Molecular dynamics simulations on the wet/dry self-latching and electric fields triggered wet/dry transitions between nanosheets:A non-volatile memory nanostructure
2022-02-24JianzhuoZhu朱键卓XinyuZhang张鑫宇XingyuanLi李兴元andQiumingPeng彭秋明
Jianzhuo Zhu(朱键卓), Xinyu Zhang(张鑫宇), Xingyuan Li(李兴元), and Qiuming Peng(彭秋明)
State Key Laboratory of Metastable Materials Science and Technology and Key Laboratory for Microstructural Material Physics of Hebei Province,Yanshan University,Qinhuangdao 066004,China
We design a nanostructure composing of two nanoscale graphene sheets parallelly immersed in water.Using molecular dynamics simulations,we demonstrate that the wet/dry state between the graphene sheets can be self-latched;moreover,the wet→dry/dry→wet transition takes place when applying an external electric field perpendicular/parallel to the graphene sheets(E⊥/E‖).This structure works like a flash memory device(a non-volatile memory): the stored information(wet and dry states)of the system can be kept spontaneously,and can also be rewritten by external electric fields.On the one hand,when the distance between the two nanosheets is close to a certain distance, the free energy barriers for the transitions dry→wet and wet→dry can be quite large.As a result, the wet and dry states are self-latched.On the other hand, an E⊥and an E‖will respectively increase and decrease the free energy of the water located in-between the two nanosheets.Consequently,the wet→dry and dry→wet transitions are observed.Our results may be useful for designing novel information memory devices.
Keywords: wet/dry properties,non-volatile memory nanostructure,molecular dynamics simulations
Wet/dry properties in nanoscale space play an important role in a number of increasingly relevant issues, such as protein folding/unfolding,[1]water or liquid processing,[2,3]catalysis,[4,5]water permeation in membrane channels,[6,7]as well as energy storage and conversion.[8—11]In this context, holding the wet/dry state and controlling the flow of water into and out of nanoscale slits are two key factors.The infiltration dynamics, the adsorption/desorption oscillation phenomenon, the critical effect on capillary infiltration,and the light-triggered wet transition phenomenon of water in mesoporous thin films have been respectively investigated through experimental studies.[12—15]Xiaoet al.reported a charge- and electric-field-induced water gating based on a nanochannel.[16]Revillaet al.studied the electrowetting phenomena on patterned poly(methylmethacrylate)surfaces using atomic force microscopy.[17]However, much detailed information at the molecular level cannot be obtained through experimental studies,which in turn can be acquired through simulation studies.[18—20]The wet/dry properties of a nanoscale slit have been investigated intensively using molecular dynamics (MD) simulations.[21—33]Huanget al.and Choudhuryet al.studied the wet/dry properties in-between two hydrophobic nanosheets.[21,24—26]They found that the wet and dry states of the system can be self-latched and the wet/dry properties were sensitive to the solute—solvent interaction.Vaitheeswaranetal.,Bratkoet al.and Englandet al.demonstrated that an external electric field had a great impact on the density of water molecules in-between two nanosheets.[27,30—32]
In this paper,using MD simulations,we demonstrate the wet and dry states self-latching as well as the external electric fields triggered dry→wet and wet→dry transitions in-between two nanosheets immersed in water.When the distance between the two nanosheets is close to a certain distance, the free energy barriers for the transitions dry→wet and wet→dry can be quite large.[25]As a result, the wet and dry states can be self-latched.On the other hand, when an external electric field perpendicular/parallel to the nanosheets is applied, the free energy of the water molecules located in-between the two nanosheets is demonstrated to be increased/decreased.Therefore,the dry/wet state turns out to be the thermodynamic stable state, and the wet→dry/dry→wet transition takes place.This structure works like a flash memory device(a non-volatile memory): the wet and dry states stored in the system not only can be kept spontaneously,but also can be rewritten by external electric fields.
The model system with two identical rectangular graphene sheets immersed parallelly in water is shown in Fig.1.Graphene sheets of three different sizes were investigated.The dimensions of the small, medium, and large graphene sheets were 20×20, 29×33, and 39×41 nm2, respectively.The distance between the two graphene sheets(D)was 6.16, 6.26, or 6.36.Each atom on the graphene sheets was constrained by a harmonic potential(k=9000 kJ/mol·2)to the initial position.An uniform external electric field perpendicular or parallel to the graphene sheets (E⊥orE‖) with a strength of 0.1 or 0.2 V/nm was exerted on the system.The OPLSAA force field was used for the carbon atoms on the graphene sheets(the type of aromatic carbon),[34]and the extended simple point charge(SPC/E)water model was adopted to model the water molecules.[35]The carbon atoms on the graphene sheets were electrically neutral.The combination of SPC/E water model and graphene sheets has been widely used in classical MD simulations to study the properties of electrical double layer capacitors (EDLC).[36—40]Generally, an external electric field may polarize water molecules.Yehet al.has demonstrated in their work that the difference between the water structures respectively obtained through SPC/E water model and the polarizable water model is rather small under an electric field of less than ~2.8 V/,[41]which is larger than the electric field strength used in this work.Therefore,we may expect that the neglect of water polarizability will not visibly impact the response to the electric field at the field strength we study.In addition, the electrical conductivity of the graphene sheets cannot be modeled in classical MD simulations.Hence, like other researchers,[36—40]we only focus on the intersheet water when the two nanosheets are in the electrostatic equilibrium state, but not in the charging or discharging process.All the MD simulations were performed using the GROMACS 2019.1 simulation package[42]in an NPT ensemble (300 K, 1 bar), with periodic boundary conditions applied in all three directions.The temperature and pressure were maintained using the V-rescale thermostat and Berendsen pressure coupling schemes, respectively.[43,44]For each system, a 300-ns simulation with a time step of 1 fs was performed.The cut-off radii of both the short-range electrostatic and the van der Waals interactions were set to 1.2 nm.The particle mesh Ewald method was applied for calculation of the long-range electrostatic interactions.[45]
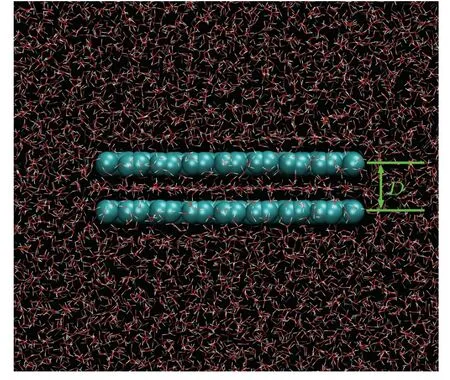
Fig.1.Snapshot of a simulation system.
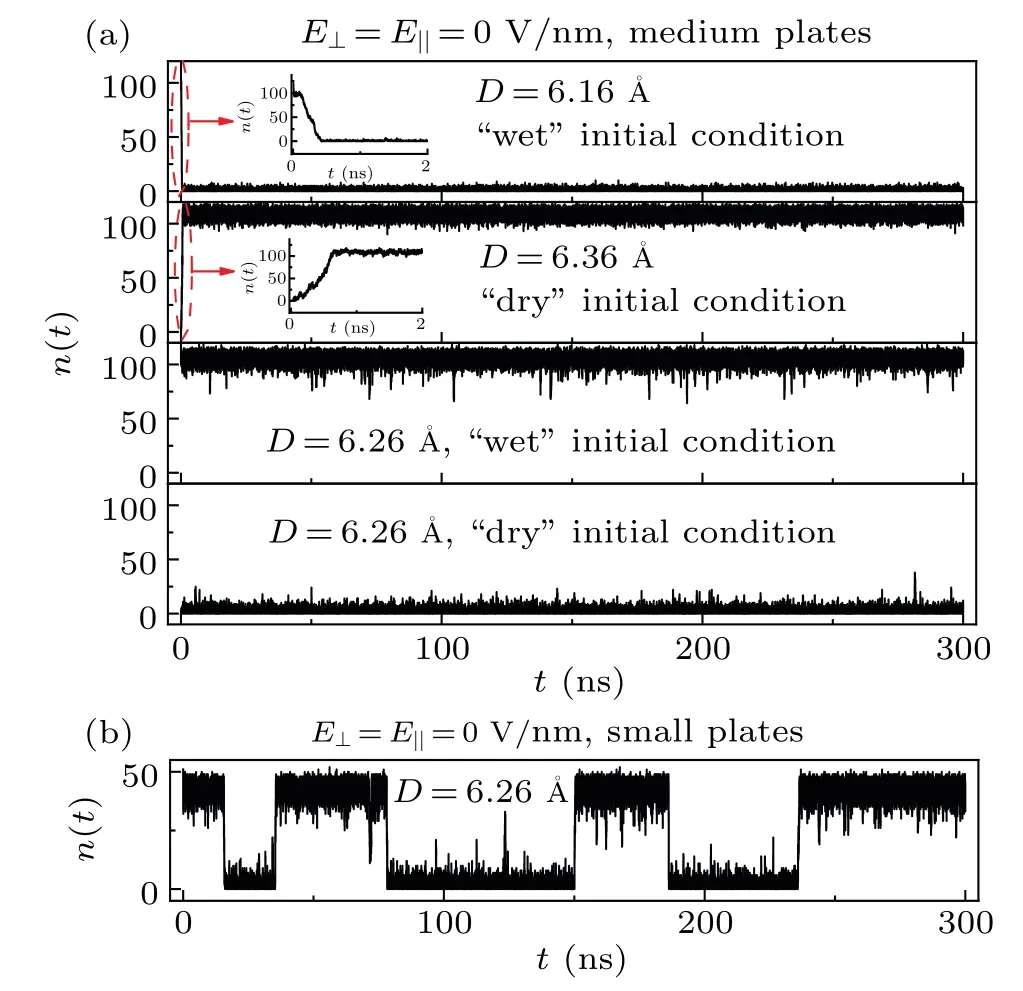
Fig.2.(a) The water molecule number n(t) between two medium graphene sheets as a function of simulation time for different D and different initial conditions without an external electric field.The insets are the enlarged diagrams.(b) The water molecule number n(t) between two small graphene sheets as a function of simulation time for D=6.26 without an external electric field.
The number of water molecules between two medium graphene sheets,n(t), was first studied.For the simulations,different initial conditions were launched for the intersheet regions: for some simulations,the intersheet regions were filled with water (“wet” initial condition); while the intersheet regions were empty for other simulations (“dry” initial condition).In Fig.2(a),we plotn(t)between two medium graphene sheets as a function of time for the systems with differentDand different initial conditions without an external electric field.In the simulation ofD=6.16with a wet initial condition, the system dries in the first 0.4 ns of the simulation,and it remains dry for the rest of the simulation(~299.6 ns);while in the simulation ofD=6.36with a dry initial condition, the system wets rapidly after the start of the simulation,and then it remains wet for the rest ~299.4 ns simulation.Thus, we may say that the dry and wet states are thermodynamically more stable for the systems ofD≤6.16andD≥6.36, respectively.For cases withD=6.26, however, simulations starting from wet initial conditions remain wet and simulations starting from dry initial conditions remain dry.From another perspective, in the case of the medium graphene sheets andD= 6.26, the system can keep the stored information (wet and dry states) spontaneously.The phenomenon of the self-latching of the wet and dry states has also been observed by Huanget al.[25]Both the theory and the simulation studies have demonstrated that there are free energy barriers for the dry→wet and wet→dry transitions in-between nanosheets.[24,25]In the simulations ofD=6.26, the free energy barriers for the transitions dry→wet and wet→dry are too large to be overcome during the 300-ns simulations.As a result, the wet and dry states of the system are self-latched.Furthermore, the free energy barriers for the dry→wet and wet→dry transitions are found to be increased with the solute size.[21,46]Figure 2(b)showsn(t)between two small graphene sheets forD=6.26without an external electric field.During the 300-ns simulation, the transition between the wet and dry states takes place several times, which indicates that the free energy barriers for the dry→wet and wet→dry transitions are lower than those between two medium graphene sheets.
Figure 3(a) showsn(t) between two medium graphene sheets as a function of simulation time for the systemD=6.26with different external electric fields and with different initial conditions.The system with wet/dry initial state can be switched to the dry/wet state within 10 ns after the start of the simulation by anE⊥/E‖of 0.2 V/nm, and then the system remains dry/wet for the rest of simulation.However,if theE⊥andE‖are reduced to 0.1 V/nm,the wet→dry and dry→wet transitions will not take place during the simulations.This may imply that the dry/wet state is thermodynamically more stable at the presence of anE⊥/E‖.Nevertheless, only anE⊥/E‖larger than a certain value (the critical electric field strength) can help the system overcome the wet→dry/dry→wet transition free energy barrier.For the medium graphene sheets, the critical electric field strength should fall in the range of 0.1—0.2 V/nm.From the perspective of information storage, in the case of medium graphene sheets andD=6.26, the information stored in the system(wet and dry states)can be electrically rewritten.Figure 3(b)showsn(t)between two large graphene sheets as a function of simulation time for the systemD=6.26with different external electric fields and with different initial conditions.The wet→dry/dry→wet transition will not take place even under anE⊥/E‖of 0.2 V/nm for the system with two large graphene sheets, which also implies that the larger of the nanosheets,the higher of the wet→dry and dry→wet transition free energy barriers.[21,46]
In the case of the medium graphene sheets andD=6.26, the initial state (wet or dry state) can be selflatched during the 300-ns simulations.Furthermore, the wet→dry/dry→wet transition takes place when applying anE⊥/E‖of 0.2 V/nm.That is, anE⊥of 0.2 V/nm makes the system dry; while anE‖of 0.2 V/nm makes the system wet.Vaitheeswaranet al.demonstrated that anE⊥can decrease the density of water between two nanosheets.[30]However,Bratkoet al.demonstrated,in their paper,that anE⊥will increase the density of water between two nanosheets.[27]Englandet al.reported that the increase and decrease of the density of water between two nanosheets under anE⊥can respectively be obtained within their respective certain parameter regimes.[32]Since the distances between the two nanosheets in their works were at or above 8, they have not observed the dry state,and thus have not further observed the wet→dry and dry→wet transitions either.[27,30]Like the flash memory, in the case of the medium graphene sheets andD=6.26,the system can keep stored information (wet and dry states) spontaneously,and can also be electrically erased and reprogrammed.
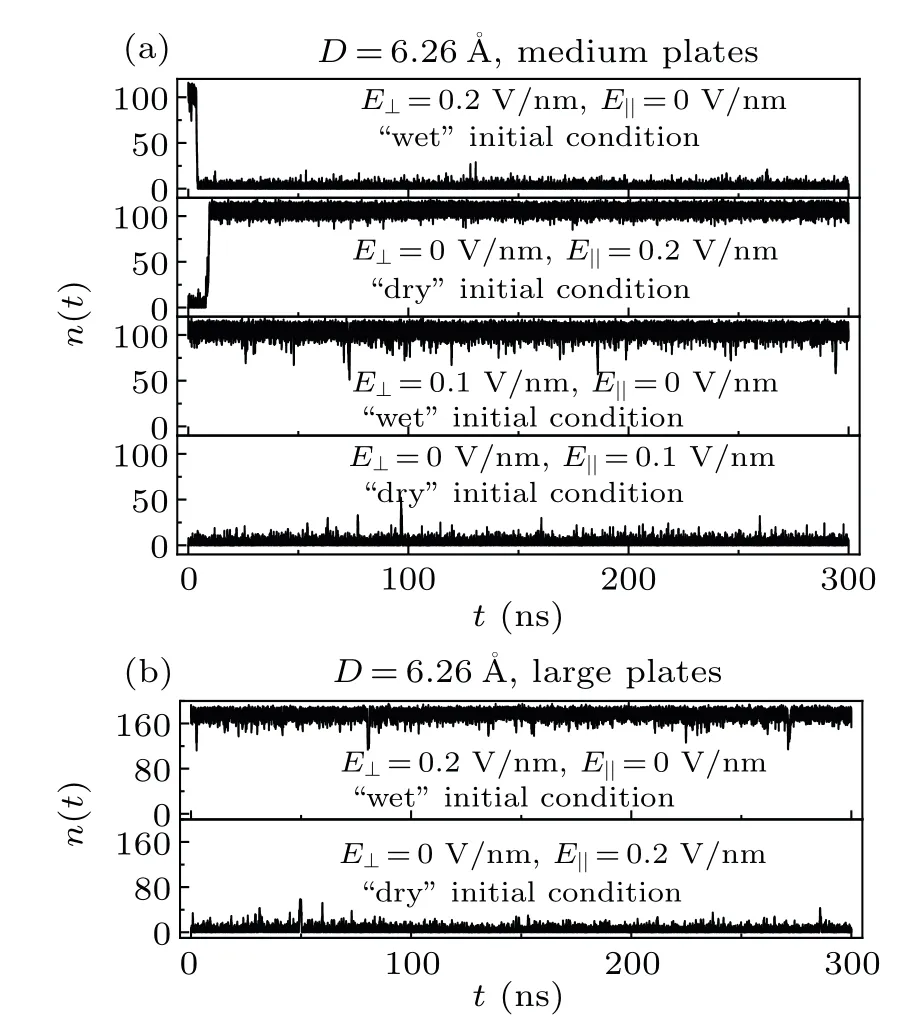
Fig.3.The water molecule number n(t)between two medium graphene sheets(a)and two large graphene sheets(b)as a function of simulation time for the system D=6.26 with different external electric fields and with different initial conditions.
Then, we studied the orientation of the water molecules in-between two medium graphene sheets withD= 6.26when the system is wet.The orientation of the water molecules is characterized by the angles ω and θ.As illustrated in the inset of Fig.4(b),ω is defined as the angle between the water dipole (μ) and the +ydirection, and θ is the angle between the vector μ and the+zdirection.The distributions of angles ω and θ are respectively displayed in Figs.4(a)and 4(b).And the snapshots of the water molecules in the intersheet region under different external electric fields are shown in Figs.4(c)—4(e),respectively.When applying aE‖of 0.2 V/nm,the distribution of ω changes much,while the distribution of θ changes little.TheE‖induces the dipoles of the water molecules in the intersheet region to align along the direction ofE‖, and makes the orientation of the water molecules more ordered(see Fig.4(d)).If anE⊥of 0.2 V/nm is applied,the distribution of ω changes little,and the distribution of θ changes slightly.TheE⊥makes the water dipoles tilt slightly towards to the+zdirection.In addition, we calculated the number of water molecules (Nwater), the average number of hydrogen bond formed by a water molecule with other water molecules(NHB),and the average interaction potential of a water molecule with other water molecules (Pwater) in the intersheet region when the system is wet or dry (Table 1).Two water molecules are considered to form a hydrogen bond if the O···O distance is less than 0.35 nm,the H—O···O angle is smaller than 30°,as well as the H···O distance is less than 0.25 nm.[47,48]When the system is dry,theE⊥/E‖decreases/increases theNHB.SoPwateris reduced/enhanced, andNwateris decreased/increased as well.It should be noted that the very few water molecules in the intersheet region should result from the water molecules near the edges of the nanosheets.When the system is wet,both theE⊥and theE‖decrease theNHB.On the other hand, anE‖of 0.2 V/nm makes the orientation of the water molecules more ordered (see Fig.4(d)).The neater arrangement of water molecules may help the intersheet region accommodate more water molecules, which implies more neighbor water molecules for a water molecule in the intersheet region.More neighbor water molecules should be beneficial for the intersheet water to obtain a strongerPwater.No matter the intersheet region is wet or dry,anE⊥/E‖of 0.2 V/nm reduces/enhances thePwaterof water molecules in the intersheet region.As a consequence,the dry/wet state becomes thermodynamically more stable when anE⊥/E‖of 0.2 V/nm is applied.Therefore,the wet→dry/dry→wet transition takes place under anE⊥/E‖of 0.2 V/nm.
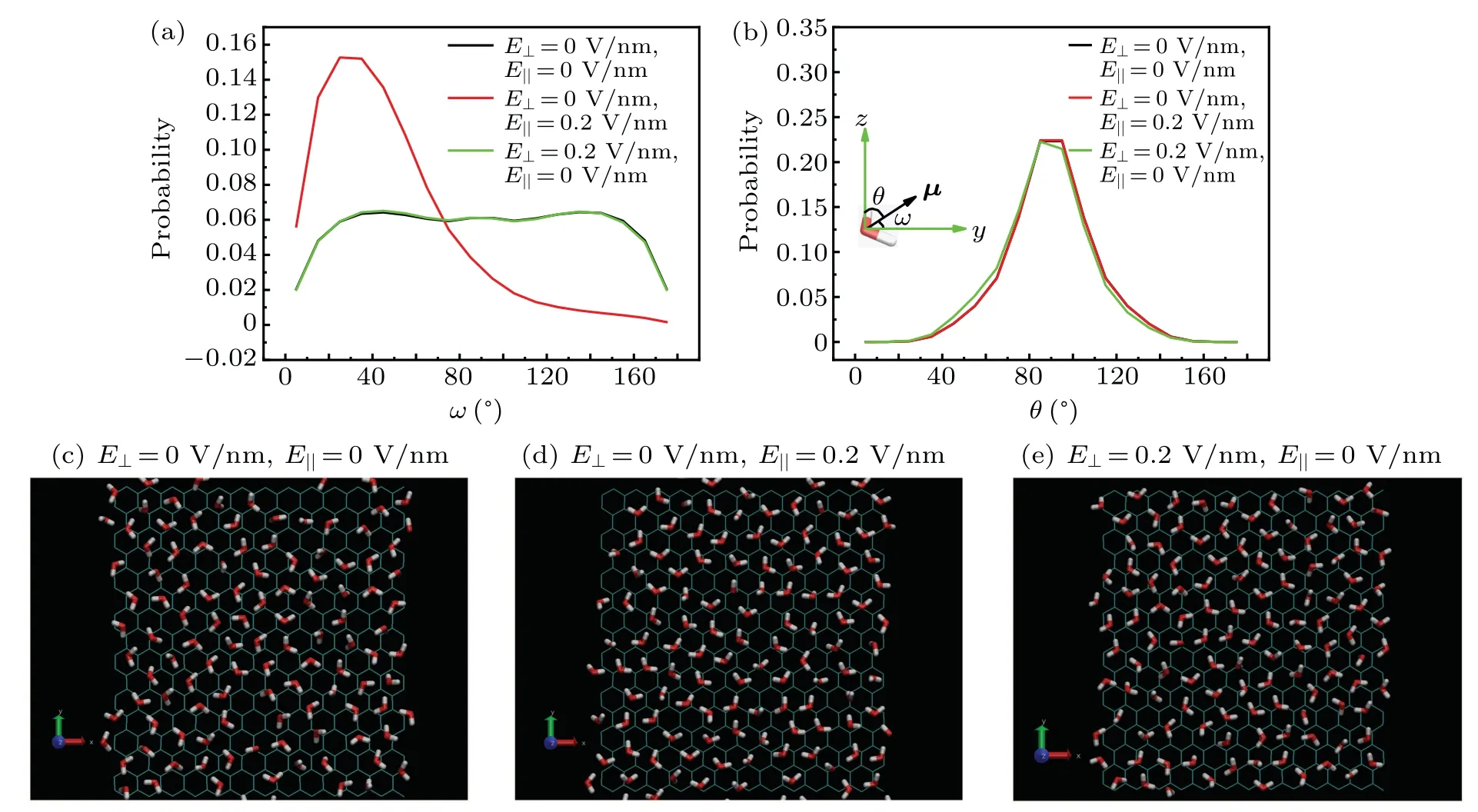
Fig.4.Probability distributions of angles ω (a)and θ (b)as well as the snapshots(c)—(e)of water molecules in-between two medium graphene sheets with D=6.26 when the system is wet under different external electric fields.
Table 1.Properties of water molecules in-between two medium graphene sheets with D=6.26 when the system is dry or wet.
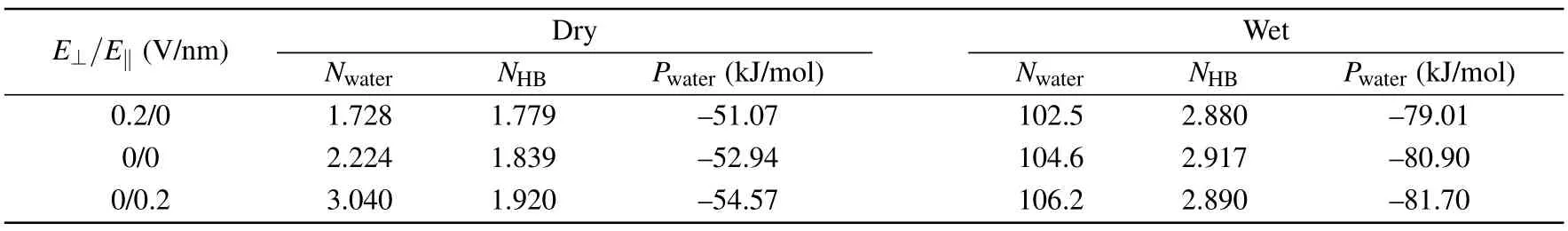
Table 1.Properties of water molecules in-between two medium graphene sheets with D=6.26 when the system is dry or wet.
E⊥/E‖ (V/nm) Dry Wet Nwater NHB Pwater (kJ/mol) Nwater NHB Pwater (kJ/mol)0.2/0 1.728 1.779 —51.07 102.5 2.880 —79.01 0/0 2.224 1.839 —52.94 104.6 2.917 —80.90 0/0.2 3.040 1.920 —54.57 106.2 2.890 —81.70
To conclude, the wet/dry properties in-between two nanoscale graphene sheets immerged in water have been studied using MD simulations.When having a proper size and a properD, the pair of graphene sheets can work like a flash memory device (a non-volatile memory): the wet and dry states between the nanosheets can be self-latched; moreover, the wet→dry/dry→wet transition will take place when anE⊥/E‖of proper magnitude is applied.The self-latching of the wet and dry states results from the large free energy barriers for the wet→dry and dry→wet transitions.On the other hand, anE⊥/E‖increases/decreases the free energy of the water located in the intersheet region, which makes the dry/wet state to be the thermodynamic stable state.Therefore,the wet→dry/dry→wet transition takes place.Our results may help understand information storage mechanisms of organism,and may also help people design novel information memory devices.
Acknowledgement
Project supported by the National Natural Science Foundation of China(Grant No.11704328).
猜你喜欢
杂志排行
Chinese Physics B的其它文章
- A broadband self-powered UV photodetector of a β-Ga2O3/γ-CuI p-n junction
- High-sensitive terahertz detection by parametric up-conversion using nanosecond pulsed laser
- High efficiency,small size,and large bandwidth vertical interlayer waveguide coupler
- High-fidelity resonant tunneling passage in three-waveguide system
- An analytical model for cross-Kerr nonlinearity in a four-level N-type atomic system with Doppler broadening
- Determine the physical mechanism and source region of beat wave modulation by changing the frequency of high-frequency waves
