Catalytic mechanism of manganese ions and visible light on chalcopyrite bioleaching in the presence of Acidithiobacillus ferrooxidans
2022-03-01ChunxiaoZhaoBaojunYangRuiLiaoMaoxinHongShichaoYuJunWangGuanzhouQiu
Chunxiao Zhao,Baojun Yang,Rui Liao,Maoxin Hong,Shichao Yu,Jun Wang,Guanzhou Qiu
Key Laboratory of Biohydrometallurgy of Ministry of Education,School of Minerals Processing &Bioengineering,Central South University,Changsha 410083,China
Keywords:Acidithiobacillus ferrooxidans Mn2+ Electrochemistry Catalysis Dissolution
ABSTRACT The bioleaching of chalcopyrite is low cost and environmentally friendly,but the leaching rate is low.To explore the mechanism of chalcopyrite bioleaching and improve its leaching rate,the effect and mechanism of manganese ions (Mn2+) and visible light on chalcopyrite mediated by Acidithiobacillus ferrooxidans (A.ferrooxidans) were discussed.Bioleaching experiments showed that when both Mn2+ and visible light were present,the copper extraction was 14.38%higher than that of the control system(without Mn2+ and visible light).Moreover,visible light and Mn2+ promoted the growth of A.ferrooxidans.Scanning electron microscopy (SEM) and energy dispersive spectrometer (EDS) analysis revealed that Mn2+ promoted the formation of extracellular polymeric substance (EPS) on the surface of chalcopyrite,changed the morphology of A.ferrooxidans,enhanced the adsorption of bacteria on chalcopyrite surface with light illumination,and thus promoted the bioleaching of chalcopyrite.UV–vis absorbance spectra indicated that Mn2+ promoted the response of chalcopyrite to visible light and enhanced the catalytic effect of visible light on chalcopyrite bioleaching.Based on X-ray photoelectron spectroscopy (XPS),the relevant sulfur speciation of chalcopyrite before and after bioleaching were analyzed and the results revealed that visible light and Mn2+promoted chalcopyrite bioleaching by reducing the formation of passivation layer Investigation into electrochemical results further indicated that Mn2+ and visible light improved the electrochemical activity of chalcopyrite,thus increasing the bioleaching rate.
1.Introduction
Chalcopyrite is the main raw material for extracting copper[1].The traditional method of extracting copper from chalcopyrite is pyrometallurgy.However,most pyrometallurgical processes release SO2and pollute the air[2].Bioleaching is a kind of mineral hydrometallurgical process.It has the characteristics of simple operation,low cost and environment friendly[3],and can be used to treat low-grade minerals effectively[4].However,the bioleaching rate of chalcopyrite is low [5],which hinders the widespread commercial application of its bioleaching process.Therefore,identifying the bioleaching mechanism of chalcopyrite is of great significance for promoting chalcopyrite bioleaching.
The bioleaching of chalcopyrite is a complex process,which is affected by the microbial community structure [6],other minerals[7],metal ions and other factors [8,9].In addition,the semiconducting properties of chalcopyrite also affect its bioleaching.It was reported that chalcopyrite is a semiconductor mineral with a band gap of 0.6 eV[10].Visible light can stimulate the photochemical reactions of semiconductor minerals and promote the growth of leaching bacteria [11].Crundwell used rotating disc technology to study the effect of visible light on the anodic dissolution of natural chalcopyrite samples in 0.3 mol·L-1sulfuric acid,and found that chalcopyrite can respond to light [10].Subsequently,Crundwell discovered that the dissolution of chalcopyrite was enhanced in the presence of light[12].Zhou et al.found that visible light illumination on chalcopyrite accelerated the cycle of Fe3+/Fe2+and promoted the release of copper [13].
Additionally,manganese has important industrial applications in steel,glass,and battery industries.The extensive industrial activities have resulted in considerable manganese-containing waste [14].Lu et al.found that a very thin manganese coating(mainly MnO2) is widely present on the surface of natural rocks[15].The manganese coating can collect solar energy and convert it into chemical energy to drive photochemical reactions of chalcopyrite.Layered manganese oxides have been reported as effective catalysts in photochemical systems [16].If this manganesecontaining waste can be effectively used,it can not only reduce environmental pollution,but also produce huge economic benefits.
Therefore,the main purposes of this study were to:(1) investigate the effects of visible light and manganese ions (Mn2+) on the bioleaching of chalcopyrite;and(2)reveal the catalytic mechanism of visible light and Mn2+on chalcopyrite bioleaching.The surface morphology and characteristics of chalcopyrite were analyzed by scanning electron microscope (SEM),ultraviolet–visible spectrophotometer,X-ray photoelectron spectroscopy (XPS) and electrochemical methods (cyclic voltammetry (CV)and electrochemical impedance spectroscopy (EIS)) to better understand the bioleaching mechanism of chalcopyrite.
2.Materials and Methods
2.1.Strains and culture conditions
Acidithiobacillus ferrooxidans (A.ferrooxidans,ATCC 23270)strain used in the bioleaching experiments was provided by the Key Lab of Biohydrometallurgy,Ministry of Education,Central South University,China.The strain was cultivated at 30 °C and 170 r·min-1in sterile 9 K medium comprising of 3 g·L-1(NH4)2SO4,0.1 g·L-1KCl,0.5 g·L-1K2HPO4·3H2O,0.5 g·L-1MgSO4·7H2O,0.01 g·L-1Ca(NO3)2and 44.7 g·L-1FeSO4·7H2O [17].The initial pH value was adjusted to approximately 2.0 with H2SO4(0.01 mol·L-1).The bacteria were harvested at the midexponential growth phase after being triple subcultured.The cell culture was filtered through the Whatman 42 filter paper to remove the precipitate,then the cell suspension was transferred to sterile centrifuge bottles (200 mL) and centrifuged at 10,000 r·min-1and 25 °C for 15 min to obtain cell pellets [18].The collected cell pellets were washed several times with dilute H2SO4(pH=2.0) to obtain pure bacteria and the pure bacteria were put in 0 K medium (iron-free 9 K medium) for subsequent experiments.
2.2.Preparation of mineral sample
Chalcopyrite samples were purchased from Daye,Hubei Province,China.The original chalcopyrite sample was ground and sieved through sieves to obtain fine particles (74 μm or less) and then stored in a nitrogen atmosphere [19].The content of copper in chalcopyrite is 32.49% determined by chemical analysis (iodometric titration) [20].The synchrotron radiation X-ray diffraction(SR-XRD)results(Fig.1)revealed that the chalcopyrite used in this study was relatively pure.The X-ray fluorescence spectral analysis(XRF)showed that the original chalcopyrite sample was composed of (mass fraction,%):Cu,33.21;S,31.59;Fe,28.21;O,4.88 and other elements,2.11.
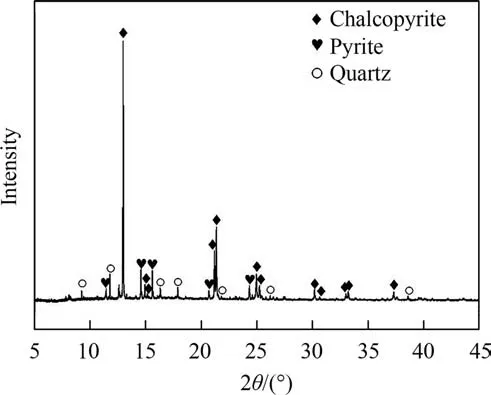
Fig.1.SR-XRD analysis of the original chalcopyrite sample(SSRF-BL14B1,Shanghai,particle size was 74 μm or less).
2.3.Leaching experiments
The leaching experiments were divided into two systems:bioleaching system and chemical leaching system.All experiments were carried out in 250 mL Erlenmeyer flasks containing 100 mL sterile 0 K medium and 2 g chalcopyrite.The initial pH of 0 K medium was controlled at 2.0[4].Each Erlenmeyer flask was placed in a constant temperature (30 °C) illumination incubator at 170 r·min-1,and the visible light intensity was set to 8500 lux (light system) or 0 lux (dark system).In the bioleaching system,A.ferrooxidans was added to each Erlenmeyer flask and the inoculum amount of bacteria was 2.0 × 107cells·mL-1.Then,different concentrations (0,50,100,150 and 200 mg·L-1) of Mn2+(manganese sulphate) were added.For comparison,the chemical leaching system was carried out with adding 0 or 100 mg·L-1Mn2+and 0.1 mol·L-1Fe3+(iron sulphate).Each experiment was repeated thrice to ensure reliability [13].Deionized water was added regularly to the Erlenmeyer flask to compensate for evaporation loss,and the sampling loss was compensated by adding sterile 0 K medium.
2.4.Analytical techniques
2.4.1.Leaching parameters
During the process of chalcopyrite leaching,200 μl supernatants were taken every three days to determine the concentration of A.ferrooxidans and metal ions (Cu2+,Fe2+and total iron).The concentration of free cells was measured with the optical microscope (CX31,400 × magnification) [8].The Cu2+concentration was determined by bis-(cyclohexanone)oxalyldihydrazone spectrophotometry,and the concentrations of Fe2+and total iron were determined by o-phenanthroline spectrophotometry [21].In addition,the pH value and redox potential(vs.Ag/AgCl)were measured by a pH meter(PHS-3C)and an ORP meter(BPP-922)[22].All the chemicals used in this experiment were of analytical grade.
2.4.2.Analysis of mineral surface morphology
After bioleaching,the residues were filtered through Whatman 42 filter paper,washed with deionized water,and then dried and stored in a vacuum drying oven(DZF-6050)for the following analysis.The surface morphologies of chalcopyrite before and after bioleaching and the feature of bacteria on the mineral surface were analyzed by SEM (NovaTMNanoSEM 230,FEI,USA) [21],and the UV–Vis absorbance characteristics of the samples were observed by UV-3600 spectrophotometer [23].
2.4.3.Chemical species of chalcopyrite surface
The chemical species of S on the surface of chalcopyrite residues were examined by XPS,which was performed with a Thermo Fisher X-ray photoelectron spectrometer (ESCALAB 250Xi).XPS spectra were recorded at the constant pass energy of 20 eV(0.1 eV/step) using the Al Kα X-ray source [24].For calibration,the binding energy of C1s peak was set at 284.6 eV[25].Considering the splitting of the spin–orbit,the S 2p peaks were divided into two peaks(S 2p3/2and S 2p1/2).The S 2p3/2peak has twice the area of the corresponding S 2p1/2peak,and the splitting energy of spin–orbit doublets was 1.19 eV.The background of the spectrum was based on the Shirley method and fitted with the Gauss-Lorentz line[26].
2.5.Electrochemical experiments
Electrochemical analysis was carried out in a three-electrode electrolyzer.The working electrode was a glassy carbon disk (φ=3 mm),the counter electrode was a platinum electrode,and the reference electrode was an Ag/AgCl(3.0 mol·L-1KCl)electrode[27].The preparation method of the working electrode was slurry coating technique:5 mg chalcopyrite residues,400 μL anhydrous ethanol and 100 μL Nafion solution (5%) were mixed,and then 10 μL mixed solution was deposited on the polished glassy carbon electrode for natural drying.The electrolyte was sterile 0 K medium(pH=2.0).After being bubbled 20 minutes of ultrapure nitrogen,the electrochemical experiments were carried out [9].Open circuit potential(OCP)was measured until the value was less than 5 mV in 60 s.After the steady OCP was observed,EIS experiments were performed with an AC perturbation of ± 5 mV in the frequency range of 0.01-100000 Hz [28].The EIS data were displayed as Nyquist plots and fitted by ZSimpWin software [9].
3.Results and Discussion
3.1.Chalcopyrite bioleaching behavior
Fig.2 shows the changes of copper concentration (a),total iron concentration (b),ferrous iron concentration (c),A.ferrooxidans concentration(d),pH value(e)and redox potential value(f)during chalcopyrite bioleaching in the presence of A.ferrooxidans.Before that,the effect of Mn2+concentrations on chalcopyrite bioleaching was explored.The dissolved copper concentrations were(2.48±0.05)g·L-1,(2.55±0.03)g·L-1,(2.62±0.00)g·L-1,(2.56±0.02)g·L-1and (2.52 ± 0.02) g·L-1when the Mn2+concentrations were 0 mg·L-1,50 mg·L-1,100 mg·L-1,150 mg·L-1and 200 mg·L-1,respectively,after 28 days of bioleaching with visible light illumination.Based on this result,it could be found that the catalytic effect was the best when the concentration of Mn2+was 100 mg·L-1.Accordingly,focus was put on the effect of 100 mg·L-1Mn2+on chalcopyrite bioleaching.
The copper concentrations in all experiments increased slowly in the first 4 days mainly because of the low concentration of A.ferrooxidans,while increased rapidly between the 4th and 8th day(Fig.2(a)).The rapid increase of copper concentration was mainly due to the rapid reproduction of bacteria after adapting to the leaching system (Fig.2(d)).Subsequently,the bioleaching rate became very slow after 8 days of bioleaching,which was primarily due to the formation of the passivation layer.After bioleaching for 28 days,when the Mn2+concentrations were 0 and 100 mg·L-1with light illumination,the concentrations of copper were (2.48 ±0.05) g·L-1and (2.56 ± 0.02) g·L-1,respectively.For comparison,the copper concentrations of the dark bioleaching systems with 0 and 100 mg·L-1Mn2+were (2.29 ± 0.02) g·L-1and (2.33 ± 0.04)g·L-1,respectively.Therefore,in the system with visible light illumination,the copper concentration was 8.51% higher than that without light and Mn2+.Moreover,when 100 mg·L-1Mn2+and visible light were both present,the copper extraction increased by 14.38%,which was the highest.These results revealed that visible light promoted the bioleaching of chalcopyrite,and Mn2+strengthened the promotion effect of visible light on chalcopyrite bioleaching.In addition,under dark conditions,the effect of Mn2+on the bioleaching of chalcopyrite was very weak.
As shown in Fig.2(b),the concentration of total iron gradually increased in the first 12 days,and then showed a downward trend.The subsequent decrease in total iron concentration was primarily due to the consumption of iron ions to form jarosite (Eq.(5)) [29].The total iron concentration was the highest in the system with Mn2+and light illumination,which was mainly on account of the dissolution of chalcopyrite (Eqs.(1)-(2)) [24].Due to the rapid growth of bacteria (Fig.2(d)) promoted the conversion of Fe2+to Fe3+(Eq.(3)),the concentration of Fe2+decreased rapidly on the 4th day and remained at a low level(Fig.2(c)).Therefore,the concentration of Fe3+in the system with Mn2+and light was the highest.Fe3+is not only the important oxidant to promote the dissolution of chalcopyrite (Eq.(1)) [30],but also can significantly accelerate the electron-transfer rate [31].In addition,the concentration of A.ferrooxidans was also the highest in the system with Mn2+and light(Fig.2(d)),which was mainly due to the ferrous ions produced by the bioleaching of chalcopyrite promoted the growth of bacteria [32].Therefore,visible light and Mn2+promoted the growth of A.ferrooxidans,accelerated the conversion between Fe2+and Fe3+,and improved the copper extraction.

where M may be K+,H3O+or Na+.
The bioleaching of chalcopyrite consumed H+,causing the pH to rise at the beginning(Fig.2(e)).And the pH value began to drop on the 4th day,which was because A.ferrooxidans multiplied (Fig.2(d))after adapting to the environment and oxidized element sulfur to produce acid (Eq.(4)).Moreover,the formation of jarosite also produced acid (Eq.(5)) [33].Finally,the pH values in the system with light dropped to about 1.9,while it dropped to about 2.0 in the system without light.Therefore,light illumination promoted the production of acids.
Fig.2(f) presents the changes of redox potential during the bioleaching experiments.Regardless of the presence or absence of Mn2+,the redox potential with visible light was higher than that without light.The higher redox potential was due to the visible light promoted the circulation between Fe2+and Fe3+and the generation of Fe3+[13].Redox potential has been well reported as the determinant in chalcopyrite bioleaching.Chalcopyrite was easy to be passivated when the redox potential was higher than 480 mV(vs.Ag/AgCl) [34–36].However,in this study,the higher redox potential did not hinder the bioleaching of chalcopyrite with visible light,indicating that the existence of visible light changed the previous mechanism of chalcopyrite bioleaching.
According to the above analysis results,it could be found that visible light and Mn2+promoted the bioleaching of chalcopyrite,the growth of A.ferrooxidans and the production of H+,Cu2+and Fe3+.
3.2.Surface morphology analysis
The surface morphologies of original chalcopyrite and residues were analyzed by SEM (Fig.3(a)-(e)).It could be found that after adding Mn2+,the surface of the residues showed some layered substances (Fig.3(c)-(d)).EDS analysis showed that the layered substances contained oxygen and carbon (Fig.3(f)),in which the presence of C was assigned to microorganisms [37].It is reported that the dominant component of extracellular polymers (EPS) is carbohydrates (sugars),and its main elements are C,H and O[38].Among them,hydrogen cannot be detected by EDS.Therefore,the layered substance was mainly EPS.EPS contribute to the attachment of bacteria on the mineral surface and provide a microenvironment that can produce favorable reaction conditions[39].Moreover,EPS can enrich Fe3+to form a high oxidation active area near the mineral surface,thus playing an active role in the dissolution of chalcopyrite [40].In addition,Lu et al.[15] proposed that the widely distributed rocks in nature had thin coatings of Mn oxides,which could harvest light energy.Layered manganese oxide was an effective catalyst in the visible light system,which could collect solar energy and convert it into chemical energy[16],thus driving the chemical reactions in the bioleaching process of chalcopyrite.Therefore,it was speculated that Mn2+could promote the response of chalcopyrite to light.Thus,the UV–vis absorbance spectra of chalcopyrite treated with Mn2+(0 mg·L-1or 100 mg·L-1) and visible light illumination were investigated(Fig.3(i)).The results showed that in the presence of visible light,Mn2+promoted the absorption of visible light by chalcopyrite,especially in the wavelength range of 400–800 nm and thus strengthened the promotion effect of visible light on chalcopyrite bioleaching.

Fig.2.Changes in (a) copper concentration,(b) total iron concentration,(c) ferrous iron concentration,(d) growth of A.ferrooxidans,(e) pH and (f) redox potential during chalcopyrite bioleaching in the presence of A.ferrooxidans (Initial pH=2.0,chalcopyrite=20 g·L-1,temperature=30 ℃,rotation speed=170 r·min-1,light intensity=0 or 8500 lux,inoculum volume=2.0 × 107 cells/ml,[Mn2+]=0 or 100 mg·L-1).
Fig.3(e) and Fig.3(f) show the morphologies of A.ferrooxidans adsorbed on the surface of chalcopyrite treated without Mn2+and with Mn2+under visible light,respectively.The results showed that the morphologies of A.ferrooxidans changed (from 2 μm in length and 0.5 μm in width to 1.2 μm in length and 0.7 μm in width) when Mn2+was added,which was probably because Mn2+adsorbed on the surface of the bacteria.Zhang et al.proposed that Mn2+could be absorbed on the cell surface with negative charges to change the morphologies of cells,increase the permeability of cell membrane and make the easier absorbability of nutrition [2].With visible light,the chalcopyrite dissolution accelerated and produced more nutrients for bacteria.Therefore,Mn2+promoted the growth of A.ferrooxidans with light illumination (Fig.2(d)).Meanwhile,more A.ferrooxidans attached to the surface of chalcopyrite in the presence of visible light and Mn2+(Fig.3(f)),which provided reaction space for the dissolution of chalcopyrite[41]and thus promoted chalcopyrite bioleaching.
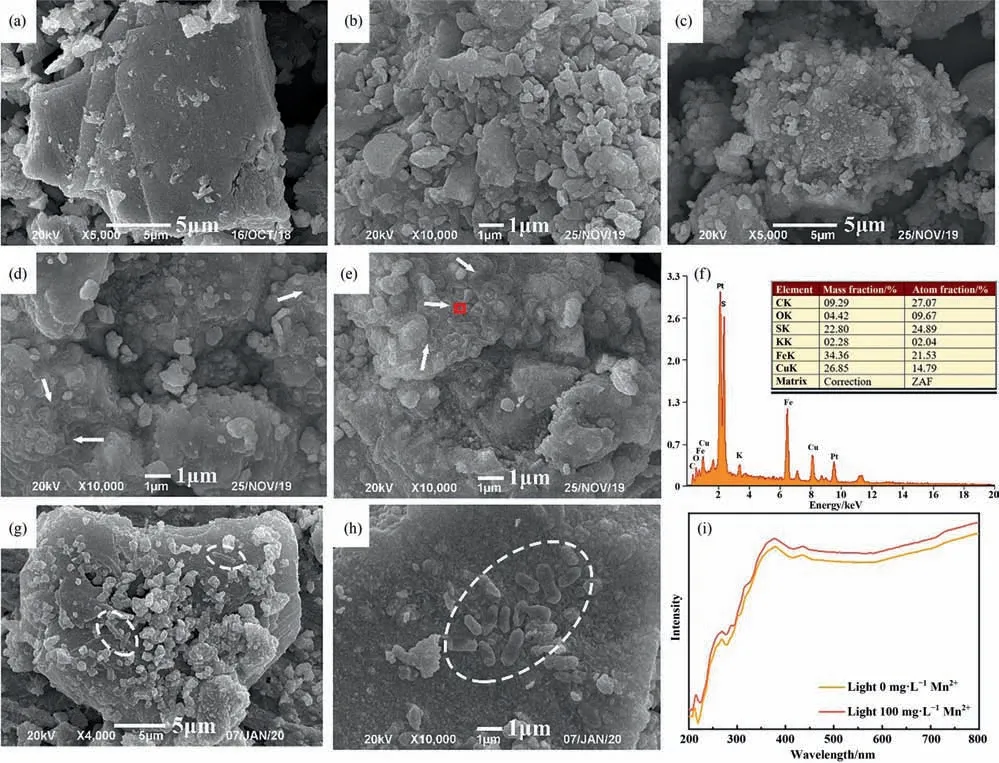
Fig.3.SEM images of the original chalcopyrite sample(a)and residues treated without Mn2+under dark(b)and light(c)conditions;residues treated with Mn2+under dark(d)and light(e)conditions;EDS spectra of the residue treated with Mn2+and light illumination(f);SEM images of A.ferrooxidans on the surface of residues without Mn2+(g)and with Mn2+(h)under visible light;UV–vis absorbance spectra of chalcopyrite residues treated with and without Mn2+under light illumination(Leaching time=28 d,light intensity=0 or 8500 lux,[Mn2+]=0 or 100 mg·L-1).
3.3.Surface species of bioleached residues
XPS is a very sensitive surface analysis method,which can be used to determine the chemical state of chalcopyrite surface [42].Therefore,the S 2p peaks of original chalcopyrite and residues were explored by XPS (Fig.4).By fitting the S 2p peaks and comparing with references,four types of S coordination and oxidation states were determined:(i)monosulfide(S2-,161.1–161.8 eV),(ii)disulfide (162.5 eV),(iii) polysulfide and elemental sulfur163.0–164.7 eV),(iv) sulfate (jarosite,,168.0–169.0 eV)[8,43,44].The relative contents of different sulfur species were obtained by assuming that the percentage of S2-,andwas proportional to the area under its S 2p peak(Fig.4(f))[45].The results showed that the sulfur on the surface of original chalcopyrite was dominated by S2-,which was consistent with the conclusions of other studies [42,46].After 28 days of bioleaching,the sulfur species on chalcopyrite surface were mainly S2-,and.Although the specific composition of the passivation material formed during chalcopyrite bioleaching is still controversial,it is generally believed that the main components of the passivation layer areand(jarosite) [34,47].In recent years,some studies have shown that jarosite would not inhibit chalcopyrite bioleaching because it is easy to peel off from the chalcopyrite surface,so the main components of the passivation layer areand S0[3,9].In comparison,the system with Mn2+and light remained less S2-and generated lessthan the system without Mn2+and light,which indicated that visible light and Mn2+promoted the bioleaching of chalcopyrite and reduced the formation of the passivation layer
3.4.Electrochemical analysis
Electrochemical methods are usually used to infer the differences in the intermediate reaction processes of minerals [47,48].Fig.5 shows the cyclic voltammetry spectra of chalcopyrite electrodes treated with different conditions.Two anodic peaks (A1,A2) and three cathodic peaks (C1,C2,C3) were observed,which represented different redox reactions.The anodic peak A1(around 0.2 V) was related to the process of chalcocite oxidation to other copper sulfide ores (Eq.(6)),and A2 (around 0.4 V) represented the oxidation of chalcopyrite to non-stoichiometric intermediate products (Eq.(7)).Concurrently,the cathodic peaks C1 (around 0.4 V),C2 (around 0 V) and C3 (around -0.4 V) were relevant to the reduction process of iron ion to ferrous ion,chalcopyrite to chalcocite and chalcocite to copper,respectively (Eqs.(8)-(10))[32,34,49].The current density measured by cyclic voltammetry was linked to the redox reaction intensity on the working electrode surface[9].Although the shape of the cyclic voltammetry curve of chalcopyrite treated with different conditions was similar,the current intensity was different.The current intensity of chalcopyrite treated with visible light and Mn2+was significantly higher than that without visible light and Mn2+.Therefore,visible light and Mn2+promoted chalcopyrite bioleaching by improving the electrochemical activity of chalcopyrite.
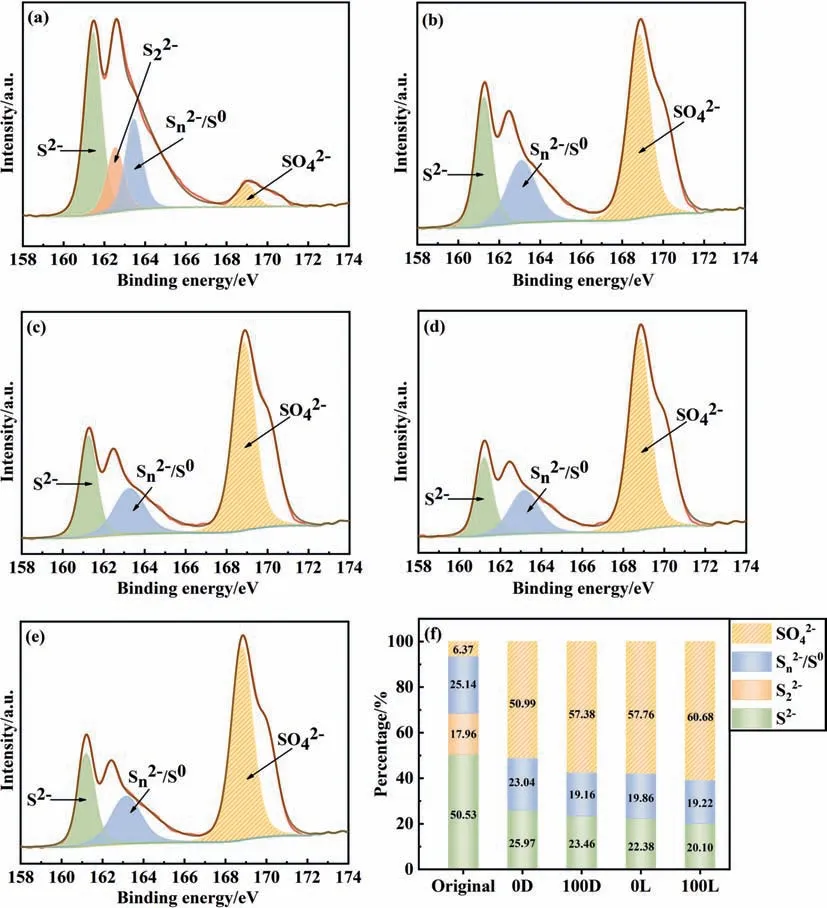
Fig.4.XPS spectra of S 2p peaks(only the fitting peaks of S 2p3/2 peaks were shown)of original chalcopyrite sample(a)and residues treated without Mn2+and light(b),with Mn2+(c),with visible light(d),with Mn2+and visible light(e);(f)contents of different sulfur species(atom%)(‘0D’means without Mn2+and light,‘100D’means with Mn2+and without light,‘0G’ means with light and without Mn2+,‘100G’ means with Mn2+ and light) (Leaching time=28 d,light intensity=0 or 8500 lux,Mn2+=0 or 100 mg·L-1).
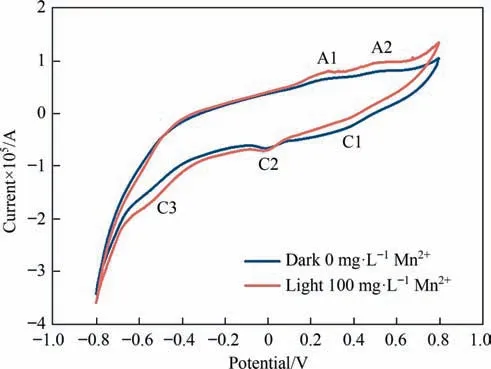
Fig.5.Cyclic voltammetry curves of chalcopyrite residues in the control group(without light and Mn2+) and in the presence of Mn2+ and visible light (Initial pH=2.0,light intensity=0 or 8500 lux,Mn2+=0 or 100 mg·L-1).
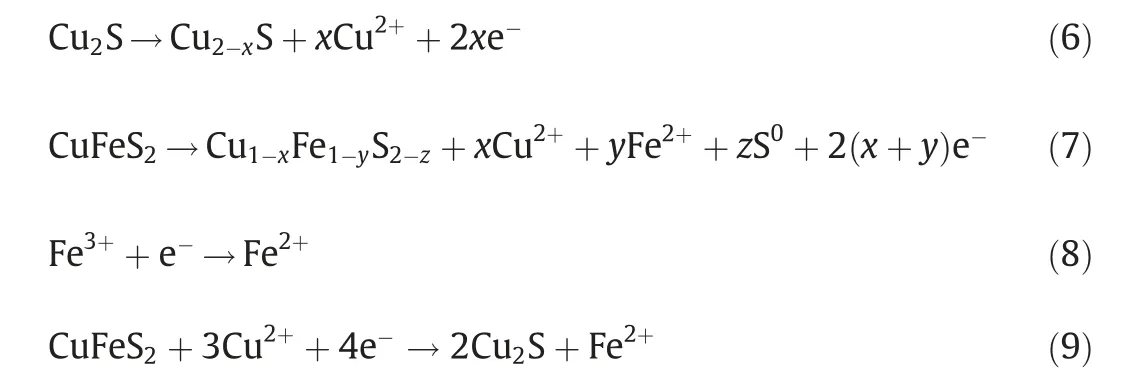
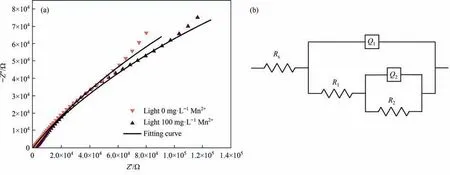
Fig.6.Nyquist diagram (a) and equivalent circuit (b) of chalcopyrite residues treated without Mn2+ and with Mn2+ under light illumination (Initial pH=2.0,light intensity=8500 lux,[Mn2+]=0 or 100 mg·L-1).
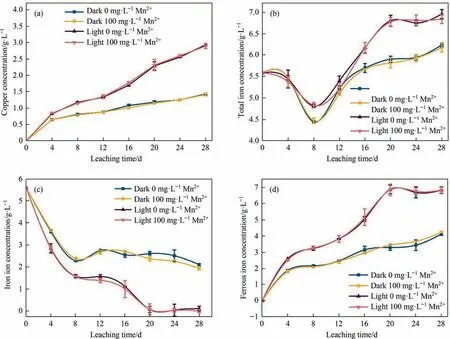
Fig.7.Changes in(a)copper concentration,(b)total iron concentration,(c)ferric iron concentration and(d)ferrous iron concentration during chalcopyrite leaching by Fe3+(Initial pH=2.0,chalcopyrite=20 g·L-1,temperature=30 ℃,rotation speed=170 r·min-1,light intensity=0 or 8500 lux,[Mn2+]=0 or 100 mg·L-1,[Fe3+]=0.1 mol·L-1).
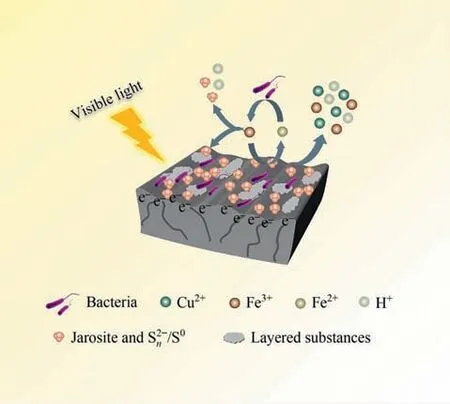
Fig.8.The mechanism of visible light and Mn2+promoting chalcopyrite bioleaching(Initial pH=2.0,chalcopyrite=20 g·L-1,temperature=30 ℃,rotation speed=170 r·min-1,light intensity=8500 lux,inoculum volume=2.0×107 cells·mL-1,[Mn2+]=100 mg·L-1).

EIS is an important method to study the interface charge transfer rate [50,51].The Nyquist plot of chalcopyrite treated with 0 mg·L-1and 100 mg·L-1Mn2+under light illumination was illustrated in Fig.6 and fitted with the equivalent circuit.The parameters fitted by EIS data were shown in Table 1,where RSwas the solution resistance;R1was the charge transfer resistance;R2was passive layer resistance;Q1represented the double-layer capacitance of the electrode–electrolyte interface and Q2stood for the passivation layer-electrolyte interface.The results indicated that the resistance of the system,especially the charge transfer resistance,was reduced by adding Mn2+under visible light.This meant that with light illumination,the addition of Mn2+accelerated the charge transfer rate and promoted the utilization of photoelectrons,thus improving the bioleaching of chalcopyrite.

Table 1 Electrochemical impedance spectroscopy parameters of chalcopyrite treated with Mn2+ (0 mg·L-1 or 100 mg·L-1) and light illumination
3.5.Effects of light illumination and Mn2+ on chalcopyrite chemical leaching
In order to clarify the direct effects of illumination and Mn2+on the iron cycle,the chemical leaching test was carried out.The results in Fig.7 revealed that the concentrations of dissolved copper,total iron and ferrous iron in the visible light system were higher than those in the dark system.The relatively higher concentration of Fe2+in the light system mainly due to the photogenerated electrons generated on the surface of chalcopyrite reducing Fe3+to Fe2+(Fig.7(c)-(d)) [13].During the chemical leaching of chalcopyrite,the addition of Mn2+had no obvious effect on the concentration of dissolved copper (Fig.7(a)).However,in the process of chalcopyrite bioleaching,Mn2+promoted the dissolution of chalcopyrite(Fig.2(a)).Therefore,it could be seen that the promotion effect of Mn2+on chalcopyrite bioleaching was mainly achieved by affecting bacteria.In addition,the redox potential mostly affected by the ratio of Fe3+/Fe2+was the decisive factor for the chalcopyrite leaching,and the relatively low redox potential was conducive to the leaching of chalcopyrite[24,34,47,52].Therefore,under light illumination,lower iron concentration (Fig.7(c))and higher ferrous iron concentration (Fig.7(d)) made the redox potential lower and promoted the leaching of chalcopyrite.
According to the above analysis results,the mechanism of visible light and Mn2+promoting chalcopyrite bioleaching was proposed (Fig.8).With visible light illumination,Mn2+increased the transfer rate of photogenerated electrons.The transfer of photogenerated electrons to chalcopyrite surface accelerated the circulation of Fe2+and Fe3+,thus improving the growth of bacteria and the adsorption of bacteria on the chalcopyrite surface.Finally,more copper ions,iron ions and acid were released,which means that the bioleaching of chalcopyrite was promoted.
4.Conclusions
Visible light and Mn2+could promote the bioleaching of chalcopyrite.When 100 mg·L-1Mn2+and visible light exist at the same time,the bioleaching rate of copper was increased by 14.38%compared with the control system.Based on the semiconductor properties of chalcopyrite,visible light facilitated the reduction of Fe3+to Fe2+,which provided more substrates for the growth of A.ferrooxidans,thus promoting the growth of bacteria.With light illumination,Mn2+not only changed the morphology of A.ferrooxidans,but also enhanced the attachment of bacteria on the chalcopyrite surface.In addition,Mn2+increased the absorption intensity of visible light by chalcopyrite,reduced the resistance of the dissolution system,and accelerated the charge transfer rate and the utilization of photoelectrons.What’s more,visible light and Mn2+enhanced the electrochemical activity of chalcopyrite.Therefore,visible light and Mn2+had a synergistic catalytic effect on the chalcopyrite bioleaching.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (51774332,51934009,U1932129),Fundamental Research Funds for the Central Universities of Central South University (2021zzts0299) and the college students innovations special project funded by Hunan province (S2021105330471).
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Struggling as in past, write a glorious future together-CJChE’s 40th anniversary
- Reduced graphene oxide modified melamine sponges filling withparaffin for efficient solar-thermal conversion and heat management
- Photocatalytic degradation of tetracycline hydrochloride with visible light-responsive bismuth tungstate/conjugated microporous polymer
- Ag nanoparticles anchored on MIL-100/nickel foam nanosheets as an electrocatalyst for efficient oxygen evolution reaction performance
- Performance improvement of ultra-low Pt proton exchange membrane fuel cell by catalyst layer structure optimization
- Anodic process of stibnite in slurry electrolysis:The direct collision oxidation
