Layered double hydroxides:Scale production and application in soil remediation as super-stable mineralizer
2022-03-01FangqiMaoPeipeiHaoYuquanZhuXiangguiKongXueDuan
Fangqi Mao,Peipei Hao,Yuquan Zhu,Xianggui Kong,Xue Duan
State Key Laboratory of Chemical Resource Engineering,Beijing University of Chemical Technology,Beijing 100029,China
Keywords:Heavy metals Pollution Remediation Layered double hydroxides Scale-up Super-stable mineralization effect
ABSTRACT Soil contamination by heavy metals has presented severe risks to human health through food chain.As one of the most promising remediation technologies,in-situ immobilization strategy has been widely adopted in practice.However,considering the large quantities of contaminated soil,it is still a huge challenge to design low-cost amendments with strong and long-term immobilization ability.Layered double hydroxides (LDHs) have drawn tremendous attention in fundamental research and practical application because of their unique properties.Moreover,owing to its super-stable mineralization effect to heavy metal ions,LDHs have exhibited great potential in the field of soil remediation.In this work,we mainly focused on the scale production strategy of LDHs with low-cost,and its application in soil remediation.Besides,several key challenges in using LDHs as amendments for immobilization of heavy metal ions are presented.We hope that this mini-review could shed light on the sustainable development of LDHs as amendment for heavy metals in future research directions.
1.Introduction
As non-renewable resource,soil plays a crucial role in sustainable development of human society though serving as medium for crop production[1-3].Unfortunately,as like water and air pollution,soil contamination has become one of the most severe problems due to the rapid growth of industrialization and urbanization,resulting in serious harm to human health and food security[4-6].Among agriculture soil pollutants,heavy metals such as cadmium,arsenic,mercury,and chromium were the major threat to human due to carcinogenicity,non-biodegradability,and bioaccumulation in food chain [7,8],which mainly come from agricultural activity,industry and mining (Fig.1).Soil pollution by heavy metals has turned into a worldwide problem.According to the Bulletin on Natural Survey of Soil Contamination in 2014,the percentage of pollutant agricultural soil is even greater than 19.4% in China,in which 82.4% of pollution was caused by heavy metals and metalloids[9].In the United States,more than 50,000 heavy metals polluted sites require urgent remediation actions base on the dates of the U.S.Environmental Protection Agency [10].Furthermore,with the growth population and improvement of living standards,the global crop production must be double to meet the demand in 2050 [11].However,despite the increasing stringency of environmental protection regulations,the quality of soil is continue exacerbating due to the increasing demand for natural resources,especially in industrialization of developing countries.Therefore,it is imperative to develop remediation technologies with effectiveness and feasibility to settle the problem of agricultural soil contamination by heavy metals.
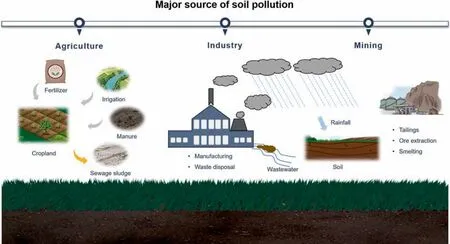
Fig.1.Schematic illustrating pollution sources of heavy metals in polluted soils.
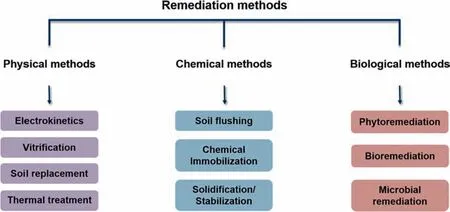
Fig.2.Remediation methods for soil contaminated by heavy metals.
It has been confirmed that the toxicity of heavy metals in agricultural soil are mainly dependent on their bioavailability,not the total amount of heavy metals[12-14].Consequently,soil remediation technologies can be classified into two categories in essence:the first category is to remove the heavy metals from the cropland(e.g.,soil washing,surface capping,electrokinetic extraction,phytoextraction);and the other category is to reduce the bioavailability and transportation of heavy metals through immobilization,vitrification,and solidification technology.All those technologies have been demonstrated specific application with advantages and limitations in field practices(Fig.2).Considering the cost,convenience,and effectiveness of those techniques,as well as the physical and chemical properties of agricultural soil,in-situ immobilization approach is being accepted in agricultural soil remediation practice with the advantages of easy-to-apply,rapid and commercial viability [15-17],which mechanism mainly rely on the reducing the bioavailability and mobility of heavy metals by the interaction between amendments and heavy metals though adsorption,complexation and precipitation.Consequently,amendments play a key role in the in-situ immobilization performance.The common amendments in research and practice were mainly containing of biochar,clay minerals,phosphates,and organic materials.However,the remediation performance of those amendments was barely satisfactory.For example,because of the poor adsorption capacity,the demand amounts of biochar and clay minerals were great and must be used every year,resulting in a high cost and imbalance of soil components[18].Compared with above contractional amendments,nanomaterials have displayed well potential to overcome those drawbacks due to the excellent surface to volume ratio,highly porous structure,desirable stability,and high surface reaction affinity[19,20].For example,the removal capacity and selectivity of sulfidated nanoscale zero-valent iron(SnZVI) to Cd can be highly improved by surface modification [21],displaying that the exchangeable Cd was decreased by over 97.6%at the dosage of 5 g·kg-1.With rational structure design,a novel dual-functional redox-responsive hydrogel was designed to capture Cu2+and Hg2+ions in soil via strong complexation between the ions and thiol groups [22],leading to a novel strategy for soil remediation.However,although those nanomaterials have shown high efficiency and extraordinary capacity for immobilization of heavy metals in soil,their practice application is limited by high cost and time-consuming operation,as well as risk assessment of nanocomposites application in soils for crop growth.Therefore,it is desirable to pay much attention to explore and design the soil amendments with cost-effective,long-term stability and high selectivity.

Fig.3.The Structure,properties and applications of LDHs.
Layered double hydroxides (LDHs) are a family of anionic clay compounds that was discovered in Sweden around 1842 as natural hydroxycarbonate [23].The first kind of synthetic LDHs called hydrotalcite (Mg6Al2(OH)16CO3·4H2O) that was fabricated by Feitknecht in 1942,and Allmann and Taylor confirmed the layered structure of LDHs by X-ray diffraction in 1960 [24].Similar to the structure of Mg(OH)2,the metal cations in LDHs located in the centers of edge-shared octahedra with six fold coordinated to hydroxyl groups.The positively charged layers of LDHs was attributed to isomorphous replacement of a fraction of divalent metal cations with trivalent metal cations.In order to balance the positive charge,various kinds of anions located into the interlayer to keep charge neutrality.Therefore,the general chemical structure formula of LDHs can be descripted asin which the M2+and M3+represent divalent and trivalent metal cations(e.g.,Mg2+,Ca2+,Ni2+,Zn2+,Al3+,Fe3+,and Ga3+),An-denotes the interlayer anion (e.g.,,Cl-,oxoanions,and organic anions),x is the molar ratio of M3+/(M2++M3+) with the value usually ranges from 0.2 to 0.33 [25-28] (Fig.3).Furthermore,the M2+can be replaced by Li+(just only find LiAl-LDH)and M3+also can be substituted by Ti4+,Zr4+,and Sn4+,respectively [29-32].In addition,LDHs containing more than two species of cations also have been synthetized [28,31].As a result,the tremendous tunability in kinds of metal cations,morphology,particle size,surface defect and the interlayer anions resulted in a versatile physical and chemical properties for LDHs,which make them deserved great attentions in the fields of catalysis,water splitting,optical materials,supercapacitors,adsorption,and drug delivery [33-38] (Fig.3).Although many reviews about LDHs have been published in recent years[27,38-41],there is no review on soil remediation applications with LDHs as soil amendments.Given that the importance of soil and the excellent performance of LDHs to immobilize cadmium (Cd) in soil remediation application in very recent date [42],a mini-review of LDHs applied in soil in-situ immobilization approach is needed and would provide a broader view for soil remediation.
This mini-review highlights the fabrication of LDHs in green technique and their new application in soil remediation.Largescale production of LDHs with green and effective approaches were introduced in the first section,which was the cornerstone of practice application.The remediation performance and mechanism of LDHs to heavy metals in soil were discussed in second section.In the final section,the opportunities and challenges in the LDHs production and soil remediation application were discussed,and some strategies were proposed at the same time.
2.Scale Production of LDHs
As functional materials,LDHs have been widely used in many fields,and lots of methods have been demonstrated to successfully fabricate of LDHs [34,37-42].Among numerous methods,coprecipitation method is the mainly technique in lab and factory due to its easy operation.However,the main drawbacks are low efficiency and uncontrollability of particle size and distribution.Duan and co-workers [43] invented a “separate nucleation and aging steps (SNAS) method” for production LDHs in large-scale,which involves an extreme rapidity mixing and nucleation process in colloid mill followed by a separate aging process.The main advantages of it as follow:1) High efficiency.The nucleation can be completely formed in a very short time;2)Narrow particle size distribution.Initial crystal nuclei were formed simultaneously in colloid mill in SNAS,and the high pressure and friction between the stator and rotor in rotating process inhibited the agglomeration of nuclei,ensuring that crystal nuclei size remains at a minimum;furthermore,all crystal nuclei aged in the same condition after nucleation,avoiding the formation of nuclei and aging take place simultaneously in traditional coprecipitation,and resulting in a well narrow particle size distribution.A pilot line for LDHs with production capacity of 1500 t·a-1has been operated in Shandong Vansinvena Material Technology Co.,Ltd based on this technique(Fig.4a and b).

Fig.4.Photos of LDHs production lines in Shandong Vansinvena Material Technology CO.,LTD.
Although the co-precipitation method has successfully used in the fabrication of LDHs,there are two significant disadvantages for this technique:Firstly,plenty of deionized water must be needed to wash the product to remove undesirable by-products such as NaCl,KCl and Na2SO4;Secondly,a large amount of acidic and basic waste water must be handled,resulting in high cost and environmental problems.
In order to reduce the production cost of LDHs,considerable research efforts have been paid in the designing of synthesis LDHs by atom economy reaction,meaning that all the raw materials changed into the final product.For instance,Roy and partners discovered that the MgAl-CO3-LDH could be fabricated by mechanical grinding method with MgO and Al2O3as raw materials [44].Although the obtained MgAl-CO3-LDH accompanied with trace of MgCO3,Mg(OH)2and Al(OH)3,which first provided a possibility that LDHs can be produced in a green technique;Inspired by above work,Lv [45] explored a new approach to synthesize MgAl-CO3-LDHs in 100% atom economy with the chemical reaction was follow:

in which all the raw materials are completely change into the final products without any by-products,exploring in a new green synthetic strategy for LDHs with atom economy of 100%.Furthermore,a pilot line with production capacity of 3000 t·a-1for LDHs has been operated in Shandong Vansinvena Material Technology CO.,LTD(Fig.4c and d).
3.Application in Soil Remediation
Although lots of soil remediation technologies have been developed to restore the function of agriculture soil,considering the properties and huge quantity of contaminated agriculture soil,insitu immobilization approach is an efficient and affordable choice for agriculture soil remediation with following advantages[10,15,16]:1)Easy-to-apply.If the amendments are water soluble,they can be sprayed in aqueous solutions with irrigation,and insoluble amendments are generally broadcast by plow incorporation;2) Relatively economical.According to reported that the total cost range of 40–65 USD·m-3for in-situ immobilization while 50–117 USD·m-3for electrokinetic remediation and 75–210 USD·t-1for soil washing;3) Rapid and effective.Owing to the amendments can quickly react with multivalent heavy metal cations to form stable speciation in natural biogeochemical environment through adsorption,chemical precipitation,surface complexation and ion exchange,minimizing the transportation of heavy metals from soil to plants.Consequently,in-situ immobilization approach has received much attention from both researchers and industry for its great promising remediation performance to heavy metals in cropland remediation.However,the heavy metals still existed in the soil by this treatment,therefore,it is critical that the immobilizing agents must have the long-term stability to immobilize the heavy metals in complicated natural conditions.
As promising environmental materials,LDHs have been extensively explored as amendments for heavy metal contaminants due to their versatility in features and structures[46].For example,heavy metal cations can be removed by LDHs in formation of precipitates based on chemical precipitation and/or surface complexation mechanism,attributing that there are abundant hydroxyl groups on the layer of LDHs;Furthermore,heavy metal oxoanions can be captured into the interlayer of LDHs through anion exchange;Moreover,owing to the intercalation property of LDHs,numerous functional LDHs have been synthesized with intercalated different anions,the heavy metal ions and oxoanions can be captured by those functional LDHs with excellent selectivity and capacity through formation of anion-metal complexes due to the strong interaction between interlayer anions and target heavy metals.
Given that the unique structure of LDHs and the excellent capture performance to heavy metals in water treatment,researchers have been focused on the LDHs as amendment for soil remediation that polluted by heavy metals in recent years.For example,in order to reduce the bioavailability of arsenite (As(III)) in soil,a ternary LDHs (CaMgFe-LDH) has been synthesized and used as the amendment for soil remediation,displaying significant removal capacity and excellent stability (removal efficiency of 47% with an initial concentration of 346 μg·L-1after 40 days)[47].The immobilization mechanism mainly attributed to the formation of As-precipitates (Ca(H2AsO3)2and CaHAsO4)though precipitation between As(III) and Ca2+that released from the LDHs matrix.Unlike the precipitation,Bagherifam founded that arsenite(As(III)) and arsenate (As(V)) mainly immobilized by the way of exchange (occurred on the surface and edges of LDHs,parts in interlayer region) with ZnAl-SO4-LDH as amendment in a simulated soil solution [48].In order to improve the immobilization performance of LDHs to heavy metals,Qiu’s group [49] obtained ultrathin nanosheets of MgAl-LDH (S-Mg-LDH) and CaAl-LDH(S-Ca-LDH) though ultrasound-assisted method in formamide solution.As soil amendments for Cr(VI),S-Mg-LDH displayed excellent performance with removal efficiency of 64.32%,while only 8.09% was obtained for the MgAl-LDH.However,there was no obvious difference in removal efficiency for S-Ca-LDH(95.69%) and CaAl-LDH (94.65%) to Cr (VI).
Considering the results of previous works,the immobilization mechanism for LDHs to heavy metals in soil was mainly depended on surface adsorption,interlayer exchange and precipitation(Table 1).However,the affinity of LDHs to heavy metals could be reduced owed to the existence of many anions in soil (e.g.,because those anions were most prone to conjugate with LDHs due to the suitable size,high charge and binding energy[56],resulting in a desorption of heavy metal ions from LDHs to soil.
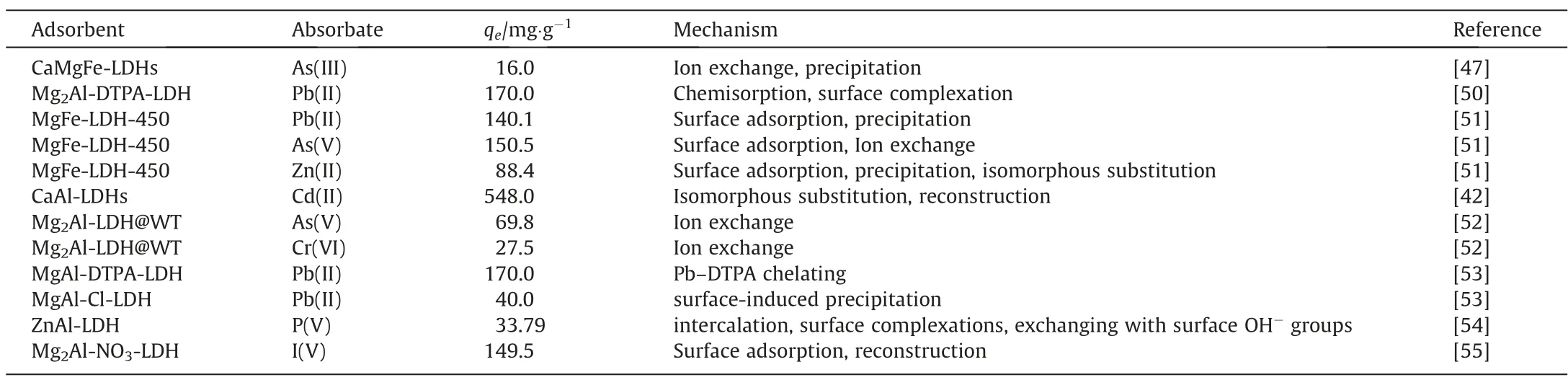
Table 1 The performance of LDHs as amendments in soil remediation
It has been confirmed that the bioavailability and mobility of heavy metal ion can be significantly reduced through anchoring it into the lattice of mineral or metal oxides by suitable approach.Inspired by the properties of LDHs with ultra-low solubility product constant(Ksp)and well buffer capacity in various pH condition[57,58].In our very recent work[42],we used CaAl-LDH as amendment to mineralize Cd2+ions in agriculture soil with the max capture capacity reach to 592 mg·g-1in a fast kinetics (less 5 min,in solution) and the immobilization efficiency up to 96.9% within 28 days in soil,much higher than that of quicklime (53.1%) and hydroxyapatite (23.8%).Furthermore,in practice application(10 ha,1 ha=104m2),the content of Cd in wheat grain obtained after remediation soil is below the standard of China in the first year,and the content of Cd continuously decreased in the followed two years,displaying well long-term stability(Fig.5a-c).Comparedwith the adsorption on the surface or in the interlayer,Cd2+was removed in the form of CdAl-LDH through isomorphic substitution of Ca2+in the layer of CaAl-LDH(Fig.5d,e),demonstrating a superstable mineralization effect in immobilization of Cd2+ions.Besides,other heavy metal ions such as Ni2+,Mn2+,and Cu2+also can be mineralized into the formation of LDHs with CaAl-LDH as mineralizer,displaying great potential as amendment in the field of heavy metals pollution.
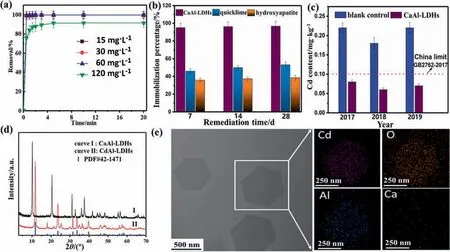
Fig.5.(a) The removal percentage of Cd2+ at different initial concentration using CaAl-LDHs as stabilizer;(b) The immobilization of Cd2+ ions in soil after remediation by CaAl-LDHs,quicklime and hydroxyapatite;(c) The content of Cd in wheat grain obtained in different years after the remediation with CaAl-LDHs as stabilizer;(d) XRD patterns of samples before and after removal of Cd2+;(e) HAADF image and the respective EDS mapping of Cd,Al,O and Ca from Ref.[42].
Despite LDHs have displayed excellent immobilization performance to heavy metals in soil remediation,the main obstacles for LDHs in practice application were the cost and the ability of scaling up production.Based on the technological innovation,several LDHs amendments have been produced in large scale through atom economical technical route,resulting in low cost and high efficiency.For instance,the CaAl-LDHs can be produced with burnt lime,calcium sulfate and aluminum hydroxide as raw materials,in which all the reactants were changed into final product without any by-products,resulting in a low cost.Moreover,scale production of CaAl-LDHs have been done through the pilot line located in Shandong Vansinvena Material Technology CO.(Fig.4),LTD.According to the field practice application (10 hectares,Jiangsu province)[42],the production cost of LDHs for each hectare of contaminated agriculture soil was estimated to be less than 4000 CNY,much more competitive than that of biochar.Furthermore,the average content of Cd in wheat grain is below the national standard with consecutive 3-years for one-time remediation,resulting in a low annual average cost,and displaying a promising competitive amendment in soil remediation market.
4.Summary and Perspective
Compared with other adsorbents,LDHs possessed various attractive properties such as large surface area,exchangeable interlayer anions,variable layer composition and structure and well buffer capacity in various pH condition.Therefore,LDHs have been widely applied in the remediation of heavy metals pollutants,exhibiting well performance.Moreover,owing to the super-stable mineralization effect to heavy metal ions,LDHs have been demonstrated to be a great promising soil amendment in in-situ immobilization technology.However,several challenging issues should be considered when scalable utilization of LDHs as amendment for soil remediation,and we also hope that those issues could serve as a further guidance to future research.
(1) The critical factor for practice application of LDHs is how to produce LDHs in scale with low cost,green technique and efficiently.Although several kinds of LDHs have been produced in green method,the vast majority of LDHs were only produced through co-precipitation method with highly costly,environmentally destructive,and extensive byproducts.Therefore,it is still a pressing but great challenging work to explore a general strategy for production LDHs with atom economy of 100%.
(2) Although LDHs has displayed excellent immobilization ability to heavy metals in soil remediation process,the real immobilization mechanism is still unclear due to the complexity and variability of soil.Therefore,it must be helpful to design and explore the well-directed amendments for heavy metals through understanding the immobilization mechanism of LDHs adsorbents by in-situ characterizations.
(3) As amendments for soil remediation,the effect of LDHs on soil properties,soil microbial community and groundwater should be evaluated by monitoring the immobilized pollutants over long periods.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (21978023),the Fundamental Research Funds for the Central Universities(XK1803-05),the Innovative Achievement Commercialization Service-Platform of Industrial Catalysis (2019-00900-2-1) and the National Basic Research Program of China (2014CB932104).The authors appreciate Prof.Haohong Duan for his constructive suggestions.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Struggling as in past, write a glorious future together-CJChE’s 40th anniversary
- Reduced graphene oxide modified melamine sponges filling withparaffin for efficient solar-thermal conversion and heat management
- Photocatalytic degradation of tetracycline hydrochloride with visible light-responsive bismuth tungstate/conjugated microporous polymer
- Ag nanoparticles anchored on MIL-100/nickel foam nanosheets as an electrocatalyst for efficient oxygen evolution reaction performance
- Performance improvement of ultra-low Pt proton exchange membrane fuel cell by catalyst layer structure optimization
- Anodic process of stibnite in slurry electrolysis:The direct collision oxidation
