The Combined Effect of Plastic Particles Size and Concentration on Rotifers’ (Brachionus plicatilis) Performance
2022-02-24SUIYanmingWANGSenyangMOHSENMohamedZHANGLongshengSHENMengyanLIUZhiquanNGUYENHaidangZHANGShengmaoLIKaixingLVLinlanandDONGXuexing
SUI Yanming, WANG Senyang, MOHSEN Mohamed, ZHANG LongshengSHEN MengyanLIU Zhiquan, NGUYEN Haidang, ZHANG Shengmao,LI KaixingLV Linlan, and DONG Xuexing
The Combined Effect of Plastic Particles Size and Concentration on Rotifers’ () Performance
SUI Yanming1),#, WANG Senyang1),#, MOHSEN Mohamed2), 3), ZHANG Longsheng1),SHEN Mengyan1), LIU Zhiquan4), NGUYEN Haidang5), ZHANG Shengmao6),LI Kaixing1), LV Linlan1), *, and DONG Xuexing1),*
1) School of Marine and Biological Engineering, Yancheng Institute of Technology, Yancheng 224002, China 2) Department of Animal Production, Faculty of Agriculture, Al-Azhar University, Nasr City, Cairo 11884, Egypt 3) CAS Key Laboratory of Marine Ecology and Environmental Sciences, Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, China 4) School of Life Sciences, East China Normal University, Shanghai 200241, China 5) Research Institute for Aquaculture No.1, Dinh Bang-Tu Son-Bac Ninh 16352, Vietnam 6) East China Sea Fishery Research Institute, Chinese Academy of Fishery Sciences, Shanghai 200090, China
The presence of microplastics in aquatic ecosystems is of increasing global concern. Nano-sized plastics, in particular, can penetrate the cell membrane and cause biological death. Our study evaluated the combined impacts of several polystyrene microspheres’ sizes and nominal concentrations on the overall performance changes of. Experimental animals were exposed to three microplastic sizes (0.08, 0.5 and 6μm) and five nominal concentrations (0, 0.5, 2, 8, 32µgmL−1) for 20d. Our results showed that the toxicological effect of particle size on rotifers did not significantly depend on the nominal concentration. The interaction between the nominal concentration and size occurred only for body length and lorica width. Specifically, high nominal concentrations of microplastics that were close to nanometer size significantly impaired the overall vitality of rotifers, embodied in shortage of body type, delay in the arrival of maturity, reduction in the cumulative number of neonates, and the advance of the death process. In comparison, fair-sized size (0.5 and 6μm) displayed non-significant damage except for individual groups. Most notably, the net reproductive yield was only a third of what it was in the original environment, implying that there was not much fertility left. Besides, with the development of rotifers, the adverse effects of polystyrene microsphere drive had become more and more serious.
microplastic; zooplankton; environmental stress; life-history parameters
1 Introduction
Plastic are organic polymer synthesized from fossil materials such as natural gas, oil, and coal, and have been mass-produced since the middle of the last century (Thompson, 2009). Up to now, they have been widely used in all aspects of our daily lives. With the global population growing at an average rate of 1.68% and the large consumption of plastic products, the total global production of plastic had reached 368 million tons since its birth (Verla, 2019; PlasticsEurope, 2020). Due to the lack of timely recovery, neglect, and contingency, an inevitable consequence was the release of plastic products into the environment (Wagner, 2014). Studies have shown that about 10% of the plastic entered the sea and remained there for an extended period because of its persistence and difficulty in degradation (Thompson, 2009). Photodegradation, thermal oxidation degradation, hydrolysis, and microbial biodegradation can eventually decompose plastic wastes to generate small plastic particles (Browne, 2008; Canesi, 2015). Plastic particles created in this way that were smaller than 5mm in size were called microplastics (Tho- mpson, 2009). More importantly, they can be further degraded to nanoscale plastics less than 1µm (Koelmans, 2015; Mattsson, 2015). While the amount of nanoplastics in the aquatic environment is unknown, microplastics can be found along coastlines, water columns, sediments, beaches, and even remote areas (Yu, 2016; Waller, 2017; Jian, 2020).
Consequently, microplastics in the ocean pose threats to the aquatic environment and marine life (Guzzetti., 2018). The accidental ingestion of microplastics was pervasive, and researches had reported traces of them in marine creatures such as seaweed, zooplankton, shellfish, shrimp, crab, and fish (Browne, 2008; Foekema, 2013; Sundbæk, 2018;Savoca, 2019; Hossain, 2020). Sjollema(2016)measured the photosynthesis capacity and the development ofin nanoscale and microplastic environments. They found that the enhancement of the negative effect was related toreducing of the plastic to the nano-level. In zooplankton, nanoscale polystyrene plastic significantly weakened the viability and reproduction of. The latter was characterized by reduced embryos and abnormal development of mortality-prone embryos (Cui, 2017). In shellfish, relevant experimental results showed that larger particles may be filtered by the oysters whereas, rather than ingested, but remained in the shell cavity by adhesion (Graham, 2019). In grass shrimp, acute exposures to various shapes and sizes increased mortality (Gray and Weinstein, 2017). Like the above results, Murray and Cowie (2011) found that lobsters were incapable of completely passing microplastic. Hence, over time, residual fibers probably became entangled inside the digestive tract, leading to biodegradable failure, causing intestinal obstruction and even mortality (Besseling, 2013). In crabs, an investigation reported that microparticles were detected in their gills and that crabs consumed less oxygen after exposure to PS beads (Watts, 2016). In one inquiry, the fish that were given food containing microplastic swam and hunted to a slower degree, displaying significant behavior contrasts to the control. There was an interference of the lipid metabolism because of microplastic uptake (Ceder- vall, 2012). Despite the growing concerns about biological consumption of microplastic, few studies had probed interactions between microplastic and zooplankton. The size and extent of microplastics’ potential effects on zooplankton are yet not well understood (Desforges, 2015).
As an intermediate between micro food chain and traditional food chain, rotifers are one of the most important secondary producers in marine ecosystems and play a pivotal role in species circulation and energy transfer in the aquatic ecosystem (Scheda and Cowell, 1988; Arndt, 1993; Devetter and Seďa, 2006; Xie, 2009). On account of feeding on algae, detritus, or other microorganisms with high assimilation efficiency, their rapid population growth rate makes rotifers the primary food source for many fish and aquatic invertebrates (Snell and Janssen, 1995; Ortaz, 2006). Due to their high protein content, some species are used as starter feeds and have a good attraction to a variety of economically farm- ed animals (Yoshimura, 1996; Wang and Mai, 2005). Even so, their value is not only in terms of specific economic efficiency, but also in terms of toxicology as a mo- del organism (Yúfera, 2001). Rotifers, usually less than 200 microns, are common zooplankton. Their culture medium volume is small, and they even can be cultured with microliter (Dahms, 2011). Therefore, the amplification effect of rotifer on the test substance is conducive to toxicological evaluation. The internal organs of the rotifer are relatively complete, with nervous, digestive, excretory, reproductive, and other organ systems (Wang, 1961). Hence, toxic and harmful substances in water can reflect obviously by the growth and reproduction of invertebrate rotifers (Preston, 1999; Radix, 2002; Faggio, 2018). The resting eggs of the rotifer can be easily stored and hatched at the same time when needed for experiments (Preston and Snell, 2001; Zhang, 2016). In this way, the physiological conditions of test materials are consistent, and experimental errors are reduced. Meanwhile, the shorter life cycle simplifies the experiment, dramatically shortens the observation time, and reduces the experimental cost (Sarma and Rao, 1991; Snell and Moffat, 1992; Luna-Andrade, 2002; Zhang, 2016).
In order to further advance our understanding of the nanoscale and microplastics potential effects on zooplankton, this study aimed to evaluate the effects of different nominal concentrations (0.5, 2, 8, 32μgmL−1(Lee, 2013)) and sizes (0.08 (Gaspar, 2018), 0.5 (Farrell and Nelson, 2013), 6μm (Deng, 2017)) of microplastics on the longevity of rotifer zooplankton, as the influences of microplastics on rotifers were reflected in body type, reproduction, as well as average life span (Besseling, 2014). This study, simultaneously, will offer a better understanding of the effects of microplastics on marine organisms (Fig.1).

Fig.1 Graphic abstract.
2 Materials and Methods
2.1 Experimental Organisms and Microbeads
The rotifer () was purchased from Dashiqiao City Xi Lin Aquarium Shop (Liaoning, China). These experimental animals were raised in the laboratory at 25℃ under light:dark 12:12 h photoperiod with a 20‰ concentration of artificial seawater. The microalgae algae(approximately 1.0×106cellsmL−1) were fed daily. Nonfunctionalized polystyrene micro- spheres with three sizes of 0.08, 0.5, and 6μm were purchased from BaseLine Chrom Tech Research Centre (Tianjin, China). The shape and size of the experimental microplastics for testingwere observed and measured under a scanning electron microscope (SEM). It was confirmed that the microplastics particles used in the experiment were spherical, with sizes ranging from nano- meter to micron (Fig.2).
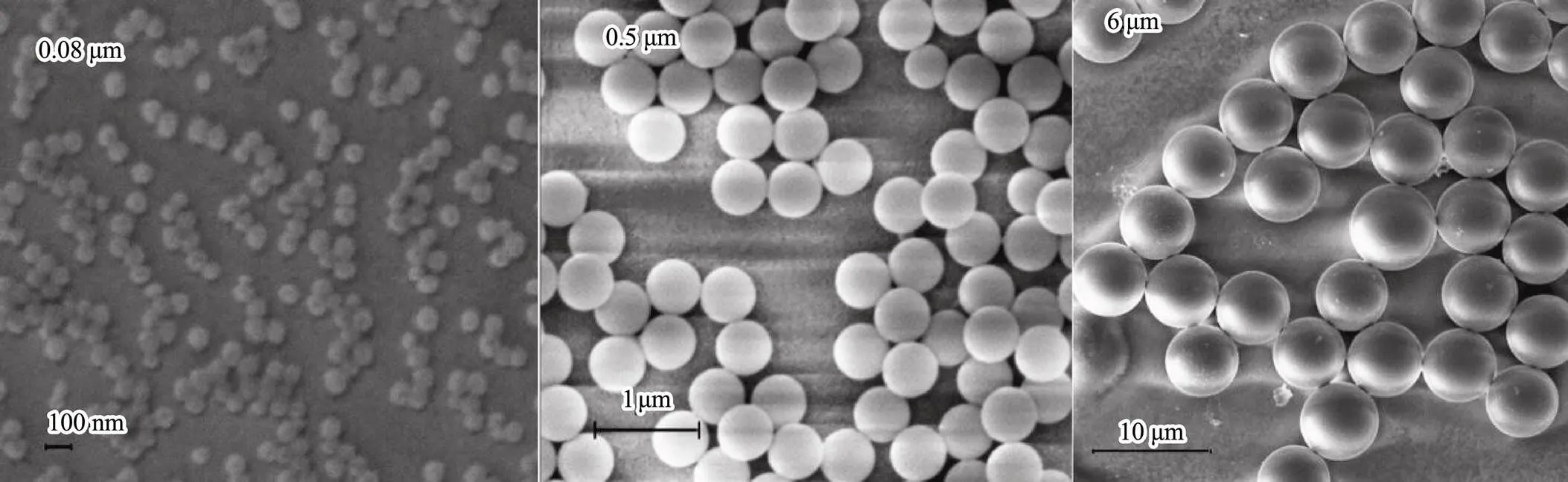
Fig.2 The SEM images showed the shape and size of the nanoscale (0.08 and 0.5μm) and microscale (6μm) plastic.
2.2 Experimental Design
As concerns about microplastic particles of small size was increasing, we used nanosized (defined here as particles less than 1µm; 0.08 and 0.5μm) and micro-sized (6 μm) polystyrene microspheres, as their widespread presence in the ocean (Browne, 2008; Canesi, 2015; Da Costa, 2016). Polystyrene was used since it was the one of the most plentiful high molecular compounds in ocean garbage (Andrady, 2011). To examine the effects of microplastic exposure onlife- history parameters, one rotifer larva less than 6h and 1mL of artificial seawater containing microalgae(approximately 1.0×106cellsmL−1) were placed into one well in a 24-well culture plate at 25℃ (=10 or 12 per replicate). This based on the report that zooplankton can mistake microplastics for food (Desforges, 2015). The culture medium was renewed daily. The experimental animals were exposed to a fully crossed design: 1) five nominal concentrations (0, 0.5, 2, 8, 32µgmL−1) within the range in experiments on the size effects of microplastic particles on marine zooplankton and the corresponding particle numbers were given in Table 1 (Lee, 2013); and 2) three sizes (0.08, 0.5 and 6μm), ranging from those similar in the size of the microalgae. They were placed in an incubator at 25℃. All exposures were conducted over 20d.
Life-history parameters were countedevery 12h under an optical microscope until the death of each capped female rotifer, including the removal of newborn offspring after each recording. The whole experiment started with newly born rotifers less than 6h old, randomly selected under an optical microscope. The experiments were incubated at 25℃ under light: dark 12:12 h photoperiod in 4000 LX with a 20‰ concentration of artificial seawater.
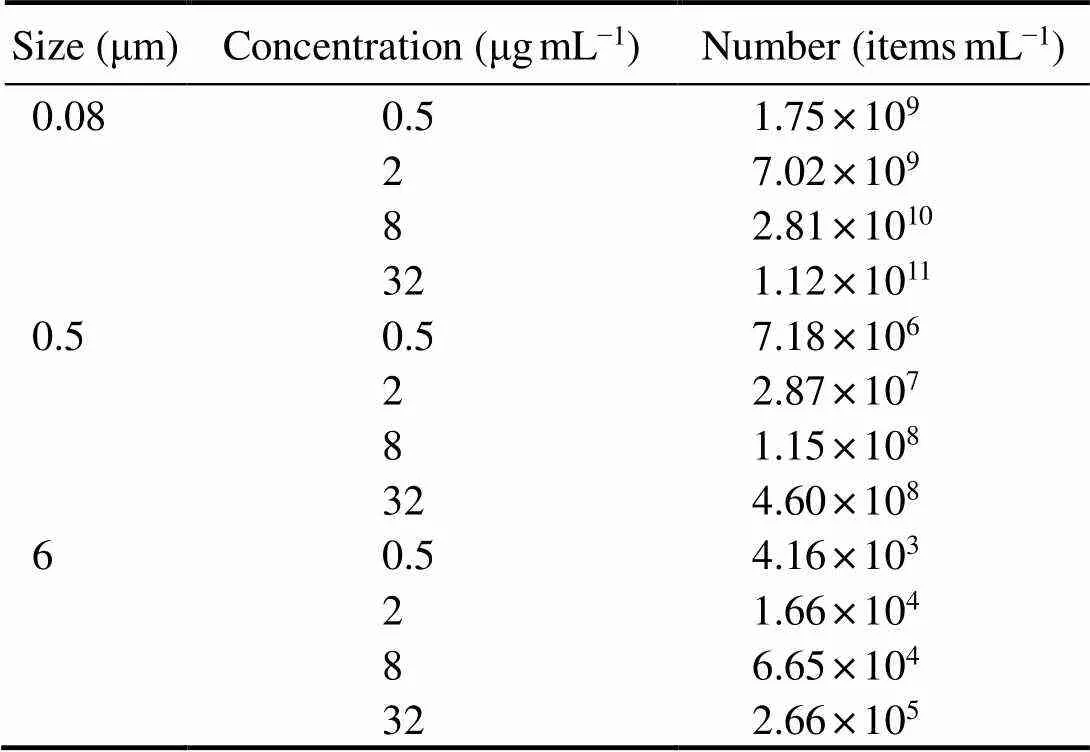
Table 1 The relationship between the size, concentration, and number of polystyrene microspheres
2.3 Measurement of Life-History Parameters
To inspect the effects of microplastics onbody sizes, three parameters were measured for each rotifer (=10 per replicate) after the first brood under the treatments: 1) the length of the body, 2) the length of the lorica, 3) the width of the lorica.
To measure the effects of microplastics onlife-history parameters, four parameters were measured for each rotifer (=12 per replicate) every 12h until the rotifer died: 1) pre-reproductive period–the time from the start of the experiment to the first spawning, 2) reproductive period–the time required for the rotifers to lay their first eggs until their last laying before death, 3) post-reproductive period–the time from the last spawning to death, 4) oviposition amount–the number of the initial maternal rotifers producing offspring. The average lifespan was calculated from the sum of the pre-reproductive period, reproductive period, and post-reproductive period.
2.4 Statistical Analysis
All averages were presented with the standard deviation ofthe mean (±SD).All the statistical analyses were carried out using the statistical software SPSS 26.0. For each measured parament, the difference between the effect of size and nominal concentration was tested using a two-way analysis of variance (ANOVA). When a significant difference was detected, a post-hoc test was carried out. For all analyses,<0.05 was considered significant. The results were expressed as the means ± SD. All data were checked for normal distribution and homoscedasticity.
3 Results
3.1 Effect of Microplastics on the Individual Size
3.1.1 Body length
Two-way ANOVA based on the growing data after the first brood displayed that 0.08μm microplastics had a more apparent adverse effect on body length than the other two sizes of microplastics (Fig.3, Table 2). Furthermore, there was a remarkable interaction between microplastics sizes and nominal concentrations on the rotifer body length (=0.010) (Fig.3, Table 2). The body length of rotifers (165.6μm±8.3μm) exposed to 32μgmL−1microplastics of 0.08-μm size exerted the most deleterious effects compared to the other nominal concentrations in identical size. Specifically, it was 14% shorter than those exposed to the control (192.8μm±6.6μm) (Fig.3). Different nominal concentration treatments of 0.5 and 6μm microplastics had no noticeable effect on body length (Fig.3).

Fig.3 Body length of B. plicatilis exposed to five microplastic concentration and three microplastic size treatments (n=10perreplicate). Different capital letters denote significant differences among three microplastic size treatments at each microplastic concentration. Different small letters denote significant differences among five microplastic concentration treatments at each microplastic size. The figure was made by Origin Pro 9 software.
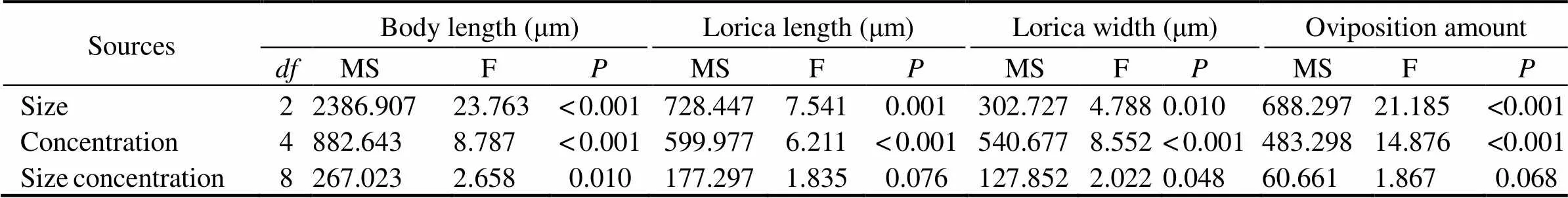
Table 2 Experimental data
Notes: Summary of two-way ANOVA results on effects of size, concentration on rotifers’ performance. The eight measured parameters were as follows: 1) body length (length in µm), 2) lorica length (length in µm), 3) lorica width (length in µm), 4) oviposition amount.
3.1.2 Lorica length
Both sizes (0.08, 0.5 and 6μm) (F=7.541,=0.001) and nominal concentrations (0.5, 2, 8, 32µgmL−1) (F=6.211,<0.001) had evident effects on lorica length, and there was no interaction of microplastic size and nominal concentration (=0.076) (Table 2). Lorica length was the longest in control, and microplastics did not exhibit overt size-dependent and nominal concentration-dependent toxicity (Fig.4). What was worth noting was that in 2µgmL−1treatment, the inhibitory effect of small size MPs on lorica length was more significant in 0.08 and 0.5 than in 6μm. In 32µgmL−1treatment, microplastics significantly inhibited the rotifers’ lorica length for both 0.08 and 6µm exposure, whereas the lowest suppression was found in 0.5µm exposure (Fig.4). The lorica length in 0.08μm under any nominal concentration was distinctly different from the control, whereas there was no difference among disparate nominal concentration treatments of 0.5 and 6 μm microplastics (Fig.4).
3.1.3 Lorica width
The influence of microplastics on the width of the loricawas examined under the five different nominal concentrations and three sizes. There was a significant interaction between MP sizes and nominal concentrations (=0.048) (Table 2). The average shortened size of lorica width ranged from a tenth to a fifth of the original lorica width, with the shortest observed width recorded in 32µgmL−10.08-μm treatment (Fig.5). Non- significant effects were observed in two other microplastic sizes (0.5 and 6μm), except for noticeable change– only 81% of the controlled width–residing in one example in 0.08μm at 32µgmL−1(Fig.5).
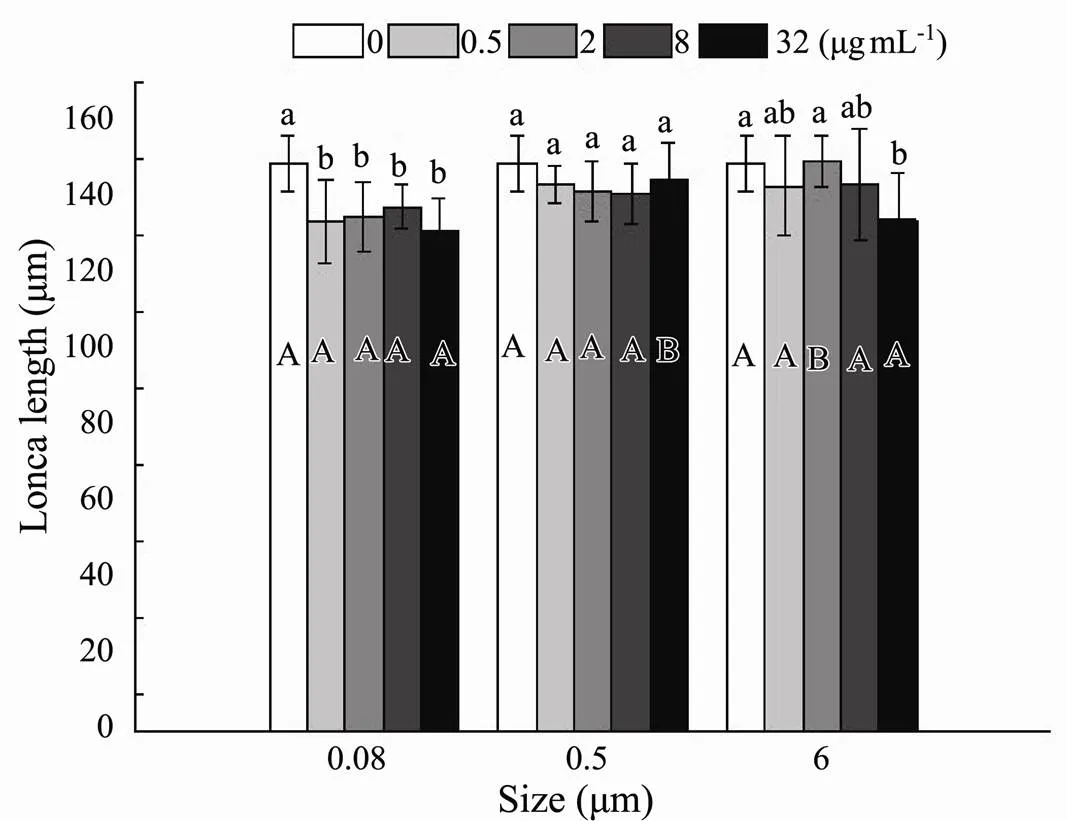
Fig.4 Lorica length of B. plicatilis exposed to five microplastic concentration and three microplastic size treatments for 19d (n=12perreplicate). Different capital letters denote significant differences among three microplastic size treatments at each microplastic concentration. Different small letters denote significant differences among five microplastic concentration treatments at each microplastic size. The figure was made by Origin Pro 9 software.
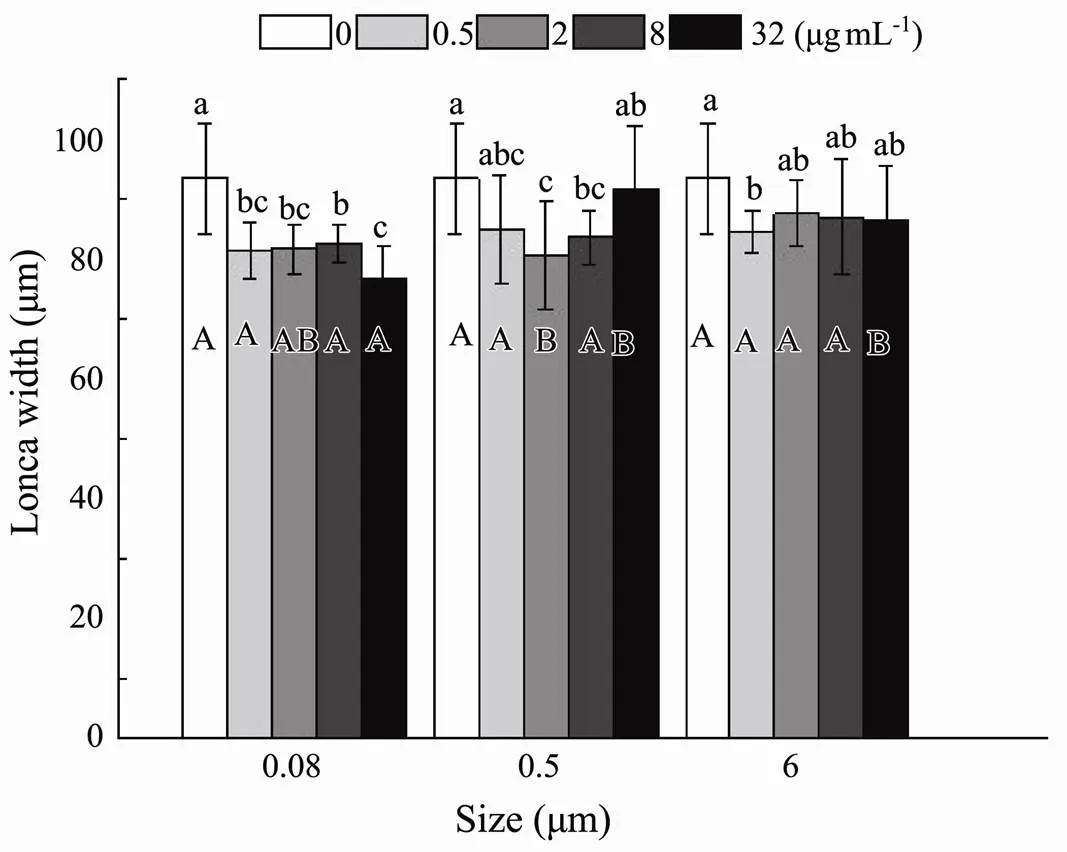
Fig.5 Same as those of Fig.4 but for lorica width of B. plicatilis.
3.2 The Effect of Microplastics on Life-History Parameters
3.2.1 Pre-reproductive period
Significant effects appeared only at five nominal concentrations (<0.001) but were not observed at three sizes (=0.050), including their interactions (=0.656) (Table 3). Demonstrable time extension was observed in 0.5µgmL−10.5-μm and 8µgmL−10.5-μm treatments ranging from 136.6% of the controlled pre-reproductive period to 154.6% of the controlled pre-reproductive period.Similarly, all sizes at the nominal concentration of 8µgmL−1significantly lengthen the pre-reproductive period (Fig.6). In contrast, a non-significant impact on the pre-reproductive period was examined in different nominal concentration treatments of 0.08 and 6μm plastic beads (Fig.6).

Fig.6 Same as those of Fig.4 but for pre-reproductive period of B. plicatilis.
3.2.2 Reproductive period
The nominal concentration of MPs, as the only factor, significantly shortened the reproductive period (<0.001) (Table 3).In 0.08μm and 0.5μm treatments, a significantchange was noticed in the reproductive at the maximum nominal concentration (32µgmL−1), and non-significant changes were observed at 0.5µgmL−1, 2µgmL−1, and 8µgmL−1(Fig.7). Interestingly, the time required for the rotifers to lay their first eggs until their last laying before death decreased slightly with increasing MP no- minal concentrations ranging from 0.5 to 8µgmL−1. In 6μm, the non-significant influence was displayed in all exposure nominal concentrations (Fig.7).
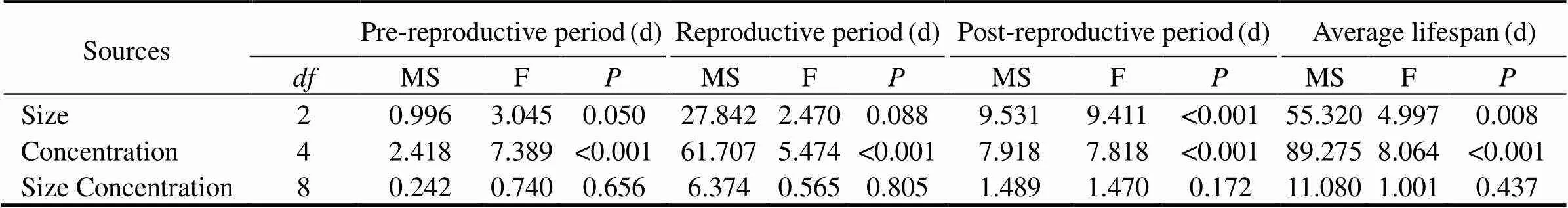
Table 3 Experimental data
Notes: Summary of two-way ANOVA results on effects of size, concentration on rotifers’ performance. The eight measured parameters were as follows: 1)pre-reproductive period (period in day), 2)reproductive period (period in day), 3)post-reproductive period (period in day), 4)average lifespan (time in day).
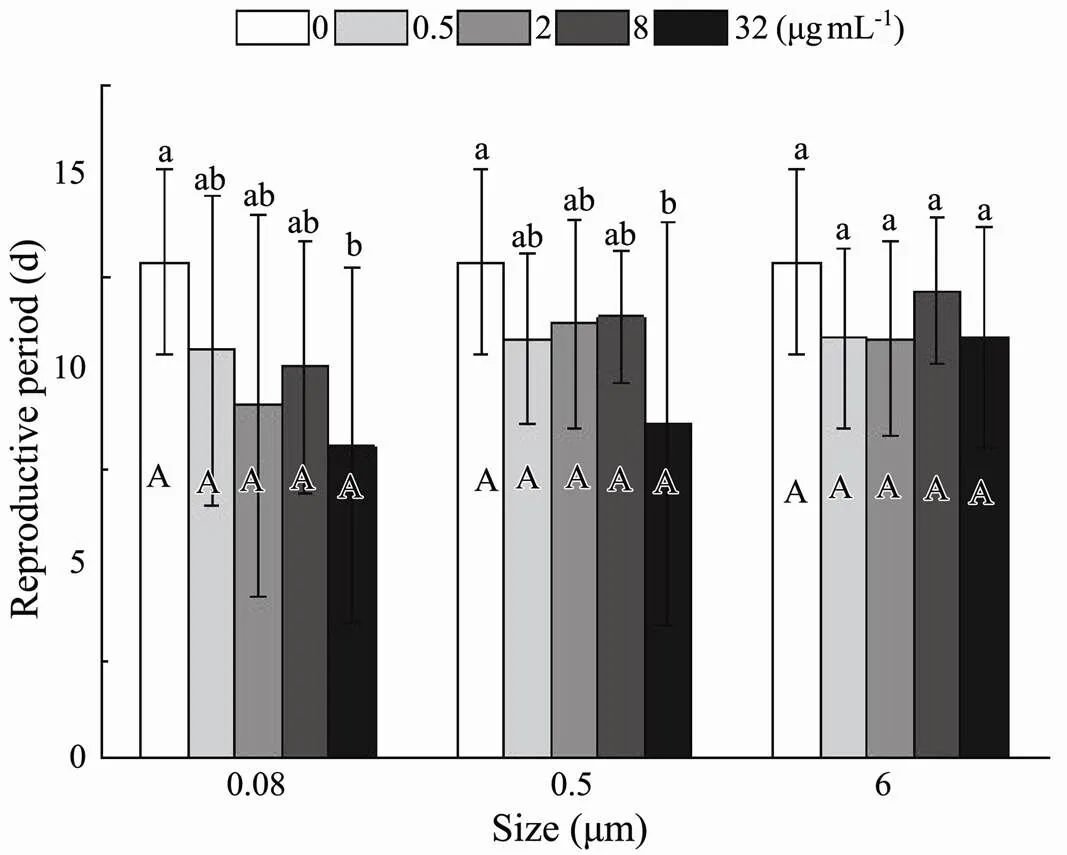
Fig.7 Same as those of Fig.4 but for reproductive period of B. plicatilis.
3.2.3 Post-reproductive period
Relevant life history data suggested that there was anoticeable effect of size (<0.001) and nominal concentrations (<0.001) on the post-reproductive period, the time from the last spawning to death, without observing an interaction between size and nominal concentrations (<0.172) (Table 3). The time from the last spawning to death was shortened significantly in the smaller size of 0.08 and 0.5μm than in 6μm at total experimental exposure nominal concentrations (Fig.8). In 0.08 and 0.5μm treatment, the process of death after the last spawning was conspicuously accelerated by different exposure nominal concentrations. However, the non-significant influence was presented at any nominal concentrations in 6μm treatment (Fig.8).
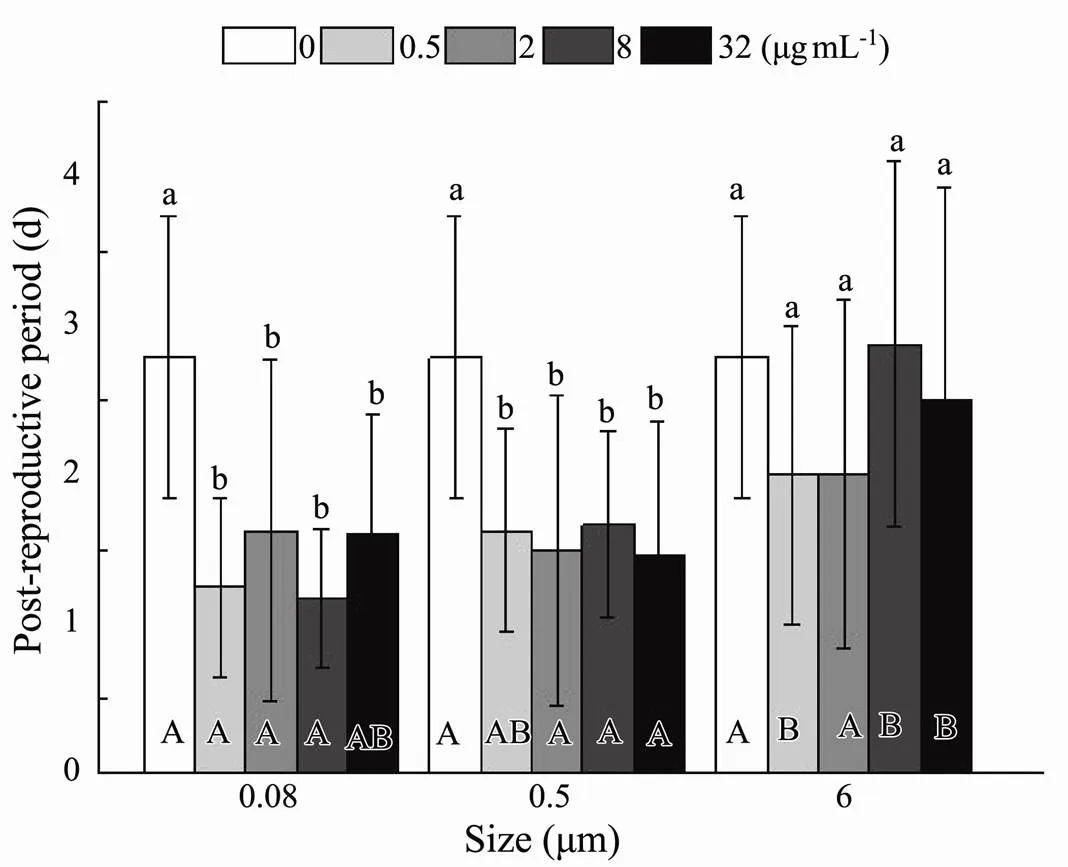
Fig.8 Same as those of Fig.4 but for post-reproductive period of B. plicatilis.
3.2.4 Average lifespan
Statistical analysis calculated by the sum of the pre- reproductive period, reproductive period, and post-reproductive period presented evidence that size (=0.008) and nominal concentration (<0.001) had a significant effect on average lifespan (Table 3). At 0.5µgmL−1, the existence of plastic exerted significant deleterious effects, which accelerated the progress towards death (Fig.9). At the highest nominal concentration of 32µgmL−1, less apparent virulence was only discovered in maximum size. The distinct toxicity presented in 0.08 and 0.5μm, while similar phenomena were observed in 6μm with 0.5 and 2µgmL−1(Fig.9). In terms of rotifers’ average lifespan, there was no interaction between the size and nominal concentration of microplastic (=0.437) (Table 3).
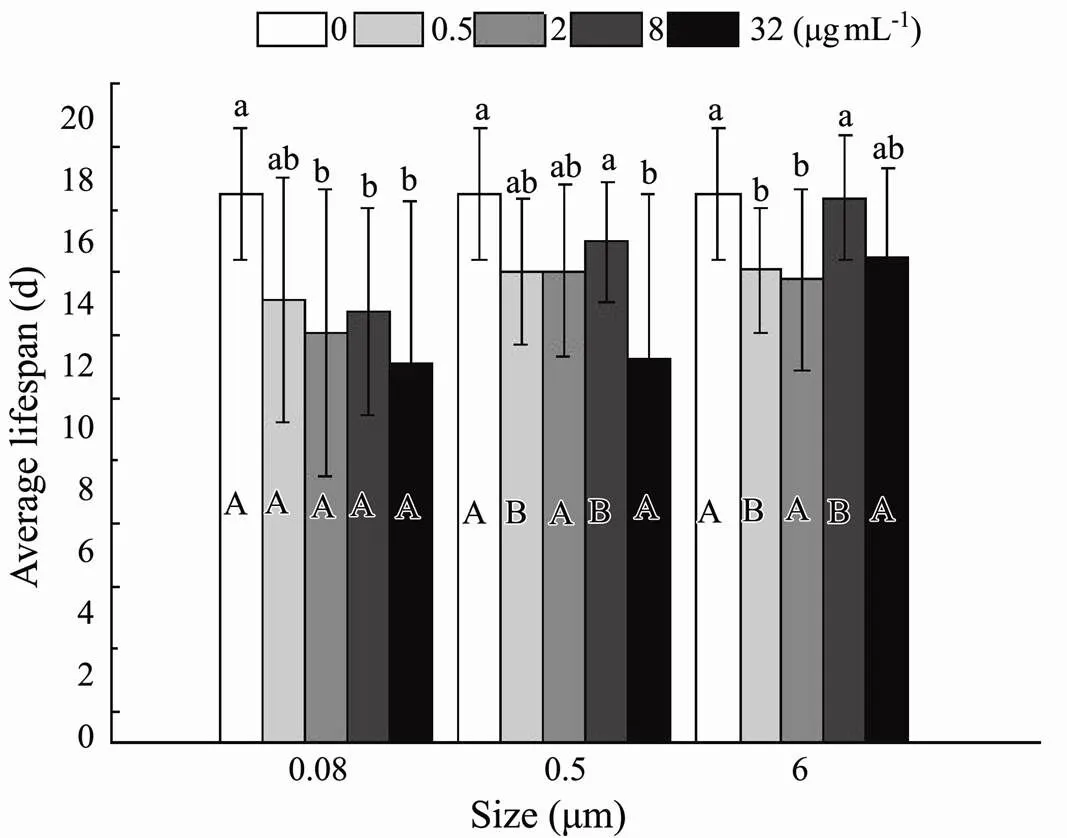
Fig.9 Same as those of Fig.4 but for average lifespan of B. plicatilis.
3.2.5 Oviposition amount
Effects of size (<0.001) and nominal concentration (<0.001) on oviposition amount were detected (Table 2). Compared to the control, the ability to lay eggs was sig- nificantly inhibited at four nominal concentrations with the minimum size (Fig.10). Different nominal concentrations in the other two sizes displayed non-significant prohibitive capability. The only exception was the impact on the number of the initial maternal rotifers producing offspring significantly in 32µgmL−10.5μm treatment. At four different nominal concentrations, the oviposition amount was different between the experimental and control groups, decreasing with increasing microplastic sizes ranging from 0.08 to 6μm (Fig.10). The interaction of plastic size and nominal concentration was not associated with decreased oviposition amount (=0.068) (Table 2).
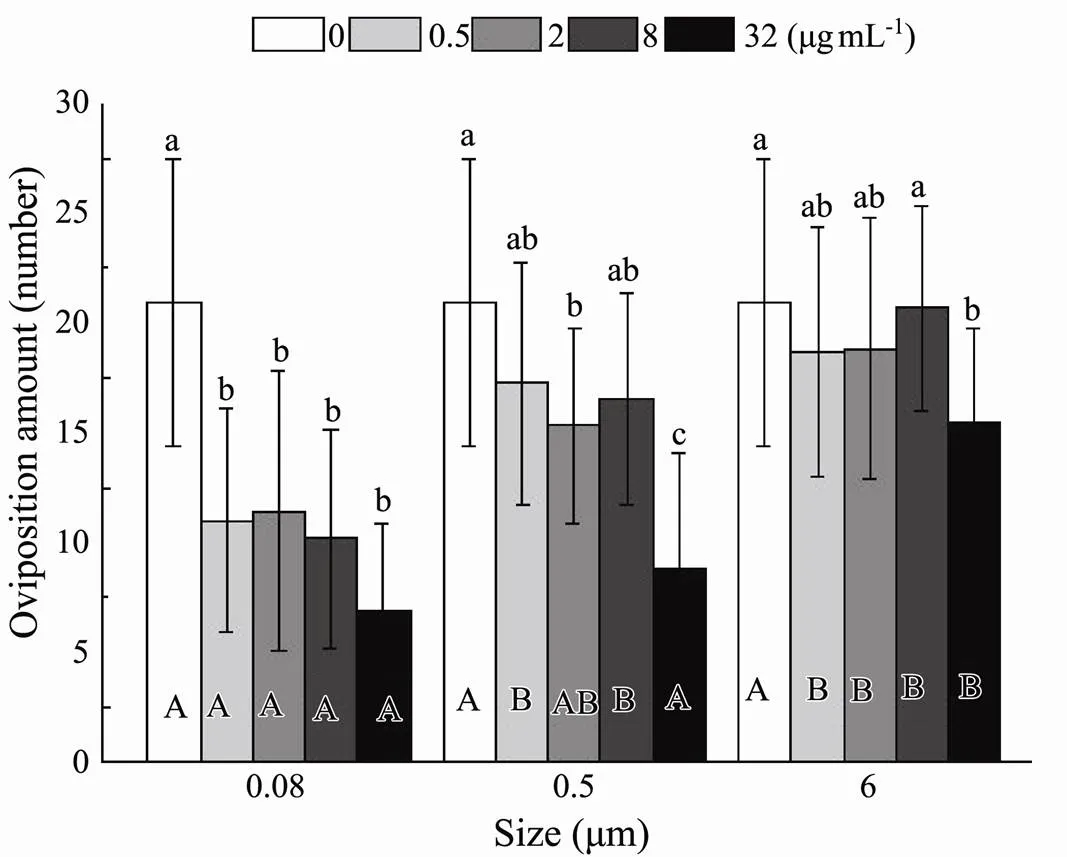
Fig.10 Same as those of Fig.4 but for the number of eggs laid byB. plicatilis.
4 Discussion
4.1 Effects of Microplastics on Individual Development
Plastic in marine, as a persistent and increasing contaminant, eventually break down into particulate pieces after undergoing solar radiation, biological degradation, or mechanical forces processes, which can inflict damage of individual growth among aquatic organisms, especially in zooplankton after ingestion (Rummel, 2017; Sun, 2019). Rotifers are filter-feeding zooplankton, and they can ingest MPs within the size of their food particles. The current study aimed to widen our understanding of the effect of MPs on a primary marine food source and a model species for ecotoxicological studies (Rico-Mar- tínez, 2016). Here, we examined the responses of the rotiferexposed to MPs at different sizes and nominal concentrations, which were similar to their feeding prey. The results showed that high nominal concentrations of small nano-plastics could negatively affect the life history parameters of.
The current study showed that microplastics surrounding rotifers suppressed growth, and this was reflected in rotifers’ bodies and lorica.Compared with the control treatment, 32µgmL−10.08μm treatment showed apparent growth inhibition. Microplastics of high nominal concentration significantly decreased body and lorica size, as measured when the spawn was first hatched, but had non-significant effects in fair-sized treatments. These results are identical to previous studies suggesting toxicity of nano- and micro-plastic act on the physical development of organisms(Besseling, 2014)and(Lei, 2018),whose body size respectively decreased about 3.1% and 4.89%. Moreover, the lorica length defined the range of particles ingested, which was attributed to a regression line=0.0896−0.033 (=0.94), which obtained between the lorica length () of theand the maximum size () of particles ingested, allowing(=93.5) to ingest granule with size under the 8.34μm (Hino and Hirano, 1980). Therefore, the microspheres’ sizes used in the current study were within the feeding range ofcultivated. Since filter feeders in the oceanic surroundings cannot successfully digest and absorb consumed microplastics, which may cause the inhibited intestine digestion and insufficient nutrition (Andrady, 2011). A widely applicable theory deciphered the dynamic energy budget framework in which the meta- bolic energy stored from comestible was used primarily for the primary functions of somatic, structural maintenance of life, and the like (Kooijman, 2001). Therefore, it was likely that energy shortage after microplastic ingestion would reduce the type of body, concretely in body length and lorica size. Insufficient energy for growth also probably arose from a drop in feeding rates due to microplastic ingestion, as found in(Ogonowski, 2016; Rist, 2017). Furthermore, inhibition in the body size may be due to the partial incorporation of microplastic into the lorica cuticle, based on the discovery about mussel that some tiny particles of polystyrene microspheres could infiltrate the structure of byssus in singles (Li, 2019).
4.2 Effects of Microplastics on Life Cycle
In the life cycle parameter determination section, we found that the time from the start of the experiment to the first spawning had been lengthened. In contrast, microplastic exposure shortened the time required for the rotifers to lay their first eggs until their last laying before death, as well as the time from the last spawning to death. Specifically, nano-plastic (0.08μm) ingested by rotifers postponed the maturity, reduced reproduction, and expedited the death process by about a third. Likewise, during the chronic toxicity of polystyrene,a decline in dry weight and a considerable loss of fecundity was observed in the freshwater benthic invertebrates microspheres (Au, 2015). Of greatest concern, however, were the phe- nomena mentioned in some studies in which short-size plastic microspheres had been found ingested in the cytoplasm by marine organisms, indicating their ability to enter multifarious cells (Browne, 2008; Von Moos, 2012). What is more, nanoparticles witha size of 0.08μm in our tests were well-known to interfere with cell metabolism and activate cytotoxicity, and may cause functional interference in specific cells, such as a finding that transfer of diminutive Carboxyl Polystyrene (CPS) latex beads to the insect endoplasmic reticulum impair the catalytic activity of CYP450 enzyme (Fröhlich, 2010). Hence, the adverse effects of small size can be interpreted as prolonged chemical reaction time in critical and biochemical reactions, because plastic microspheres can easily diffuse from milieu interieur into cells when the size is reduced. Microplastic may also damage the cytoskeleton and lead cellular deformation, which are fundamental to life. Posteriorly, the ability to filtering algae can be weakened because of the presence of polystyrene beads (Cole, 2013). Another research on four freshwater invertebrates showed that the number of 1 and 10µm particles plastic microspheres ingested byincreased steadily increased with age (Scherer, 2017). These observations indicated that several sta- ges of invertebrates development did vary in terms of energy intake. Specifically, the consumption of nanoscale and microplastic increases as the larvae mature. The possible reason may be that it is difficult for the larvae to effectively filterthat provided energy for individual growth and development from the microplastics-rich environment, leading to a delay in the emergence of the adults. More importantly, in other studies, some species had exhibited the act of giving up fecundity, which was thought to be in order to continue to survive in such harsh environmental conditions that they must rationally utilize low energy, resulting in a shortened bree- ding period (Won and Lee, 2014; Han, 2015). Thus, itisinteresting to note that it is more important to be aware of the increase in virulence, which was corresponded to the growth ofduring pre-repro- ductive, reproductive and post-reproductive periods, with life-history parameters changing 31.14%, 37.11% and 42.65%, respectively.
4.3 Effects of Microplastics on Reproduction and Spawn
Owing to reducing the energy intake and then depriving the breeding time, this brought to a usually worse state, with the cumulative number of neonates significantly reduced on nanoscale by about 70% at higher nominal concentrations treated with control offspring. Similarly, research on the progeniture index of micro- plastic about pacific oysters suggested a41% decrease in Dlarval yield born from plastic-treated parents (Sussarellu, 2016). However, the middle-large size plastic microspheres displayed a slim impact, only 87.48% of the control group (Canniff and Hoang, 2018). Microplastics tend to gather in the artificial seawater, which cause agglomeration to form, makingless likely to filter them and weaken toxicity. As a final result, including the aforementioned premature death, these poor performances will greatly reduce the natural feed intake of zooplankton, allowing the algae to obtain greater abundance. Therefore, the occurrence frequency of marine red tides may be closely related to the microplastics in seawater. Primary producers and creatures that prey on zooplankton and consumers with higher nutritional levels may also be negatively affected through the food chain (Cedervall, 2012; Mattsson, 2015). Perhaps in the future, nanoplastic-rich waters will continue to expand, because uncontrollable natural decomposition process will still occur in marine plastic debris, leaving currently damaged marine ecosystems in a state of devastation (Mattsson, 2015).
5 Conclusion
In summary, we showed here that individual size at maturity, different breeding periods, average lifetime, and generative yield ofwere on a sticky wicket during exposure to polystyrene microspheres, especially the smaller nano-sized plastics. Proceeding from the whole physiological reaction, the behavior of rotifers exposed to 0.08-μm nanoplastic at a nominal concentration of 32µgmL−1was greatly poor. At the same time, the larger microspheres slightly altered the rotifers’ performance. Another observation was that the toxicity of experimental polystyrene microspheres to life-history parameters was positively correlated with the increase in the nominal concentration of microplastics and the decrease in microplastics size. In addition, the developmental toxicity of microplastics to organisms is of concern. These experimental date complement the understanding of the interactions between microplastic and zooplankton, which are usually hard to observe, and provide a reference for future researchers.
Acknowledgements
This study was funded by research grants from the Natural Science Foundation of China (Nos. 41706142 and 619360 14), the National Modern Agricultural Industry Technology System Construction Project (No. CARS-49) and the National Key R&D Program during the 13th Five-Year Plan Period (No. 2018YFD900603). Dr. Yanming Sui is supported by a fellowship from China Scholarship Council.
Andrady, A. L., 2011. Microplastics in the marine environment., 62 (8): 1596-1605, https://doi.org/ 10.1016/j.marpolbul.2011.05.030.
Arndt, H., 1993. Rotifers as predators on components of the microbial web (bacteria, heterotrophic flagellates, ciliates)-a review., 255 (1): 231-246, https://doi.org/10. 1007/BF00025844.
Au, S. Y., Bruce, T. F., Bridges, W. C., and Klaine, S. J., 2015. Responses ofto acute and chronic microplastic exposures., 34 (11): 2564-2572, https://doi.org/10.1002/etc.3093.
Besseling, E., Wang, B., Lürling, M., and Koelmans, A. A., 2014. Nanoplastic affects growth ofand reproduction of., 48 (20): 12336-12343, https://doi.org/10.1021/es503001d.
Besseling, E., Wegner, A., Foekema, E. M., Van Den Heuvel- Greve, M. J., and Koelmans, A. A., 2013. Effects of micro- plastic on fitness and PCB bioaccumulation by the lugworm(L.)., 47 (1): 593-600, https://doi.org/10.1021/es302763x.
Browne, M. A., Dissanayake, A., Galloway, T. S., Lowe, D. M., and Thompson, R. C., 2008. Ingested microscopic plastic translocates to the circulatory system of the mussel,(L.)., 42 (13): 5026-5031, https://doi.org/10.1021/es800249a.
Canesi, L., Ciacci, C., Bergami, E., Monopoli, M. P., Dawson, K. A., Papa, S.,., 2015. Evidence for immunomodulation and apoptotic processes induced by cationic polystyrene na- noparticles in the hemocytes of the marine bivalve., 111: 34-40, https://doi.org/ 10.1016/j.marenvres.2015.06.008.
Canniff, P. M., and Hoang, T. C., 2018. Microplastic ingestion byand its enhancement on algal growth., 633: 500-507, https://doi. org/10.1016/j.scitotenv.2018.03.176.
Cedervall, T., Hansson, L. A., Lard, M., Frohm, B., and Linse, S., 2012. Food chain transport of nanoparticles affects behaviour and fat metabolism in fish., 7 (2): e32254- e32254, https://doi.org/10.1371/journal.pone.0032254.
Cole, M., Lindeque, P., Fileman, E., Halsband, C., Goodhead, R., Moger, J.,., 2013. Microplastic ingestion by zooplankton., 47 (12): 6646-6655, https://doi.org/10.1021/es400663f.
Cui, R., Kim, S. W., and An, Y. J., 2017. Polystyrene nanoplastics inhibit reproduction and induce abnormal embryonic development in the freshwater crustacean., 7 (1): 12095, https://doi.org/10.1038/s415 98-017-12299-2.
Da Costa, J. P., Santos, P. S. M., Duarte, A. C., and Rocha-San- tos, T., 2016. (Nano)plastics in the environment-sources, fates and effects., 566-567: 15-26, https://doi.org/10.1016/j.scitotenv.2016.05.041.
Dahms, H. U., Hagiwara, A., and Lee, J. S., 2011. Ecotoxicology, ecophysiology, and mechanistic studies with rotifers., 101 (1): 1-12, https://doi.org/10.1016/j. aquatox.2010.09.006.
Deng, Y., Zhang, Y., Lemos, B., and Ren, H., 2017. Tissue accumulation of microplastics in mice and biomarker responses suggest widespread health risks of exposure., 7 (1): 46687, https://doi.org/10.1038/srep46687.
Desforges, J. P. W., Galbraith, M., and Ross, P. S., 2015. Ingestion of microplastics by zooplankton in the Northeast Pacific Ocean., 69 (3): 320-330, https://doi.org/10.1007/s00244-015- 0172-5.
Devetter, M., and Seďa, J., 2006. Regulation of rotifer community by predation of(Copepoda) in the Rimov Reservoir in spring., 91 (1): 101-112, https://doi.org/10.1002/iroh.200510810.
Faggio, C., Tsarpali, V., and Dailianis, S., 2018. Mussel digestive gland as a model tissue for assessing xenobiotics: An overview., 636: 220-229, https://doi.org/10.1016/j.scitotenv.2018.04.264.
Farrell, P., and Nelson, K., 2013. Trophic level transfer of microplastic:(L.) to(L.)., 177: 1-3, https://doi.org/10.1016/j.envpol.2013.01.046.
Foekema, E. M., De Gruijter, C., Mergia, M. T., Van Franeker, J. A., Murk, A. J., and Koelmans, A. A., 2013. Plastic in North Sea fish., 47 (15): 8818- 8824, https://doi.org/10.1021/es400931b.
Fröhlich, E., Kueznik, T., Samberger, C., Roblegg, E., Wrighton, C., and Pieber, T. R., 2010. Size-dependent effects of nanoparticles on the activity of cytochrome P450 isoenzymes., 242 (3): 326-332, https://doi.org/10.1016/j.taap.2009.11.002.
Gaspar, T., Chi, R., Parrow, M., and Ringwood, A., 2018. Cellular bioreactivity of micro- and nano-plastic particles in oysters., 5: 345, https://doi.org/10. 3389/fmars.2018.00345.
Geyer, R., Jambeck, J. R., and Law, K. L., 2017. Production, use, and fate of all plastics ever made., 3 (7): e1700782-e1700782, https://doi.org/10.1126/sciadv.1700782.
Graham, P., Palazzo, L., Andrea De Lucia, G., Telfer, T. C., Baroli, M., and Carboni, S., 2019. Microplastics uptake and egestion dynamics in pacific oysters,(Thun- berg, 1793), under controlled conditions., 252: 742-748, https://doi.org/10.1016/j.envpol.2019.06. 002.
Gray, A. D., and Weinstein, J. E., 2017. Size- and shape-depen- dent effects of microplastic particles on adult daggerblade grass shrimp ()., 36 (11): 3074-3080, https://doi.org/10.10 02/etc.3881.
Guzzetti, E., Sureda, A., Tejada, S., and Faggio, C., 2018. Microplastic in marine organism: Environmental and toxicological effects., 64: 164-171, https://doi.org/10.1016/j.etap.2018.10.009.
Han, J., Won, E. J., Lee, M. C., Seo, J. S., Lee, S. J., and Lee, J. S., 2015. Developmental retardation, reduced fecundity, and modulated expression of the defensome in the intertidal copepodexposed to BDE-47 and PFOS., 165: 136-143, https://doi.org/10.1016/j. aquatox. 2015.05.022.
Hino, A., and Hirano, R., 1980. Relationship between body size of the rotiferand the maximum size of particles ingested., 46 (7): 1217-1222, https://doi.org/10.2331/ suisan.46.1217.
Hossain, M. S., Rahman, M. S., Uddin, M. N., Sharifuzzaman, S. M., Chowdhury, S. R., Sarker, S.,., 2020. Microplastic contamination in penaeid shrimp from the northern Bay of Bengal., 238: 124688, https://doi.org/10.1016/j. chemosphere.2019.124688.
Jian, M., Zhang, Y., Yang, W., Zhou, L., Liu, S., and Xu, E. G., 2020. Occurrence and distribution of microplastics in China’s largest freshwater lake system., 261: 128186, https://doi.org/10.1016/j.chemosphere.2020.128186.
Koelmans, A. A., Besseling, E., and Shim, W. J., 2015. Nanoplastics in the aquatic environment. Critical review. In:. Bergmann, M., and Gutow, L., eds., Springer International Publishing, Cham, 325-340, https://doi. org/10.1007/978-3-319-16510-3_12.
Kooijman, S. A., 2001. Quantitative aspects of metabolic organization: A discussion of concepts., 356 (1407): 331-349, https://doi.org/10.1098/rstb. 2000.0771.
Lee, K. W., Shim, W. J., Kwon, O. Y., and Kang, J. H., 2013. Size-dependent effects of micro polystyrene particles in the marine copepod., 47 (19): 11278-11283, https://doi.org/10.1021/ es401932b.
Lei, L., Wu, S., Lu, S., Liu, M., Song, Y., Fu, Z.,., 2018. Microplastic particles cause intestinal damage and other adverse effects in zebrafishand nematode., 619-620: 1-8, https://doi. org/10.1016/ j.scitotenv.2017.11.103.
Li, Q., Sun, C., Wang, Y., Cai, H., Li, L., Li, J.,., 2019. Fusion of microplastics into the., 252: 420-426, https://doi.org/10.1016/j.env pol.2019.05.093.
Luna-Andrade, A., Aguilar-Duran, R., Nandini, S., and Sarma, S. S. S., 2002. Combined effects of copper and microalgal () concentrations on the population growth ofMüller (Rotifera)., 141 (1): 143-153, https://doi.org/10.1023/A:10213 46512560.
Mattsson, K., Ekvall, M. T., Hansson, L. A., Linse, S., Malmendal, A., and Cedervall, T., 2015. Altered behavior, physiology, and metabolism in fish exposed to polystyrene nano- particles., 49 (1): 553- 561, https://doi.org/10.1021/es5053655.
Mattsson, K., Hansson, L. A., and Cedervall, T., 2015. Nano- plastics in the aquatic environment., 17 (10): 1712-1721, https://doi.org/10. 1039/c5em00227c.
Murray, F., and Cowie, P. R., 2011. Plastic contamination in the decapod crustacean(Linnaeus, 1758)., 62 (6): 1207-1217, https://doi.org/ 10.1016/j.marpolbul.2011.03.032.
Ogonowski, M., Schür, C., Jarsén, Å., and Gorokhova, E., 2016. The effects of natural and anthropogenic microparticles on individual fitness in., 11 (5): e0155063, https://doi.org/10.1371/journal.pone.0155063.
Ortaz, M., González, E., and Peñaherrera, C., 2006. Depredación de peces sobre el zooplancton entres embalses neotro- picales con distintos estados tróficos., 31 (7): 517-524.
PlasticsEurope, 2020. Plastics–The facts 2020: An analysis of european plastic production, demand and waste data. PlasticsEurope, https://www.plasticseurope.org/en.
Preston, B. L., and Snell, T. W., 2001. Full life-cycle toxicity assessment using rotifer resting egg production: Implications for ecological risk assessment., 114 (3): 399-406, https://doi.org/10.1016/S0269-7491(00)00232- 3.
Preston, B. L., Snell, T. W., and Kneisel, R., 1999. UV-B exposure increases acute toxicity of pentachlorophenol and mercury to the rotifer., 106 (1): 23-31, https://doi.org/10.1016/S0269-7491 (99)00065-2.
Radix, P., Severin, G., Schramm, K. W., and Kettrup, A., 2002. Reproduction disturbances of(Rotifer) for the screening of environmental endocrine disrupters., 47 (10): 1097-1101, https://doi.org/10.1016/S0 045- 6535(01)00335-6.
Rico-Martínez, R., Arzate-Cárdenas, M. A., Robles-Vargas, D., Pérez-Legaspi, I. A., Jesús, A. F., and Santos-Medrano, G. E., 2016. Rotifers as models in toxicity screening of chemicals and environmental samples. In:–. Larramendy, M. L., and Soloneski, S., eds., IntechOpen, London, 57-99, https://doi.org/ 10.5772/61771.
Rist, S., Baun, A., and Hartmann, N. B., 2017. Ingestion of micro- and nanoplastics in–quantification of body burdens and assessment of feeding rates and reproduction., 228: 398-407, https://doi.org/ 10.1016/j.envpol.2017.05.048.
Rummel, C. D., Jahnke, A., Gorokhova, E., Kühnel, D., and Schmitt-Jansen, M., 2017. Impacts of biofilm formation on the fate and potential effects of microplastic in the aquatic environment., 4 (7): 258-267, https://doi.org/10.1021/acs.estlett.7b00164.
Sarma, S. S. S., and Rao, T. R., 1991. The combined effects of food and temperature on the life history parameters ofmuller(Rotifera)., 76 (2): 225-239, https://doi.org/10.1002/iroh.19910760207.
Savoca, S., Capillo, G., Mancuso, M., Bottari, T., Crupi, R., Branca, C.,., 2019. Microplastics occurrence in the Tyrrhenian waters and in the gastrointestinal tract of two congener species of seabreams., 67: 35-41, https://doi.org/10.1016/j.etap.2019.01. 011.
Scheda, S. M., and Cowell, B. C., 1988. Rotifer grazers and phytoplankton: Seasonal experiments on natural communities., 114 (1): 31-44.
Scherer, C., Brennholt, N., Reifferscheid, G., and Wagner, M., 2017. Feeding type and development drive the ingestion of microplastics by freshwater invertebrates., 7 (1): 17006, https://doi.org/10.1038/s41598-017-17191-7.
Sjollema, S. B., Redondo-Hasselerharm, P., Leslie, H. A., Kraak, M. H. S., and Vethaak, A. D., 2016. Do plastic particles affect microalgal photosynthesis and growth?, 170: 259-261, https://doi.org/10.1016/j.aquatox.2015.12.002.
Snell, T. W., and Janssen, C. R., 1995. Rotifers in ecotoxicology: A review., 313 (1): 231-247, https://doi.org/10. 1007/BF00025956.
Snell, T. W., and Moffat, B. D., 1992. A 2-d life cycle test with the rotifer., 11 (9): 1249-1257, https://doi.org/10.1897/ 1552-8618(1992)11[1249:ADLCTW]2.0.CO;2.
Sun, Y., Xu, W., Gu, Q., Chen, Y., Zhou, Q., Zhang, L.,., 2019. Small-sized microplastics negatively affect rotifers: Changes in the key life-history traits and rotifer-phaeocystis population dynamics., 53 (15): 9241-9251, https://doi.org/10.1021/acs.est.9b02893.
Sundbæk, K. B., Koch, I. D. W., Villaro, C. G., Rasmussen, N. S., Holdt, S. L., and Hartmann, N. B., 2018. Sorption of fluorescent polystyrene microplastic particles to edible seaweed., 30 (5): 2923-2927, https://doi.org/10.1007/s10811-018-1472-8.
Sussarellu, R., Suquet, M., Thomas, Y., Lambert, C., Fabioux, C., Pernet, M. E. J.,., 2016. Oyster reproduction is affected by exposure to polystyrene microplastics., 113 (9): 2430-2435, https://doi.org/10.1073/pnas.15 19019113.
Thompson, R. C., Moore, C. J., Saal, F. S. V., and Swan, S. H., 2009. Plastics, the environment and human health: Current consensus and future trends., 364 (1526): 2153- 2166, https://doi.org/10.1098/rstb.2009.0053.
Thompson, R. C., Swan, S. H., Moore, C. J., and Vom Saal, F. S., 2009. Our plastic age., 364 (1526): 1973-1976, https://doi.org/10.1098/rstb.2009.0054.
Verla, A. W., Enyoh, C. E., Verla, E. N., and Nwarnorh, K. O., 2019. Microplastic-toxic chemical interaction: A review study on quantified levels, mechanism and implication., 1 (11): 1400, https://doi.org/10.20944/preprints201 908.0260.v1.
Von Moos, N., Burkhardt-Holm, P., and Köhler, A., 2012. Uptake and effects of microplastics on cells and tissue of the blue musselL. after an experimental exposure., 46 (20): 11327-11335, https://doi.org/10.1021/es302332w.
Wagner, M., Engwall, M., and Hollert, H., 2014. Editorial: (Micro) plastics and the environment., 26 (1): 16, https://doi.org/10.1186/s12302-014-0016- 3.
Waller, C. L., Griffiths, H. J., Waluda, C. M., Thorpe, S. E., Loaiza, I., Moreno, B.,., 2017. Microplastics in the Antarctic marine system: An emerging area of research., 598: 220-227, https://doi.org/10. 1016/j.scitotenv.2017.03.283.
Wang, J. J., 1961.. Science Press, Beijing, 38-41, http://ir.ihb.ac.cn/handle/342005/ 10660 (in Chinese).
Wang, X., and Mai, K., 2005. A successful microbound diet for the larval culture of Chinese shrimp., 4 (3): 267-271, https://doi.org/10.1007/s11802-005-0046-y.
Watts, A. J. R., Urbina, M. A., Goodhead, R., Moger, J., Lewis, C., and Galloway, T. S., 2016. Effect of microplastic on the gills of the shore crab., 50 (10): 5364-5369, https://doi.org/10. 1021/acs.est.6b01187.
Won, E. J., and Lee, J. S., 2014. Gamma radiation induces growth retardation, impaired egg production, and oxidative stress in the marine copepod., 150: 17-26, https://doi.org/10.1016/j.aquatox.2014. 02.010.
Xie, Z., Xiao, H., Tang, X., and Cai, H., 2009. Experimental stu- dy on the interspecific interactions between the two bloom- forming algal species and the rotifer., 8 (2): 203-208, https:// doi.org/10.1007/s11802-009-0203-9.
Yoshimura, K., Hagiwara, A., Yoshimatsu, T., and Kitajima, C., 1996. Culture technology of marine rotifers and the implications for intensive culture of marine fish in Japan., 47 (2): 217-222, https://doi.org/10.1071/ mf9960217.
Yu, X., Peng, J., Wang, J., Wang, K., and Bao, S., 2016. Occurrence of microplastics in the beach sand of the Chinese inner sea: The Bohai Sea., 214: 722-730, https://doi.org/10.1016/j.envpol.2016.04.080.
Yúfera, M., 2001. Studies on(Rotifera): An example of interaction between fundamental and applied research., 446 (1): 383-392, https://doi.org/10.1007/978- 94-010-0756-6_49.
Zhang, L., Niu, J., and Wang, Y., 2016. Full life-cycle toxicity assessment on triclosan using rotifer., 127: 30-35, https://doi.org/10.1016/j.ecoenv.2015.12.043.
January 29, 2021;
May 24, 2021;
June 12, 2021
© Ocean University of China, Science Press and Springer-Verlag GmbH Germany 2022
#The two authors contributed equally to this work.
. E-mail: lvlinlan77@126.com
E-mail:ycitdong@126.com
(Edited by Ji Dechun)
杂志排行
Journal of Ocean University of China的其它文章
- Probabilistic Methods for Reliability Assessment of Floating Structures Based on Extreme Wave Simulation
- Structure of the Potential Vorticity of an Explosive Cyclone over the Eastern Asian Region in Late November 2013
- Enzyme-Induced Carbonate Precipitation for the Protection of Earthen Dikes and Embankments Under Surface Runoff: Laboratory Investigations
- Isolation, Identification, and Quantitative Determination of Saponin in Apostichopus japonicus by HPLC-DAD
- Transcriptome Analysis of Pacific White Shrimp (Litopenaeus vannamei) Under Prolonged High-Salinity Stress
- Evaluation of the Bioavailability of Metals in Sediment from the Southern Coastal Wetland of the Qiantang Estuary by Using Diffusive Gradients in Thin Films Technique
