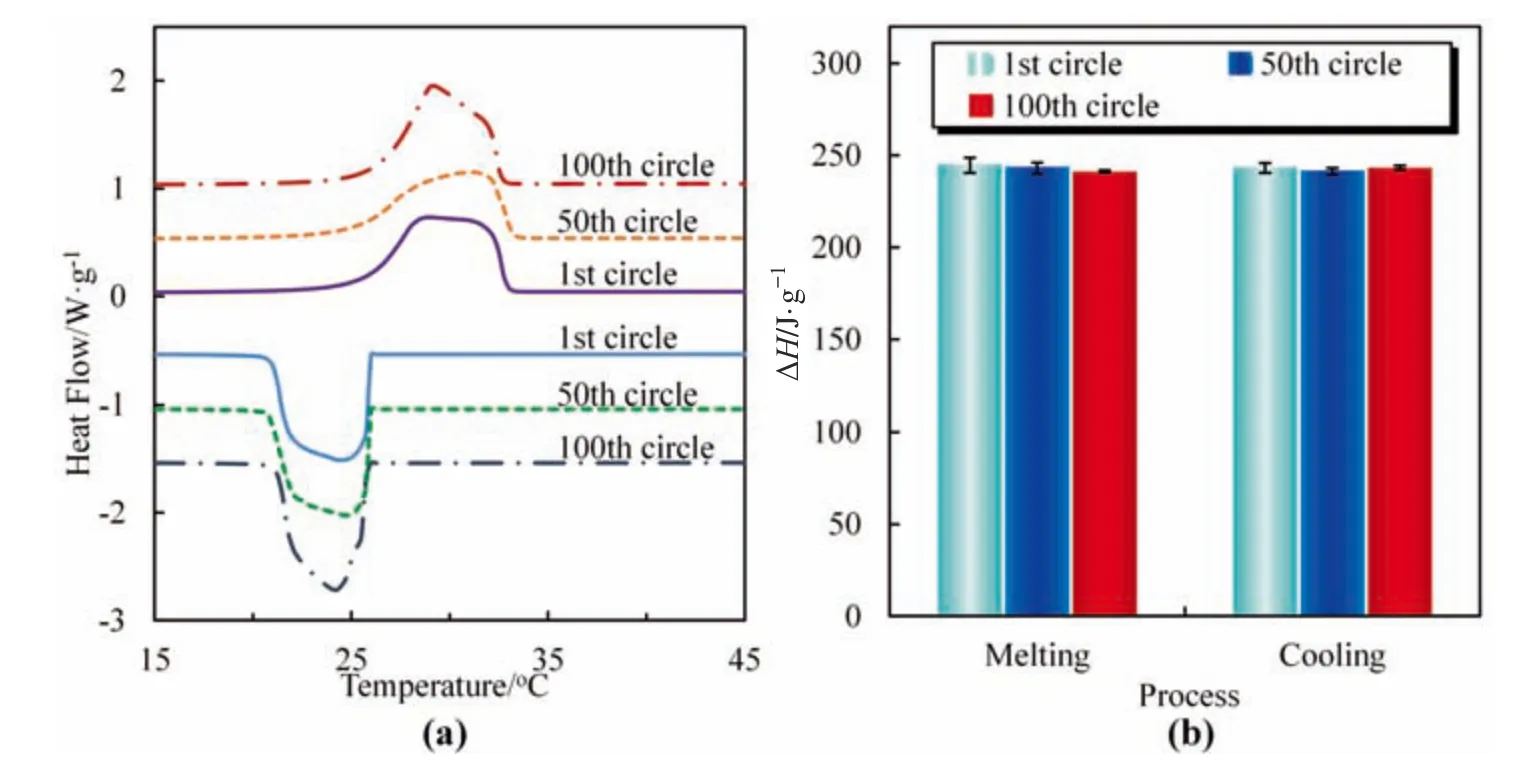
Fig.7.(a)DSC curves of rGS-pf tested for 1st,50th and 100th heating/cooling cycles and(b)melting enthalpy(left)and crystallization enthalpy(right)of rGS-pf in 1st,50th and 100th heating/cooling cycles.
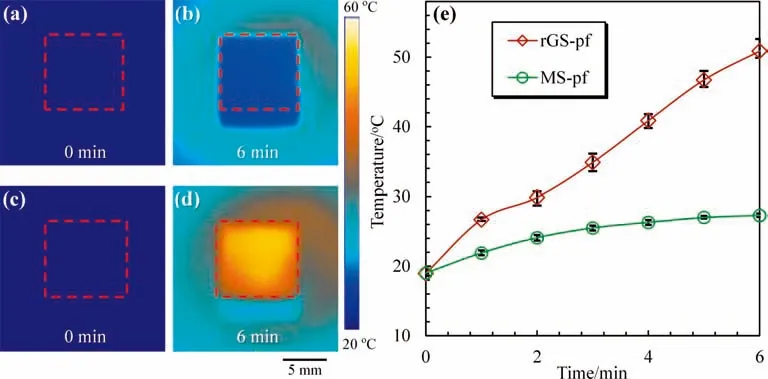
Fig.8.Fast energy storage of rGS-pf.(a-d)Infrared thermal images of surface temperature distributions of MS-pf(a,b)and rGS-pf(c,d)by applying the simulated sunlight for 6 min.The scale bar is 5 mm.(e) The temperature–time curves of surface average temperatures of MS-pf and rGS-pf labeled by red point frames in (a-d).
The thermo-regulation experiments are carried out by constructing an energy storage/release system with the prepared rGS-pf.In the tests,two model houses are constructed by 3D printing,and their walls and roofs are equipped with and without rGSpf,respectively (Fig.9a).The optical images of two model houses are shown in Fig.9b.The model house equipped without rGS-pf is labeled as House A,which is the control group;while the model house equipped with rGS-pf is labeled as House B.The thermocouple with dual channels is used to record the temperatures inside both House A and House B.
At the beginning of the heating process (Fig.9c,t=0 min),the initial indoor temperatures of the two model houses are 18 ºC,the same as the environment temperature.When the simulated sunlight is turned on,the temperature inside House A increases rapidly (green line in Fig.9c),while that inside House B keeps a lower temperature (red line in Fig.9c).Paraffin in the rGS-pf of House B can absorb the thermal energy converted from solar energy by rGS.When rGS-pf equipped in House B melts completely,the temperature inside House B continues to increase,but keeps lower than that inside House A until the end of the heating process (Fig.9c,t=20 min).The maximum difference of the inside temperatures between the two houses is as high as 11.6ºC upon turning on the simulated sunlight for 20 min.
When the simulated sunlight is turned off,the temperatures inside both Houses A and B decrease,while the temperature inside House A decreases sharply compared to that inside House B.The temperatures inside the two houses become almost the same after turning out the simulated sunlight for 10 min.However,the temperature inside House A continues to decrease sharply,while the temperature inside House B decreases much slower.Because the paraffin in the rGS-pf crystallizes and releases the energy to prevent the decrease of the temperature inside House B.As shown in Fig.9c,the thermo-regulation performances of rGS-pf for the temperatures inside houses are repeatable.The results confirm the stable and excellent thermo-regulation performances of the proposed rGS-pf,which ensure long-term serviceability.The proposed rGS-pf could buffer the environmental temperature variation via absorbing or releasing the energy in time to provide a more comfortable microclimate.

Fig.9.Schematic illustrations(a)and optical images(b)of the model houses equipped without(House A)and with(House B)rGS-pf for thermo-regulation under simulated sunlight.(c)Temperature evolution curves of the temperature changes inside model houses equipped without and with rGS-pf during repeated heating and cooling circles by exposing model houses to periodically “on–off” simulated solar radiation.
4.Conclusions
In summary,the reduced graphene oxide modified melamine sponges filling with paraffin (rGS-pf) have been successfully developed for solar-thermal conversion and heat management.The porous structures of the rGS and the oleophilic property of the rGO nanosheets help trap the liquid paraffin in the rGS,ensuring no leakage of paraffin during the repeated phasechange processes.The content of rGO nanosheets coated on the skeleton of rGS-pf is only 0.11%,but the loading content of paraffin in the rGS-pf is as high as 97.53%.The proposed rGSpf provides excellent performance for heat management to buffer the environmental temperature variation via absorbing or releasing the energy in time for providing a more comfortable microclimate inside the house.The efficiency of solar-thermal conversion could reach up to 92.5%.The thermo-regulation provided by the rGS-pf is real-time,repeatable and long-term stable,and thus is of great potential in providing a comfortable environment.In the daytime,the rGS-pf converts solar energy to thermal energy in time and stores the heat to impede the rapid increment of the indoor temperature.While at night,the rGSpf releases the heat to keep the indoor temperature.Moreover,the strategy for fabrication of the proposed rGS-pf in this study can be easily scaled up for preparing large-sized rGS-pf or other PCMs filled rGS for thermo-regulation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors gratefully acknowledge support from the National Natural Science Foundation of China (22022810),the Program for Changjiang Scholars and Innovative Research Team in University(IRT15R48) and Sichuan University (2020SCUNG112).
Supplementary Material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cjche.2021.09.017.
Nomenclature
R the enthalpy ratio
Tc,ecrystallization end temperature,°C
Tc,ocrystallization onset temperature,°C
Tc,pcrystallization peak temperature,°C
Tm,emelting end temperature,°C
Tm,omelting onset temperature,°C
Tm,pmelting peak temperature,°C
ΔHccrystallization enthalpy,J·g-1
ΔHmmelting enthalpy,J·g-1
ΔHm,paraffinmelting enthalpies of the paraffin,J·g-1
ΔHm,x-pfmelting enthalpies of the x-pf,x refers to either MS or rGS,J·g-1
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Struggling as in past, write a glorious future together-CJChE’s 40th anniversary
- Photocatalytic degradation of tetracycline hydrochloride with visible light-responsive bismuth tungstate/conjugated microporous polymer
- Ag nanoparticles anchored on MIL-100/nickel foam nanosheets as an electrocatalyst for efficient oxygen evolution reaction performance
- Performance improvement of ultra-low Pt proton exchange membrane fuel cell by catalyst layer structure optimization
- Anodic process of stibnite in slurry electrolysis:The direct collision oxidation
- Catalytic mechanism of manganese ions and visible light on chalcopyrite bioleaching in the presence of Acidithiobacillus ferrooxidans
