Performance improvement of ultra-low Pt proton exchange membrane fuel cell by catalyst layer structure optimization
2022-03-01JinyanXiKangMengYingLiMengWangQiangLiaoZidongWeiMinhuaShaoJianchuanWang
Jinyan Xi,Kang Meng,Ying Li,Meng Wang,,Qiang Liao,Zidong Wei,Minhua Shao,Jianchuan Wang,
1 School of Chemistry &Chemical Engineering,Chongqing University,Chongqing 400044,China
2 School of Energy and Power Engineering,Chongqing University,Chongqing 400044,China
3 School of Chemistry and Bioengineering,HongKong University of Science and Technology,Hong Kong,China
Keywords:Catalyst Mass transfer Optimization Fuel cell Ultra-low platinum Carbon nanotubes
ABSTRACT Reducing the loading of noble Pt-based catalyst is vital for the commercialization of proton exchange membrane fuel cell(PEMFC).However,severe mass transfer polarization loss resulting in fuel cell performance decline will be encountered in ultra-low Pt PEMFC.In this work,mild oxidized multiwalled carbon nanotubes(mMWCNT)were adopted to construct the catalyst layer,and by varying the loading of carbon nanotubes,the catalyst layer structure was optimized.A high peak power density of 1.23 W·cm-2 for the MEA with mMWCNT was obtained at an ultra-low loading of 120 μg·cm-2 Pt/PtRu (both cathode and anode),which was 44.7% higher than that of MEA without mMWCNT.Better catalyst dispersion,low charge transfer resistance,more porous structure and high hydrophobicity of catalyst layer were ascribed for the reasons of the performance improvement.
1.Introduction
To address the global warming issue,new energy sources with high energy density and environmentally-friendly are highly attractive.Among which,proton exchange membrane fuel cell(PEMFC) is considered to be the most promising one.It has the advantages of high energy conversion efficiency,zero emission,low noise,etc [1].However,the commercialization of PEMFC is challenged by the high cost,which is ascribed to the utilization of noble platinum(Pt)as catalysts[2–5].In order to fulfill the commercialization of PEMFC,a target of lower than 0.1 g·kW-1for Pt loading (both anode and cathode) was set up by the US Department of Energy.Nevertheless,the performance of PEMFC will normally decline when the Pt loading decreases.Therefore,how to maintain the performance of ultra-low Pt PEMFC is vital and extensive efforts have been done.The related research mainly focused on two fields:catalyst design and the structure of membrane electrode assembly (MEA).
For Pt-based catalyst design,it includes increasing the number of active sites,alloying with non-noble metal,ordered alloying(intermetallic)/surface de-alloying,single atom Pt catalyst.Through surface morphology optimization of the anisotropic nanomaterials,such as construction of Pt nanowires [6,7],highly concave Pt nanoframes [8] and Pt multicubes [9],more unsaturated active sites can be generated on the specific high-index facet,thereby it improved the catalytic activity of Pt-based catalyst.After alloyed with other metal,the geometric and electronic structure of Pt will change[10–12],three surface effects were induced:ensemble effect,ligand effect and geometric effect.Ensemble effect was always accompanied with ligand effect,which greatly improved the catalytic activity of Pt catalyst[13].Geometric effect especially the distortion of crystal lattice can influence the electron structure of multilayer atom,leading to successful adjusting of Pt catalyst[14].Although alloying largely improves the mass activity of catalyst,the dissolution of non-noble metal in the alloy accelerated the decline of MEA.To increase the activity and stability,ordered alloy[15,16] and surface de-alloy [17] catalyst were developed,which has been a research hotspot in Pt-based catalyst field these years.Single atom catalyst [18,19] was a quite important route to increase the utilization of Pt,because theoretically it possesses almost 100% dispersion of atom and unique coordination environment.Wei et al.[20] reported a single atom layer catalyst which possess high catalytic activity and stability,as well as good antipoisoning (for CO,NOx,SOx) properties.
The performance of PEMFC is not only dependent on the catalytic activity of catalyst,but also highly rely on the structure of MEA (core component of fuel cell).In ultra-low Pt PEMFC,mass transfer polarization loss is much more severe than common Pt loading PEMFC,including gas transfer,proton transfer issue,and so on.To improve the performance of ultra-low Pt PEMFC,efforts have been done on high efficient “gas-solid-liquid” interface construction,ordered catalyst layer design and anti-flooding catalyst layer preparation.A well “gas-solid-liquid” interface was prepared by alternative ion-exchange/electrodeposition method,and the asprepared MEA with a Pt loading of 0.014 mg·cm-2exhibited peak power density of approximately 2.2 times larger than that of conventional MEA[21].In 2002,Middelman proposed an ideal ordered electrode [22],in which electron,proton,gas will transfer within their own channels,thus the mass transfer resistance will be greatly reduced.This ideal was partially realized with vertical aligned carbon nanotubes supported Pt catalyst [23,24],the total mass transfer efficiency of the as-prepared MEA was quite high,at an ultra-low loading of 35 μg·cm-2Pt,the H2/O2fuel cell can reach a high peak power density of 1.03 W·cm-2.Shao et al.[25]developed an ultrathin catalyst layer based on open-walled PtCo bimetallic nanotubes arrays,as an oxygen reduction reaction(ORR) catalyst,the utilization of metal catalyst was as high as 14.38 kW·g-1.In fuel cell,water should be excluded in time,or it will block the transfer of reaction gas,resulted in output drop of fuel cell,so called “water flooding”.Increasing the hydrophobicity of catalyst layer was a good way to alleviate the “water flooding” issue,for example,by adding of simethicone in the catalyst layer,flooding in pores with a diameter of 20–70 nm was solved [26].
Carbon nanotubes is a 1D nanomaterials with high aspect ratio and specific surface area,as well as unique electronic property.Usually it has interaction with the surface supported metal catalyst,improving the catalytic and stability of catalyst[27–31].It also has been applied in the gas diffusion layer preparation[32–34],by reducing the thickness of gas diffusion layer and inducing more porous structure,better fuel cell performance was achieved.With a high aspect ratio,carbon nanotubes formed a more porous network in catalyst layer,together with high hydrophobicity induced by PTFE,a peak power density of 949 mW·cm-2was achieved with 0.3 mg·cm-2Pt loading [35].Toyota has developed electrodes based on vertically aligned CNTs (VACNTs),which effectively enhanced the gas diffusivity,water drainage and effective utilization of Pt (0.1 mg·cm-2) [24].However,the VACNTs in-situ grown by chemical vapor deposition method,the sophisticated process and high cost resulted difficulty in practical application.In this work,a facile route was shown to promote the performance of fuel cell by simply adding of mild surface modified multiwalled carbon nanotubes (mMWCNTs) to the catalyst ink.The catalyst layer structure was optimized,finally a high peak power density of 1.23 W·cm-2was obtained at an ultra-low loading of 120 μg·cm-2Pt/PtRu (sum of cathode and anode).
2.Materials and Methods
2.1.Materials
MWCNTs (TNIM1,95% (mass),diameter=5–15 nm,length=10–30 μm) were purchased from Timesnano,China.Pt/C (Hispec 9100,60%(mass),Johnson-Matthey),PtRu/C (Hispec 10000,60%(mass),Johnson-Matthey) catalysts,carbon paper (HCP120),Nafion HP membrane (20 μm,Dupont) and Nafion ionomer (5%(mass),Dupont) were purchased from Shanghai Hesen company,China.Carbon paper was soaked in ethanol to remove impurities and pollutants before use.Acetone (AR),sulfuric acid (AR),isopropanol (AR) and ethanol (AR) were purchased from Chuandong Chemical Group,China.Hydrogen and oxygen were purchased from Chongqing Risinggas,Ltd.
2.2.Preparation of modified MWCNT (mMWCNT)
MWCNTs were firstly immersed in acetone and refluxed for 12 h,then MWCNTs were collected and transferred to 3 mol·L-1H2SO4,stirred at room temperature for 48 h.After that,the suspension was left overnight,filtered and washed with deionized water for several times,at last,it was dried in a vacuum oven at 80 °C obtain mMWCNTs.
2.3.Preparation MEA
The MEAs were prepared by gas diffusion electrode (GDE)method.Pt/C (cathode) or PtRu/C (anode),Nafion ionomer,isopropanol and mMWCNT were mixed together and ultrasonicated for 1 h to obtained uniform dispersed catalyst ink.In which,the mass ratio of Pt/C (or PtRu/C):ionomer:isopropanol=2.5:1:2.The effective area of electrodes were both 5.0 cm2for anode and cathtode,and the Pt/PtRu loading on the cathode and anode were both 60 μg·cm-2.The loading of mMWCNT varied from 0 to 500 μg·cm-2.The catalyst ink was dropped onto the carbon paper and dried,then the Nafion HP membrane was sandwiched between the as-prepared cathode and anode carbon paper,followed by hot pressing at 135°C,5 MPa for 3 min to obtained MEA.Samples were designated as mMWCNT-x μg·cm-2(x=0,62.5,125,250,500,corresponding to the loading of mMWCNT).
2.4.Characterizations
The X-ray Photoelectron Spectroscopy (XPS) was done by a,ESCALAB250Xi (Thermo Fisher Scientific),the light source was Al Kα X-ray(hv=14486.6 eV).The morphologies of catalyst ink were observed with a transmission electron microscopy (TEM,FEI Talos F200s),the accelerating voltage was 200 kV,samples were dropped on copper mesh and dried.The morphologies of catalyst layer were observed by scanning electron microscopy (SEM,JEOL JSM-5900LV)at an accelerating voltage of 5 kV.The contact angle measurement was carried out with a JC2000D1 instrument,at room temperature.For catalyst layer measure,the sample was prepared by dropping the catalyst ink on a PET film to form a uniform coating;for the hydrophilicity test of carbon nanotubes,the carbon nanotubes powder was pressed to be a wafer with a diameter of 16 mm.The H2/O2fuel cell performance was tested on an 850e fuel cell test system (Scriber associates).The cell temperature was 80 °C,pure H2and O2were supplied with varied humidity (40%RH,70%RH,100%RH),anode gas flow rate was 0.25 L·min-1,cathode gas flow rate was 0.35 L·min-1,the back pressure was kept at 0.2 MPa.Before the test,the catalysts were activated for a period of time (generally 2–3 h) at constant voltage of 0.5 V.The electrochemical impedance spectroscopy of the MEA was also studied with 850e fuel cell test system,at different current densities of 0.1 A·cm-2,0.5 A·cm-2,0.8 A·cm-2,1.0 A·cm-2,in the frequency range of 0.1 Hz to 10 kHz.The thermogravimetric (TG) test was conducted with a TA Q500 TGA,heating from 25 to 600°C at a rate of 10°C·min-1,at N2atmosphere.BET was done with a Quadrasorb 2 MP analyzer (Quantachrome).Before the test,the sample was degassed in vacuum at a high temperature of 200 °C for at least 12 h.
3.Results and Discussion
MWCNT was quite hydrophobic and hard to dispersed,thus it was necessary to increase its polarity.The most common way to increase the hydrophilicity of MWCNT was oxidation.However,oxidation will destroy the conjugated π electron structure,resulting in conductivity decline,which was harmful for electron transfer in PEMFC.Besides,too much high hydrophilicity will bring difficulty to water management in fuel cell operation,and finally the fuel cell performance comprised.Therefore,in this work,MWCNT was mildly oxidized at room temperature.From the XPS data in Fig.1,there was hardly oxygen-containing groups,while the oxygen atom ratio was 3.19% for mMWCNT.It suggested that,after oxidation,the surface of mMWCNT was successfully functionalized with a small amount of oxygen-containing groups.TGA curves also demonstrated that there were unstable oxygencontaining functional groups induced by oxidation for mMWCNT(Fig.S1 in Supplementary Material).Consequently,the hydrophilicity of mild-oxidized mMWCNT was improved as shown in contact angle test (Fig.S2).This mildly oxidized mMWCNT not only possessed increased hydrophilicity,but also maintained the intrinsic high electron conductivity of MWCNT.
The single cell H2/O2performance was demonstrated in Fig.2.At an ultra-low loading of 120 μg·cm-2Pt/C (or PtRu/C),the conventional MEA without mMWCNT added showed a peak power density of 0.85 W·cm-2.Intriguingly,by adding a small amount of mMWCT,the performance of fuel cell largely improved.Especially for mMWCNT-125 μg·cm-2,the peak power density elevated to 1.23 W·cm-2,which was 44.7% higher than that of mMWCNT-0 μg·cm-2.However,for higher loading of mMWCNT (250,500 μg·cm-2),the fuel cell performance dropped,it might be ascribed to thicker catalyst layer and narrower gas transfer channel resulting in higher gas transfer resistance.From the limiting current cures,by adding a small amount of mMWCNT (62.5,125 μg·cm-2),the mass transfer polarization loss was alleviated at high current density.In order to find out the reason for the remarkable improvement in fuel cell performance,systematic studies were done and analyzed as follows.
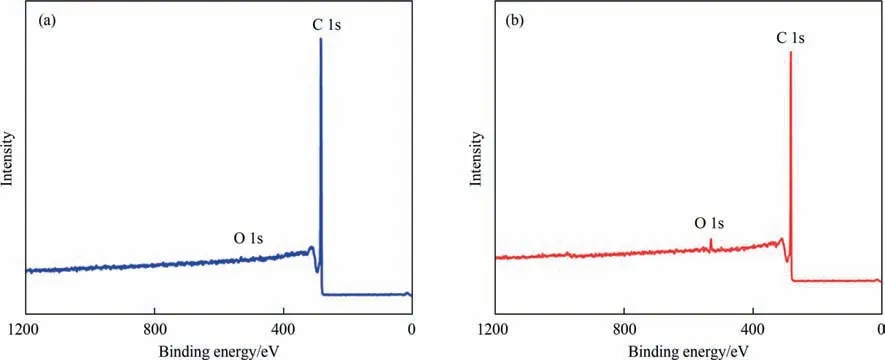
Fig.1.The XPS spectra of (a) unmodified MWCNT and (b) modified MWCNT (mMWCNT).

Fig.2.H2/O2 fuel cell performance of ultra-low Pt PEMFC with different loading of mMWCNT,the catalyst loading for all the samples were:anode:PtRu/C 60 μg·cm-2,cathode:Pt/C 60 μg·cm-2.

Fig.3.Humidity dependence of H2/O2 fuel cell performance of ultra-low Pt PEMFC:(a) mMWCNT-0 μg·cm-2 (b) mMWCNT-125 μg·cm-2.The catalyst loading for all the samples were:anode:PtRu/C 60 μg·cm-2,cathode:Pt/C 60 μg·cm-2.
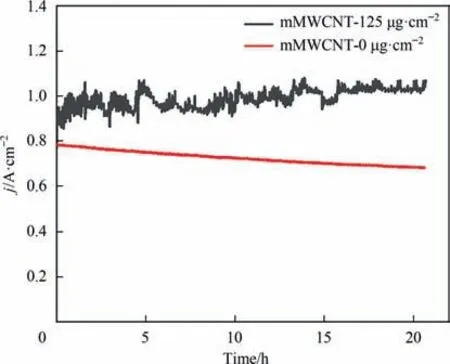
Fig.4.Short-term durability of MEA based on mMWCNT-0 μg·cm-2 and mMWCNT-125 μg·cm-2.
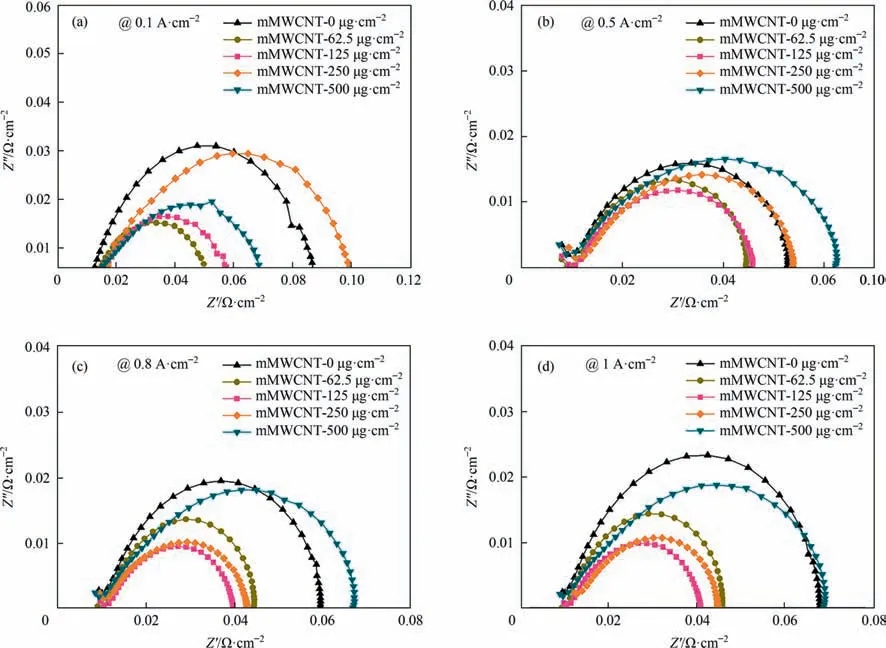
Fig.5.Electrochemical impedance spectroscopy (EIS) of MEAs with different loading of mMWCNT,at (a) 0.1 A·cm-2,(b) 0.5 A·cm-2,(c) 0.8 A·cm-2 and (d) 1 A·cm-2.

Fig.6.TEM images of catalyst ink,(a) anode catalyst ink mMWCNT-0 μg·cm-2,(b)cathode catalyst ink mMWCNT-0 μg·cm-2,(c) anode catalyst ink mMWCNT-125 μg·cm-2,(d) cathode catalyst ink mMWCNT-125 μg·cm-2.

Fig.7.SEM images of electrode(carbon paper supported),(a)anode mMWCNT-0 μg·cm-2,(b)anode mMWCNT-62.5 μg·cm-2,(c)anode mMWCNT-125 μg·cm-2,(d)anode mMWCNT-250 μg·cm-2,(e)anode mMWCNT-500 μg·cm-2,(f)cathode mMWCNT-0 μg·cm-2,(g)cathode mMWCNT-62.5 μg·cm-2,(h)cathode mMWCNT-125 μg·cm-2,(i)cathode mMWCNT-250 μg·cm-2,(j) cathode mMWCNT-500 μg·cm-2.
The cell performance was also conducted at different humidity.As shown in Fig.3,for both mMWCNT-0 μg·cm-2and mMWCNT-125 μg·cm-2,the peak power density increased with the humidity decreased(from 100%RH to 40%RH).Normally,the conductivity of proton membrane will decrease with the decrease of humidity,which may result in drop of cell performance.However,at lower humidity,the water drainage was better,leading to better performance at high current density.Especially for MEA with such an ultra-low loading of catalysts,the influence of water drainage was greater than that of conductivity drop,thus the peak power density promoted with the humidity decreased.We also found that at low humidify condition,the performance of the mMWCNT 125 μg·cm-2was still better than that of the conventional catalyst layers without mMWCNT,which implied that there was no severe dehydration of electrolytes by adding of mMWCNT.
It is well known that durability of catalyst is an important performance indicator for fuel cells.The short-term durability of MEA based on mMWCNT-0 μg·cm-2and mMWCNT-125 μg·cm-2was conducted at a constant voltage of 0.6 V.As shown in Fig.4,because of ultra-low loading of catalyst,the mMWCNT-0 μg·cm-2showed a decrease of 100 mA·cm-2after 20 h.Intriguingly,there was hardly decline for that of mMWCNT-125 μg·cm-2,indicating an excellent fuel cell stability.Therefore,by adding of mild-oxidized MWCNT,the life-time of ultra-low Pt fuel cell can be improved.
EIS spectra were first done and shown in Fig.5,the diameter of the semicircle represented the charge transfer resistance (Rct).Obviously,no matter at low current density or high current density,the Rctof MEA adding a small amount of mMWCNT were lower than that of MEA without mMWCNT.Especially at a high current density of 1 A·cm-2,the Rctof mMWCNT-125 μg·cm-2was only 57% of that of mMWCNT-0 μg·cm-2,which can explain the less mass transfer polarization loss at high current density in Fig.2.The lower Rctof MEA with mMWCNT can be ascribed to the excellent conductivity of mMWCNT,because the conjugated π structure of MWCNT was not destroyed by mild oxidation.
The morphology of catalyst and mMWCNT in catalyst ink was observed in TEM images.As shown in Fig.6,generally the dispersion of mMWCNT was excellent,and most importantly the dispersion of catalysts with mMWCNT were much better than that of without mMWCNT.The catalyst of anode and cathode were different,the dispersion and morphology of anode and cathode catalyst layer were different at microscale.However,at macroscale latter in SEM pictures,the catalyst layer looked the same.For anode catalyst ink,large PtRu/C clusters of over 100 nm were observed.After adding of 125 μg·cm-2mMWCNT,the dispersion of PtRu/C was largely improved,there was hardly large catalyst clusters,all the PtRu/C particles were “attached” on the surfaces of mMWCNT.For cathode Pt/C catalyst ink,there seemed to be little improvement of dispersion after adding of 125 μg·cm-2mMWCNT.It can be concluded that there was good interaction between PtRu/C and mMWCT,which might be ascribed to the small amount of oxygencontaining functional group on the surface of mMWCT,leading to excellent dispersion of PtRu/C.The good dispersion of catalyst especially anode PtRu/C catalyst,increased the utilization of PtRu/C,which facilitated the fuel cell performance at an ultralow loading of Pt-based catalyst.

Fig.8.Contact angles of catalyst ink with various loading of mMWCNT:(a) Anode,(b) Cathode.
The structure of catalyst layer was studied by SEM,the surface of carbon paper was after coated with catalyst ink was directly applied for SEM observation.As shown in Fig.7,either for anode catalyst layer or cathode catalyst layer,the dispersion of catalyst was somewhat improved by adding of mMWCNT.What’s more,the catalyst structure changed with the incorporation of high aspect mMWCNT,more porous structure was achieved.By varying the loading of mMWCNT,the structure of catalyst layer was optimized,mMWCNT-125 μg·cm-2possessed the best porous structure.BET test was also done to show the change of porous structure(see Table S1,Fig.S3 and Fig.S4).In order to get information under operational condition,catalyst layer with carbon paper support was adopted for BET test.The results showed that,generally,the specific area increased after mMWCNT added,but the average pore size was dominated by carbon paper.The optimized porous structure was quite important for the mass transfer in catalyst layer,especially for the reaction gas transfer,which can accelerate the electrochemical reaction process.
The anti-water flooding properties in catalyst layer was vital for fuel cell.Commonly,catalyst layer with effective pores and high hydrophobicity was beneficial for water draining.The porous structure has been studied in SEM morphologies,indicating that the fine porous structure of mMWCNT-125 μg·cm-2was positive for the water management in fuel cell.The hydrophobicity of catalyst layer was another concern,as shown in Fig.8,the hydrophobicity of catalyst ink was studied.Obviously,by adding mMWCNT,the contact angle increased for both anode and cathode,thus higher hydrophobicity achieved,which was helpful to the water draining.Although the MWCNT was oxidized,only a few functional polar groups were induced,thereby the mMWCNT was highly hydrophobic,which was the explanation to the increase of hydrophobicity for catalyst ink with mMWCNT.
4.Conclusions
Modified mutiwalled carbon nanotubes(mMWCNT)with a few oxygen-containing groups was synthesized by mild oxidation,the as-prepared mMWCNT was incorporated into the catalyst layer of PEMFC.A high peak power density of 1.23 W·cm-2for the MEA with 125 μg·cm-2mMWCNT was obtained at an ultra-low loading of 120 μg·cm-2Pt/PtRu (both cathode and anode),which was 44.7%higher than that of MEA without mMWCNT.The reason for the remarkable improvement in PEMFC performance was systematically studied.As the hydrophilicity of the mMWCNT was improved,there was better interaction between the catalyst and mMWCNT,especially for anode PtRu/C and mMWCNT,leading to an excellent dispersion of catalyst particle,which promote the utilization of Pt-based catalyst.Furthermore,the intrinsic high electron conductivity of mMWCNT was maintained as a result of mild oxidation,the charge transfer resistance of MEA greatly reduced by adding a few amount of mMWCNT.Compared the catalyst layer without mMWCNT,more porous structure in catalyst layer was obtained with appropriate loading of mMWCNT due to the high aspect ratio of mMWCNT,contributed to largely improved gas transfer and water draining properties.The hydrophobicity of the catalyst layer with mMWCNT was also increased obviously,which was highly beneficial for the anti-water flooding.In a word,better catalyst dispersion,low charge transfer resistance,more porous structure and high hydrophobicity of catalyst layer were ascribed for the reasons of the performance improvement.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Thanks for the financial supports by the National Key Research and Development Program of China (2019YFB1504500),the National Natural Science Foundation of China (22078031,91834301,21761162015),the Fundamental Research Funds for the Central Universities,CQU (2020CDJQY-A032,2020CDJLHZZ-064),the Natural Science Foundation of Chongqing(cstc2020jcyjmsxmX0637).
Supplementary Material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cjche.2021.11.013.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Struggling as in past, write a glorious future together-CJChE’s 40th anniversary
- Reduced graphene oxide modified melamine sponges filling withparaffin for efficient solar-thermal conversion and heat management
- Photocatalytic degradation of tetracycline hydrochloride with visible light-responsive bismuth tungstate/conjugated microporous polymer
- Ag nanoparticles anchored on MIL-100/nickel foam nanosheets as an electrocatalyst for efficient oxygen evolution reaction performance
- Anodic process of stibnite in slurry electrolysis:The direct collision oxidation
- Catalytic mechanism of manganese ions and visible light on chalcopyrite bioleaching in the presence of Acidithiobacillus ferrooxidans
