Anodic process of stibnite in slurry electrolysis:The direct collision oxidation
2022-03-01YongluZhangDingfanQiuChengyanWangYongqiangChenZhichaoYaoXiaowuJieWeiGaoShufengRuan
Yonglu Zhang,Dingfan Qiu,Chengyan Wang ,Yongqiang Chen ,Zhichao Yao,Xiaowu Jie,Wei Gao,Shufeng Ruan
1 State Key Laboratory of Advanced Metallurgy,University of Science and Technology Beijing,Beijing 100083,China
2 School of Metallurgical and Ecological Engineering,University of Science and Technology Beijing,Beijing 100083,China
3 BGRIMM Technology Group,Beijing 100160,China
Keywords:Slurry electrolysis Stibnite Potential range Potential interval Steady-state polarization curve
ABSTRACT Mineral oxidation leaching in the anode area is the key step in slurry electrolysis.By adopting the slow linear potential scanning method during slurry electrolysis,this study investigated the steady-state polarization curve of a pure stibnite mineral on a graphite anode.In addition,the influence of the mineral particle size,liquid–solid ratio,stirring speed,and temperature on the collision oxidation of the mineral with the anode was studied.Based on the different oxidation reactions,the potential range can be divided into three intervals:the low-potential interval with a potential lower than 0.75 V,an intermediatepotential interval with a potential within 0.75–1.2 V,and a high-potential interval with a potential higher than 1.2 V.The collision oxidation of the mineral with the anode occurred in all three intervals.The oxidation of Sb(III) also appeared in the intermediate-and high-potential intervals,and chlorine evolution occurred in the high-potential interval.Therefore,the low-potential interval was determined to be a suitable potential interval for the slurry electrolysis process.In the low-potential interval,the particle size,liquid–solid ratio,and stirring speed had little effect on the oxidation rate of the minerals.As the temperature increased,the stibnite oxidation rate and exchange current density increased.Overall,the direct collision oxidation rate of stibnite was relatively low and the current densities under all the investigated conditions were lower than 0.4 mA·cm-2.This indicates that it is difficult to realize industrial production while relying solely on this process.
1.Introduction
Arsenic-containing antimony gold concentrate(As-Sb-Au ore)is an important raw material for antimony and gold extraction[1–3].Antimony often exists in the form of stibnite and usually needs to be removed before gold extraction[4–7].Zhang et al.[8]proposed a slurry electrolysis (SE) method to pretreat this As-Sb-Au ore.In the proposed method,stibnite is selectively oxidized to release antimony ions in the anode region,whereas metallic antimony is generated by electrodeposition in the cathode region.In addition,gold is enriched in the residue for further treatment.In the SE process,the oxidation leaching reaction of ore replaces the anodic reaction of traditional electrodeposition.The anodic reaction consumes a very large amount of energy during the normal electrodeposition process,which in turn results in effective leaching of the metal [9–13].Therefore,mineral oxidation leaching in the anode area is the key step in SE,and an understanding of this process is necessary for optimizing the control of the SE process.This subject has been extensively investigated in previous studies [14–17].
Previous research on the electrooxidation process of arsenopyrite in H2SO4and H2SO4–NaCl solutions [18] indicated that oxidation of arsenopyrite particles by direct contact with the anode is inefficient.Fe(III) oxidizes arsenopyrite at atmospheric pressure between 49 °C and 81 °C.However,the current is utilized inefficiently at lower temperatures.In addition,Cl2,which is generated from the Cl-ion,is an efficient oxidant between 49 °C and 81 °C.The mechanism of electrooxidation of pyrite in NaCl solutions was achieved in two steps [19]:hypochlorite production on the anode and pyrite oxidation by hypochlorite.Pyrite oxidation was dependent on the production of hypochlorite,which had a current efficiency of up to 97%.
In the Dextec-Cu process,Spencer and Harris[20]reported that chalcopyrite in slurry could be directly electrooxidized or electrochemically leached.This electrochemical reaction occurred between the mineral particles,electrolyte,an air bubble,and the anode surface.Everett[21]also reported that the collision between the chalcopyrite particles and the anode surface caused them to lose electrons and be oxidized;hence,this is the main reason for the leaching of chalcopyrite.These two studies demonstrated that oxidation leaching of Cu(II) and Fe(III) is weak during the SE process,and is not the main cause for chalcopyrite leaching.In contrast,Yang and Zhang [22] demonstrated that in NaCl solution,the probability of a collision oxidation reaction between chalcopyrite and the anode was low.In addition,the leaching of chalcopyrite was mainly due to the oxidation of Cu(II) and Fe(III).Additionally,Zhang et al.[23] reported that in the SE process of the gold concentrate,the contribution of direct anodic oxidation of mineral particles to sulfide leaching was small,and leaching was mainly dependent on non-electrode processes (chemical oxidation or chemical dissolution).
Wang et al.[24] studied the SE process of bismuthinite in the “NaCl–HCl” system.In the absence of iron ions in the electrolyte,the anode reaction mainly consisted of the formation of chlorine,which is the main oxidant in the oxidation leaching reaction of bismuthinite.In the presence of iron ions,the oxidation reaction on the anode was mainly Fe(II),and the oxidation of bismuthinite was mainly achieved by Fe(III).The direct collision oxidation of bismuthinite with the anode was not the main reaction in both cases.
Regarding antimony sulfides,Zhang and Chen [25] demonstrated that for a system consisting of 2–3 mol·L-1of HCl and 4–6 mol·L-1of Cl-,stibnite leaching in the anode area of the SE process depended mainly on chemical oxidation dissolution.The oxidation by Fe(III) and Sb(Ⅴ) only occurred toward the end of the reaction.During the SE of jamesonite in HCl–NH4Cl solution [26],the anode reaction mainly consisted of a direct collision oxidation between the mineral and the anode when the anode potential was lower than 1.10 V (SCE).When the anode potential was greater than 1.10 V (SCE),the chlorine and oxygen evolution reactions played a leading role.In the presence of the iron ions,the main reaction that occurred on the anode was the oxidation of Fe(II),and the oxidation of the minerals was mainly conducted by Fe(III).
In summary,due to the differences in the sulfide minerals,test conditions,and research methods,the SE anode process was different.In general,the anode reaction of SE can be divided into the following stages:(1) direct collision oxidation of the minerals and electrodes;(2) indirect electrooxidation of the minerals through redox couples,such as Fe(III)/Fe(II),Cu(II)/Cu(I),and Cl2/Cl-;and(3) chemical dissolution of minerals.Despite these advances,it is apparent that there still much that is unknown about the anode reaction of the SE process.
For the HCl-NaCl system with a HCl concentration below 1.5 mol·L-1,the SE anode process for treating As-Sb-Au ore has not been reported.Mineralogical analysis had previously shown that the main antimony-bearing mineral in the As-Sb-Au ore was stibnite [8].Therefore,pure stibnite was taken as the research object in this study.Furthermore,the direct collision oxidation reaction of minerals with an electrode during the SE process in the HCl-NaCl system was studied.
2.Experimental
The stibnite samples used in this study consisted of natural crystals with a high purity (from Hunan Lengshuijiang,China).The X-ray diffraction (XRD) test results are shown in Fig.1,where no other obvious impurity phases were detected except for the stibnite phase.The minerals were crushed,ground,and screened to obtain the test samples.Unless stated otherwise,the particle size of the samples used in the test ranged from 0.048 to 0.074 mm.
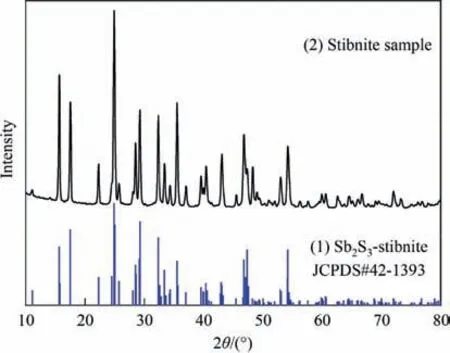
Fig.1.XRD pattern of the stibnite samples.
Electrochemical measurements were performed using a system with three electrodes.The working electrode (WE) consisted of graphite with a purity of 99.99% and an area of 1 cm2.The WE was prepared by performing wet-grinding with emery paper and rinsing with deionized water before each use.The reference electrode (RE) was a silver/silver chloride electrode (saturated KCl).In addition,a titanium plate was used as the counter electrode(CE).The RE was obtained from Shanghai Chenhua Instrument Co.,Ltd.The WE and CE were customized by Jinko Instruments.All the potentials were relative to the standard hydrogen electrode(SHE).The potential against Ag/AgCl plus the standard electrode potential of Ag/AgCl at a specific temperature equals the potential against SHE at that temperature.The base electrolyte contained 150 g·L-1NaCl and 1 mol·L-1HCl.All the reagents were analytical grade,and all the aqueous solutions were prepared using deionized water.The electrolyte was de-aerated with nitrogen before each experiment.The solution was heated by a thermostatic water bath and stirred by a stirring motor.The electrochemical experiments were carried out using a CHI660E electrochemical workstation from the Shanghai Chenhua Instrument Co.,Ltd.The experimental arrangement is shown in Fig.2.
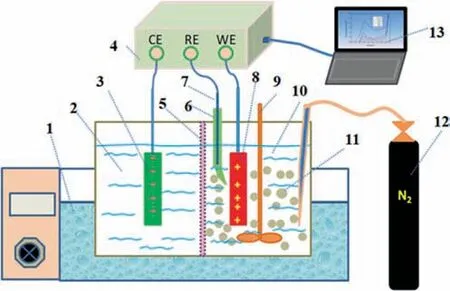
Fig.2.Schematic representation of the experimental arrangement:1—Thermostatic water bath;2—Cathode part;3—Counter electrode;4—Electrochemical workstation;5—Polypropylene diaphragm;6—Luggin capillary;7—Reference electrode;8—Working electrode;9—Stirring system;10—Anode part;11—Minerals;12—Nitrogen;13—Date recorder.
3.Results and Discussion
The steady-state polarization curve of the electrode reaction was measured by performing slow linear potential scanning.The relationship between the polarization curves and scanning speed,which ranged from 0.8–50 mV·s-1,was studied in the “HCl-NaCl” and “HCl-NaCl-stibnite” systems (Fig.3).The polarization curve was measured by successively reducing the scanning speed.The polarization curve no longer changed significantly when the scanning speed fell below 1 mV·s-1,and the electrode process was considered to have reached steady state.Therefore,in this study,a scanning speed of 1 mV·s-1was used to measure the steadystate polarization curve.In addition,unless otherwise stated,the basic electrolyte composition in this study consisted of 1 mol·L-1HCl and 150 g·L-1NaCl.
3.1.Steady–state polarization curve of stibnite
As shown in Fig.4,graphite was used as the anode to study the polarization curves for base electrolyte(line 1)and base electrolyte with stibnite(line 2).When the potential was lower than 1.2 V(vs.SHE),the current density of line 1 was extremely low and exhibited almost no variation when the potential increased.When the potential was larger than 1.2 V,the current density increased dramatically.At this point,chlorine evolution started to occur at the anode (Eq.(1)).Overall,line 2 appeared to be above line 1.When the potential was larger than 0.45 V,a current was generated.This indicates that stibnite was oxidized and the process generated a current,which agrees with a previous study [27].
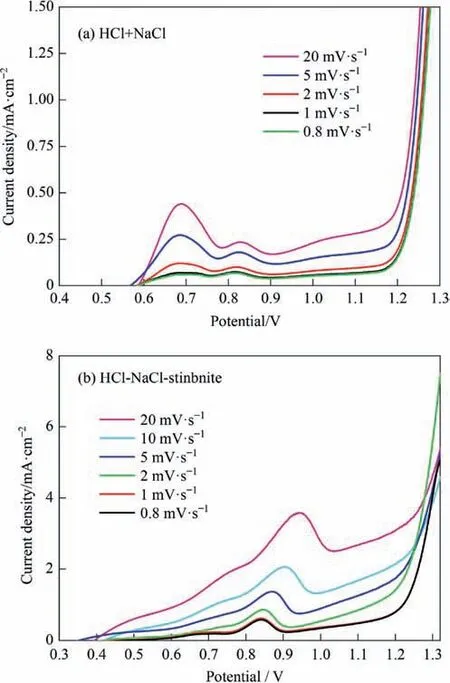
Fig.3.Steady-state polarization curves for anode reaction at different scanning speeds.
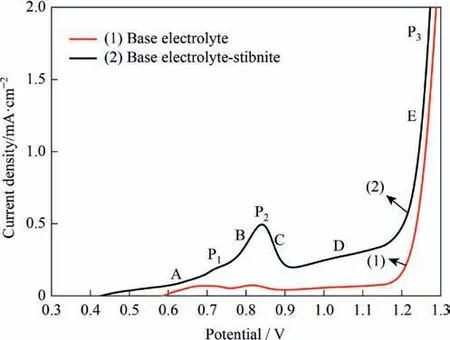
Fig.4.Polarization curves for (1) base electrolyte and (2) base electrolyte with stibnite (50 °C,L/S=10,agitation speed of 500 r·min-1).

There were three anode current peaks (P1,P2,and P3) in the investigated potential range.According to the types of chemical reactions,this range can be divided into three intervals.Section A was the low-potential interval,where the current increased slowly with an increase in the potential.The reaction-rate determining step was attributed to the kinetics at the electrode.The main electrode reaction was the collision electrooxidation of the stibnite particles,(Eq.(2)).Sections B,C,and D were the intermediatepotential interval.As the scanning potential increased,the reaction driving force increased,and the electrochemical reaction accelerated.However,when the potential reached a certain level,the consumed reactants could not be replenished quickly enough by diffusion.This limited the reaction,and current peak P1 appeared.Thus,the reaction-rate determining step became the diffusion of mineral particles.In section B,a current peak (P2) appeared with an increase in the potential.This peak was due to oxidation of trivalent antimony that accumulated near the electrode surface,(Eq.(3)).The oxidation current was superimposed on Eq.(2).When the potential in section B reached point P2,the current reached its peak value.Afterwards,following the consumption of the accumulated Sb(III) near the electrode,and the limitation of the mineral particles,the current in section C gradually decreased.Section D showed the process of continuous oxidation of stibnite to produce Sb(III) and of Sb(III) to simultaneously produce Sb(V).In this section,the rate of collision contact of mineral particles with the electrode was the reaction-rate determining step.Section E was the high-potential interval.While the reactions depicted in Eqs.(2)and (3) continued,the main electrode reaction in this interval was the evolution of chlorine.As the potential increased,the current density significantly increased.
During the SE process,a large amount of Sb(V)in the electrolyte will increase the electrodeposition energy consumption.In addition,it is also likely to form pentavalent antimony oxide precipitation,thereby reducing the antimony leaching rate [8].Therefore,the electrolysis of the stibnite slurry should be controlled in the low-potential interval;that is,the potential should not exceed 0.75 V to avoid the formation of Sb(V).

3.2.Influence of particle size on the polarization curve of stibnite
The effect of particle size on the polarization curve of stibnite is shown in Fig.5.The stibnite minerals were crushed,ground,and screened to obtain samples of a specific particle size.The current densities of the polarization curve with a particle size of 0.096–0.120 mm were higher than when the particle size was 0.150–0.212 mm.This trend became more obvious when the potential was higher than 0.7 V.However,when the particle size was further reduced to less than 0.074 mm,the polarization current density showed a slight decrease.This indicates that within a certain particle size range,reducing the particle size can increase the polarization current density.However,when the particle size is further reduced,this trend is no longer valid,and even the opposite effect can be observed.When experimenting on minerals with small particle sizes,an adhesion layer of extremely fine minerals on the surface of the electrode can be found (Fig.6).This might adversely affect the contact oxidation of the suspended minerals in the solution with the electrode.
3.3.Influence of the liquid–solid ratio on the stibnite polarization curve
The effect of the liquid–solid (L/S) ratio on the polarization curve of stibnite is shown in Fig.7(a).When the potential was less than 0.75 V (the low-potential interval),there was no clear influence of the liquid–solid ratio on the current density of the polarization curve.When the potential exceeded 0.75 V,a peak in the polarization current density appeared when the liquid–solid ratio was 10:1,as shown in Fig.7 (b).When the liquid–solid ratio decreased from 25:1 to 10:1,the current density increased.It is theorized that as the liquid–solid ratio decreased,the solid content in the solution became higher.This enhanced the contact collision between the mineral particles and the anode,leading to an increase in the polarization current density.When the liquid–solid ratio was further reduced to 5.0:1,the polarization current decreased instead.As the liquid–solid ratio was too small,the slurry contained more minerals,and it was more likely to deposit an adhesion layer of extremely fine minerals on the electrode surface,as described in Section 3.2.This can inhibit the contact oxidation of the minerals with the anode and reduce the polarization current.
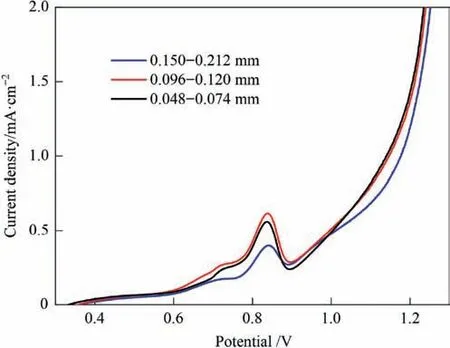
Fig.5.Effect of particle size on polarization curve of stibnite (50 °C,L/S=10,agitation speed of 500 r·min-1).

Fig.6.Optical photograph of adhesion layer.
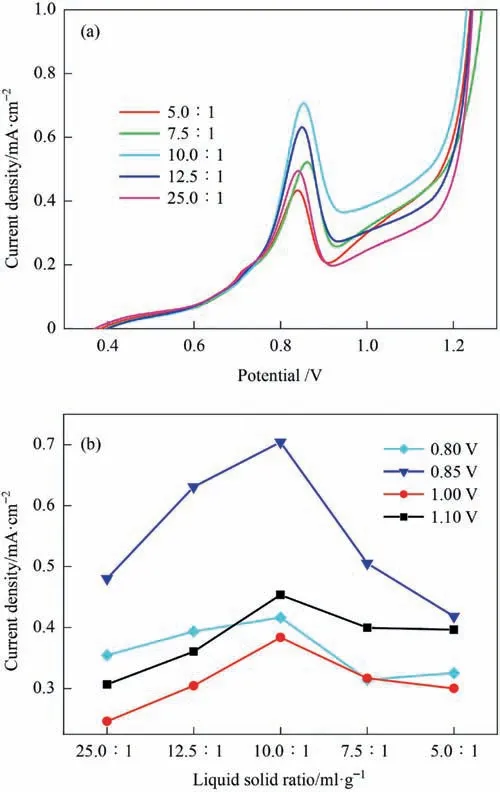
Fig.7.Effect of liquid–solid ratio on polarization curve of stibnite (50 °C,agitation speed of 500 r·min-1).
3.4.Influence of stirring speed on the polarization curve of stibnite
The effect of stirring speed on the polarization curve of stibnite is shown in Fig.8.When the potential was lower than 0.75 V (the low-potential interval),the electrode reaction rate was extremely slow,and the reaction rate-determining step was associated with the electrode kinetics.In this section,only reaction (2) took place,and the stirring speed did not show a clear influence on the polarization current.
When the potential was larger than 0.75 V (the intermediatepotential interval),besides reaction (2),the oxidation reaction (3)of Sb(III) also occurred.When the potential was around 0.85 V,due to the concentrated oxidation reaction of the previously accumulated Sb(III) over a short period,the positive influence of the stirring speed on the current appeared to be more obvious.As the accumulated Sb(III)was consumed,the influence of the stirring speed change on the polarization current tended to flatten out.
At the current peak P1,the rate of collision contact of mineral particles with the electrode was the reaction-rate determining step.Consequently,in sections B,C,and D,the higher stirring speed causes more mineral particles to collide with the anode,and the current density of the polarization curve showed a slight increase with an increase in stirring speed.
Mineral particles need enough kinetic energy to cross the liquid boundary layer and collide with the electrode.Within the investigated range,the stirring speed did not significantly improve the reaction speed,indicating that mechanical stirring provided limited kinetic energy for mineral particles.It is difficult to pass the relatively large mineral particles through the liquid boundary layer on the electrode surface to achieve contact with the electrode.If the stirring speed is extremely high,a vortex can form between the electrodes and a large number of bubbles can enter the solution.This can hinder the contact between the mineral particles and the electrode [28].
To further confirm that within a certain potential range the collision between mineral and anode will be a reaction-rate determining step,ultrasound was applied to the study system.The results from this are shown in Fig.9.When in sections A and B,ultrasound had little effect on the current density,but starting from P2,ultrasound noticeably affected the current density.Compared with mechanical stirring,ultrasound can provide mineral particles with more kinetic energy.This increases the probability of collisions with the anode,and causes the current density of the polarization curve to increase significantly.
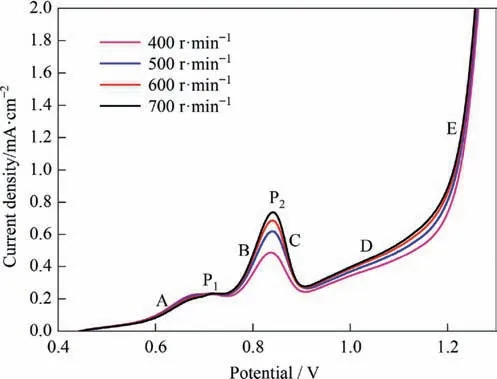
Fig.8.Effect of stirring speed on the polarization curve of stibnite(50°C,L/S=10).
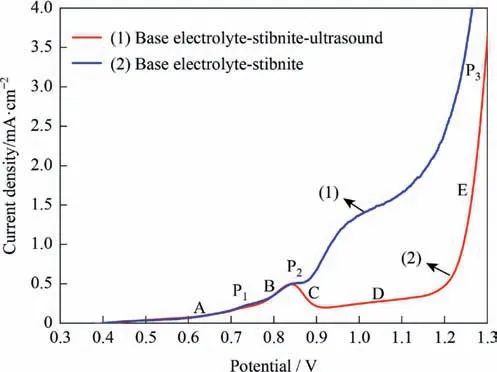
Fig.9.Effect of ultrasound on polarization curve of stibnite (50 °C,L/S=10,agitation speed of 500 r·min-1).
3.5.Influence of temperature on the polarization curve of stibnite
The effect of temperature on the steady-state polarization curve of stibnite is shown in Fig.10.The polarization current density increased with an increase in temperature,and the steady-state polarization curve and oxidation peaks P1 and P2 all moved toward the negative direction.Although the increase in temperature helps reduce the stibnite oxidation potential,these results indicate that it also helps accelerate the dissolution rate.With a potential of 0.70 V,the plot of the current density at different temperatures,lgJ -1/T,is shown in Fig.11 and the linear fitting equation is as follows.

The apparent activation energy is 16.85 kJ·mol-1according to

where J is the current density at the potential,mA·cm-2;A is a constant;E is the apparent activation energy,J·mol-1;T is the temperature,K;and R is the gas constant,8.314 J·mol-1·K-1.
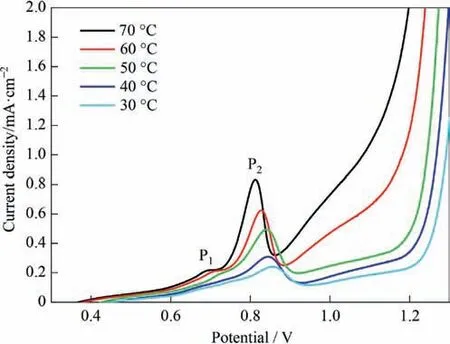
Fig.10.Effect of temperature on the steady-state polarization curves of stibnite(L/S=10,agitation speed of 500 r·min-1).
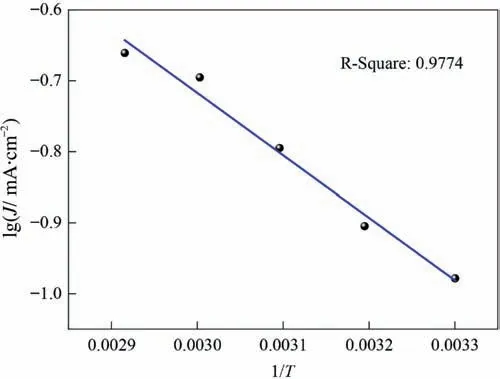
Fig.11.lgJ vs.1/T curve at a potential of 0.70 V.
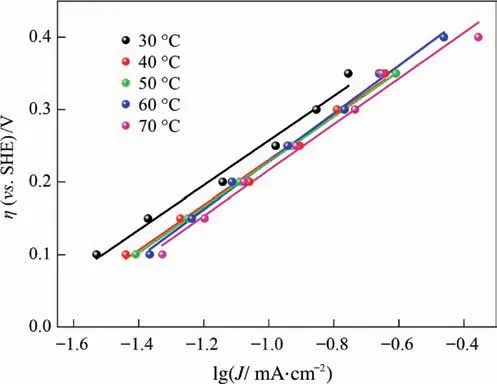
Fig.12.η-lgJ curve at different temperatures.
The apparent activation energy is relatively low,and it is speculated that the controlled process consists of diffusion of the ores[29,30].In other words,the collision between the mineral particles and the electrode surface is the step in the reaction that controls the speed.
Fig.10 shows the steady-state polarization curves of the stibnite electrooxidation decomposition.Data with potential from 0.40 to 0.70 V were chosen to calculate the overpotential and then fit the Tafel equation,and the current densities J under the same overpotential were used to plot the η-lgJ relationship diagram,as shown in Fig.12.
The Tafel equations obtained by linear fitting(shown in Fig.12)are as follows.

where βn is the apparent transfer coefficient;F is Faraday’s constant(96485.3 C·mol-1);T is the temperature;η is the anode overpotential;J0is the exchange current density;and J is the anodic current density.
The value of βn can be obtained from the linear fitting slope,and J0can be obtained from the interception of the linear fitting and the value of βn.The calculated values for βn and J0at different temperatures are listed in Table 1.

Table 1 Values of J0 and βn at different temperatures
As shown by the data,even though the exchange current density increases with an increase in temperature,it can only reach 2.057 × 10-2mA·cm-2even at 70 °C,which is a very small value.At the same time,the apparent transfer coefficient βn is also small.This indicates that the direct collision oxidation reaction of stibnite on the anode is a very slow reaction with an extremely weak reversibility.The above-mentioned experimental results also showed that in the low-potential interval (potential lower than 0.75 V),the current density was lower than 0.4 mA·cm-2,which is a very slow reaction rate.Therefore,it is difficult to meet the requirements for industrial production by relying solely on the collision reaction of the stibnite particles with the anode.Therefore,the addition of redox couples,such as Fe(III)/Fe(II),into the reaction system—which may improve the reaction speed of the mineral in the anode region—will be studied in future research.
4.Conclusions
This study examined the anodic behavior of natural stibnite in chloride solutions.The most important conclusions from this study are as follows:
(1) According to the reactions,the investigated potential range can be divided into three intervals:a low-potential interval(<0.75 V),in which the main reaction is the collision oxidation dissolution of stibnite with the electrode;an intermediate-potential interval (0.75–1.2 V),in which the main reactions are the collision oxidation dissolution of stibnite with the electrode and the oxidation reaction of Sb(III);and a high-potential interval (>1.2 V),in which a large amount of chlorine evolution occurs in addition to the above-mentioned reactions.
(2) In the low-potential interval,stirring speed and L/S ratio had almost no effect on the current density,as seen in the polarization curve.However,the current density increased withincreasing temperature.In the intermediate-potential interval,stirring speed and temperature helped increase the current density,while particle size had little influence.An L/S ratio of 10:1(ml/g)had the maximum effect on current density;increasing or decreasing the L/S ratio above or below 10 decreased the current density.
(3) The low-potential interval was suitable for the stibnite SE process.However,in this interval,the direct collision oxidation rate of stibnite was extremely slow,and the current densities under all the investigated conditions were lower than 0.4 mA·cm-2.Adding a redox couple to the solution,such as Fe(III)/Fe(II),was considered as a possible means to increase the electron transfer rate.
The results of this study could serve as a guide to further understand the mineral reaction process and optimize control conditions for slurry electrolysis.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors gratefully acknowledge BGRIMM MTC Technology Co.,Ltd.for their assistance with the chemical composition analysis.This work was supported by the National Key Research &Development Program of China (2019YFC1908404),the Major Science and Technology Projects of Qinghai Province (2018-GXA7),and the National Natural Science Foundation of China(51604030).
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Struggling as in past, write a glorious future together-CJChE’s 40th anniversary
- Reduced graphene oxide modified melamine sponges filling withparaffin for efficient solar-thermal conversion and heat management
- Photocatalytic degradation of tetracycline hydrochloride with visible light-responsive bismuth tungstate/conjugated microporous polymer
- Ag nanoparticles anchored on MIL-100/nickel foam nanosheets as an electrocatalyst for efficient oxygen evolution reaction performance
- Performance improvement of ultra-low Pt proton exchange membrane fuel cell by catalyst layer structure optimization
- Catalytic mechanism of manganese ions and visible light on chalcopyrite bioleaching in the presence of Acidithiobacillus ferrooxidans
