Evaluation of the Bioavailability of Metals in Sediment from the Southern Coastal Wetland of the Qiantang Estuary by Using Diffusive Gradients in Thin Films Technique
2022-02-24FENGWeihuaWANGZhifuZHUWenzhuoZHENGFangqinZHANGDongrongandXUHengtao
FENG Weihua, WANG Zhifu, ZHU Wenzhuo, ZHENG Fangqin, ZHANG Dongrong, and XU Hengtao, *
Evaluation of the Bioavailability of Metals in Sediment from the Southern Coastal Wetland of the Qiantang Estuary by Using Diffusive Gradients in Thin Films Technique
FENG Weihua1), WANG Zhifu1), ZHU Wenzhuo2), ZHENG Fangqin1), ZHANG Dongrong1), and XU Hengtao1), *
1) Key Laboratory of Engineering Oceanography, Second Institute of Oceanography, MNR, Hangzhou 310012, China 2) College of Marine Science & Technology, Zhejiang Ocean University, Zhoushan 316022, China
Metal pollution has become an major issue governing the wetland ecosystem health. The southern coastal wetland of the Qiangtang Estuary are facing unusual perturbation due to rapid development along the embayment in recent decades. This study evaluated the bioavailability of metals (Cu, Pb, Cd, Cr and Zn) in the sediment of the southern coastal wetland of the Qiangtang Estuary using diffusive gradients in thin films (DGT) techniques and compared with several methods based on total metal content. The results showed that the contents of Cr, Pb, Cd and Cu in sediment, as detected using DGT, were considerably correlated with the exchangeable fraction and the content inroots, while a weak correlation was observed for Zn. Therefore, DGT analysis could be used to evaluate the bioavailability and potential risk of Cr, Cd, Pb and Cu for. Quantitative indices, such asconcentration, bioaccumulation in, geoaccumulation index (geo) and potential ecological risk index (), revealed that Cd was a major potential ecological risk factor along the southern coast wetland of the Qiantang Estuary, especially in the upstream region, which is potentially more vulnerable to the anthropogenic pollution.
diffusive gradients in thin films; estuarine wetland; metal; bioavailability
1 Introduction
Estuarine wetlands are an effective sink of metal pollutants and play an increasingly important role in reducing the increasing pollution load of estuarine and offshore waters (Feng., 1998; Nedell and Taffaelli, 1999; Rees., 1999). However, under certain physical, che- mical and biological actions, metal pollutants accumulated on beaches can be released to the overlying water through exchange between sediment and water, which makes the wetland of estuaries very important secondary pollution sources (Gambrell., 1991; Riedel., 1999). The migration and transformation of metals are affected by the pH value, redox potential (Eh), acidic volatile sulfide (AVS) content, organic matter (OM) content, dissolution of iron and manganese oxides and other factors of sediments (Olaniran., 2013). The changes of these biogeochemical environmental factors in sediments can affect the bioavailability of metals (Shaifullah., 2008), thus affecting the survival of plants and animals in sediments. Previous studies (Bi., 2003;Zhang., 2019) have found that the total metal content can be used to evaluate the overall pollution level of sediments, but it is difficult to assess the bioavailability of heavy metals. The analysis of the occurrence form and effective state of heavy metals can be used to evaluate the risk of metal pollution in sediment environment. Chemical fractionation can distinguish metals of natural origin from those derived from anthropogenic sources. Metal fractionation is essential for potential toxicity and fluidity (Maiz., 2000). To this end, sequential extraction me- thod was used to provide information on the strength and manner of metal binding to sediments (Tessier., 1979). Metals in exchangeable fraction are considered to be the most sensitive to changes in environmental conditions due to their weak binding to sediments; metals in this fraction are the most likely to migrate and transform; and are most easily absorbed by plants (Pardo., 1990). The higher the proportion of metals, the more liquid they are and the greater the risk they pose to the environment (Islam., 2015). Metals from anthropogenic sources are found mainly in the most unstable sediment components, which are susceptible to minor changes in environmental conditions (Alves., 2007). However, the process of sequential extraction method is too tedious and usually not sensitive enough to reflect the contamination level of metals in sediments (García- Hernández., 2005; Baptista., 2014; Chen., 2019). Therefore, it is necessary to explore a more effective method to evaluate the bioavailability of heavy metals in sediments, which is of great theoretical and practical significance to evaluate the ecological effects of metal pollutants on estuarine wetlands, coastal estuarine wetlands and maintain the health of estuarine ecosystems.
The diffusive gradients in thin films (DGT) is a useful method for predicting the bioavailability of metals in soil (Zhang and Davison, 1998; Harper, 2000; Ernstherger., 2005; Wang., 2012; Xu., 2013; Yin., 2014; Song., 2018). This technique takes into account the migration of metals between solid and liquid phases, which can be used to simulate the absorption process of metal by plant roots (Warnken., 2008; Williams., 2009). Therefore, DGT has an excellent ability to predict the bioavailability of metals in soil compared with the methods based on total content and chemical fraction. Although DGT is a promising method for evaluating the bioavailability of metals, it is ineffective in accommodate some factors that affect metal uptake in plants, such as the effect of protective/competitive ions, which may lead to inaccurate results (He., 2009; Richau., 2009). The metal content in plants is controlled by the activity of free ions in the pore water of the soil around the roots (Gaw., 2008; Song., 2018). The concentration of free ions at the root-pore water interface is often very high, which results in the transfer of metal complexes from soil to plant (Zhang., 2001). For estuarine wetland sediment, which is affected by tidal dynamics and has a high moisture content, the process of metal uptake by plants is usually more complex than that of soil on land. It is worth studying whether DGT technology can be used to predict the bio-availability of metals in estuary sediments.
The coastal wetland of the Qiangtang Estuary, with a vast area, not only is a place for migratory birds to replenish energy in the Asia-Pacific region and to survive the winter, but also breeds extremely rich ecological resources (Liyan., 2008).which grows in the middle and high tide area of the beach, is characterized by strong and rapid growth, is the main vegetation species of coastal tidal beach wetlands in Qiangtang Estuary, and has the functions of eliminating waves, protecting beaches and promoting silt (Ren., 2014). However, with the development of the urban eco- nomy and the acceleration of urbanization around the Qiangtang Estuary in recent years, the development and utilization of tidal flats have also exhibited an extraordinary development. At present, many wetlands on the southern coast of the Qiangtang Estuary are being used for the development and construction of ports, industrial parks, sewage outlets,. Furthermore, due to natural erosion, the area of natural beaches is gradually being reduced, and the ecosystem of the area is being affected to varying degrees (Liyan., 2008).
The purpose of this study was to test the applicability of the DGT technology to assess the bioavailability of metals in estuarine wetland sediment. Four-dominated wetland regions contaminated with metals were selected. The distribution of Cr, Cd, Zn, Pb and Cu in sediments androots was investigated. The DGT method was compared with the traditional methods of total metal content and sequential extraction. Based on these data, we evaluated the bioavailability of metals in the sediments from this estuarine wetlands of the Qiangtang Estuary.
2 Materials and Methods
2.1 Study Area and Sampling Sites
The study area is a large coastal wetland located at the southern coast of the Qiangtang Estuary in China (Fig.1). Four sampling regions, whereplants are large and exhibit consistent growth, were selected along the southern coast of the Qiangtang Estuary, and ten sampling sites were arranged according to the distribution of coastal industrial areas and large sewage outlets. Region A (S1), with less macrophyte vegetation, is located downstream of the confluence of the Cao’e River and Qiantang River, where it is mainly affected by the nearby Shangyu industrial park (SYI) and the sewage outlet upstream of the Cao’e River. The B region (S2, S3 and S4), with,Loisel and shellfish farming, is located 20km downstream of the A region and near Hangzhou Bay national wetland (HZBNW), and the sewage deep treatment project (SDTP), which is used to treat the primary treated sewage in the wetland. In the C region (S5, S6 and S7), there are large areas of macrophyte vegetation and tidal flat aquaculture; this region is located downstream of the Hangzhou Bay new zone sewage treatment plant sewage outlet (HZBSO). The D re- gion (S8, S9 and S10), located 30km upstream of Ningbo port which is one of the largest ports in China, is largely affected by many small outlets of industrial factories along the coast (Table 1).
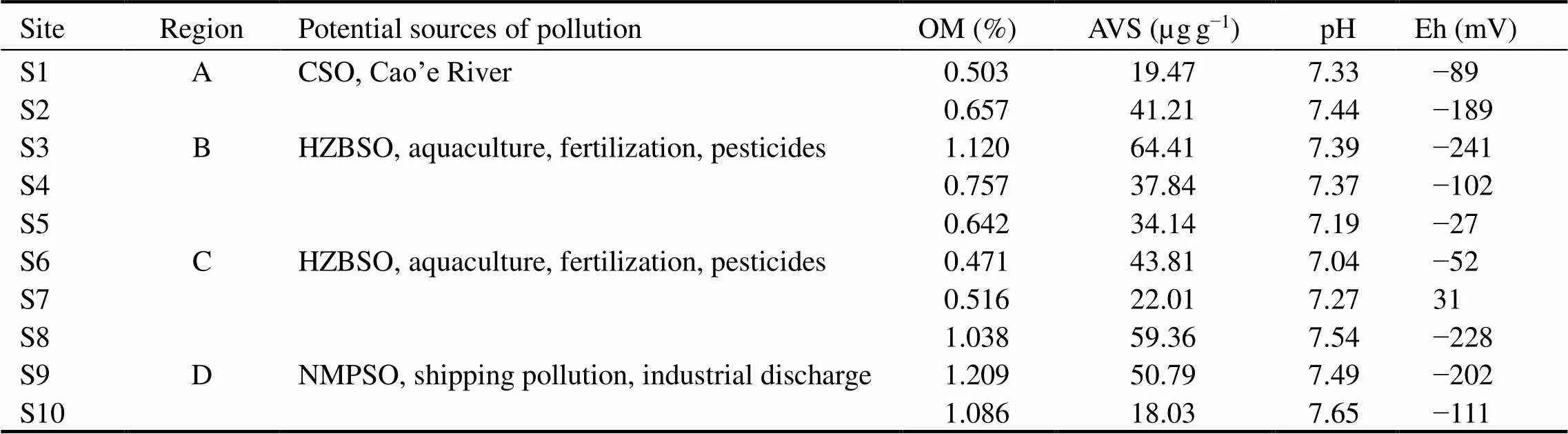
Table 1 The chemical properties in sediment collected from ten sampling sites within four regions along the southern coast wetland of the Qiantang Estuary
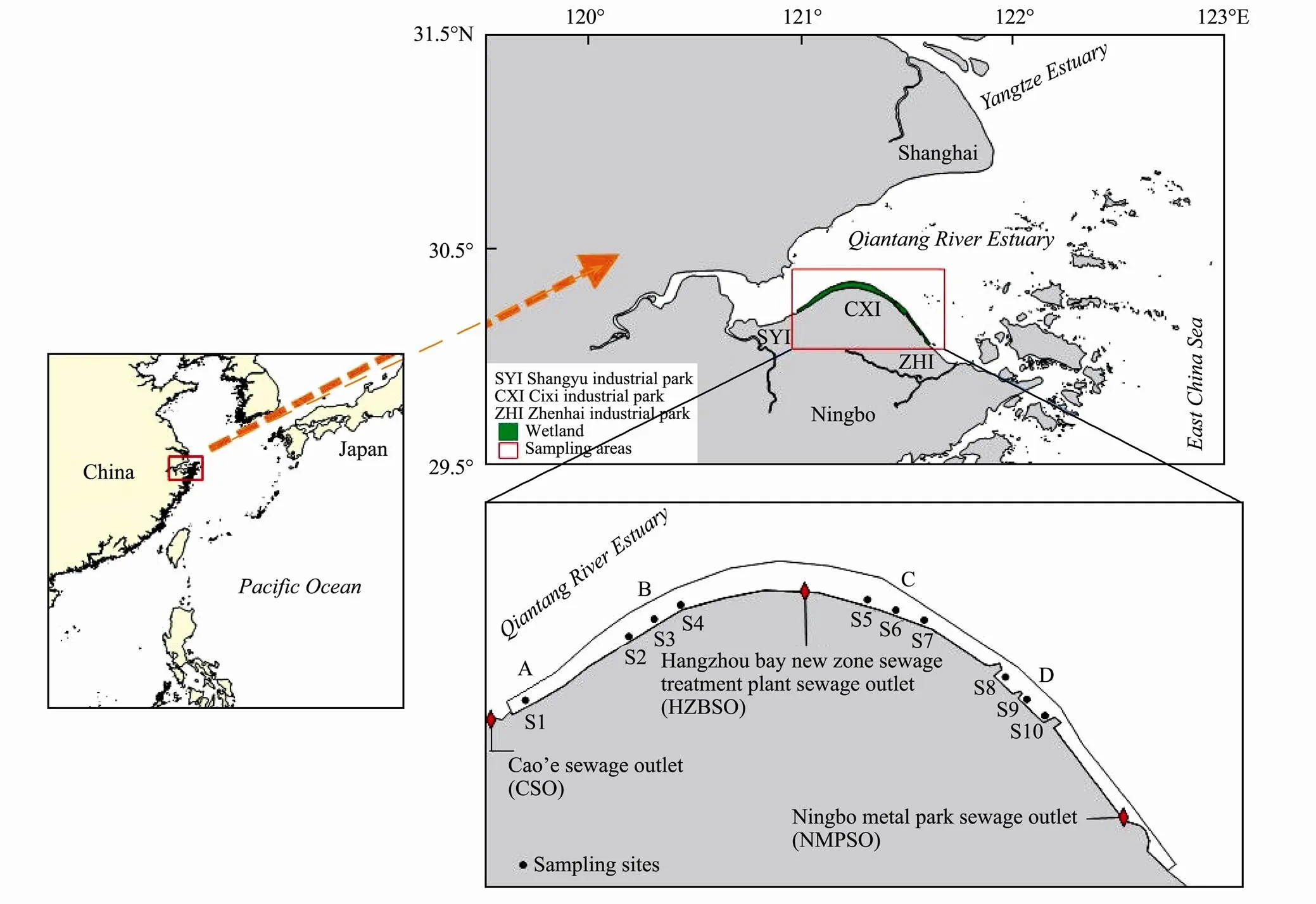
Fig.1 Map of the study area and sampling sites along the southern coast wetland of the Qiantang Estuary.
2.2 Sampling and Sample Pretreatment
In November 2019,samples were collected from each site. The belowground plant issue was collected and cleaned using fresh water. At each plant sampling site, approximately 500g of sediment was collected at a depth of 30cm near the root of thesample. Sediment and plant samples were collected in washed plastic boxes, transported to the lab for pretreatment. The samples were freeze-drying, ground into a powder using a grinder. These subsamples were stored in a drier until analyzed. The other portions of the wet sediment samples were stored at 4℃ until analyzed.
2.3 Sample Digestion and Metal Quantification Methods
Metal analysis of plant samples was carried out according to the method described by the specification for marine monitoring (GB17378, SOA, 2007). Approximately 0.4g of dry powder sample was mixed with 9mL 98% nitric acid and 2mL 30% hydrogen peroxide in a polytetrafluoroethylene vessel and let to stand for 0.5–1h at room temperature for preoxidation, then digested in a microwave digestion system (Anton Paar, Austria). Instrument parameter for microwave digester were: the first step is to take 10min to heat up to 120℃ and keep it for 5min, the second step is to take 10min to heat up to 175℃ and keep it for 25min. After digestion, the sample was transferred to a heater and heated until the residual acid was reduced to 0.5–1mL at 120℃. The samples were diluted to 25mL with Milli-Q water, then heavy metals were determined by atomic absorption spectrometry (AAS, PE 900T, US). The certified reference material (, GBW (E) 100395, Chinese Academy of Sciences) were analyzed in sync with the sample to verify the method accuracy. The recovery percent rang- ed from 85% to 112%.
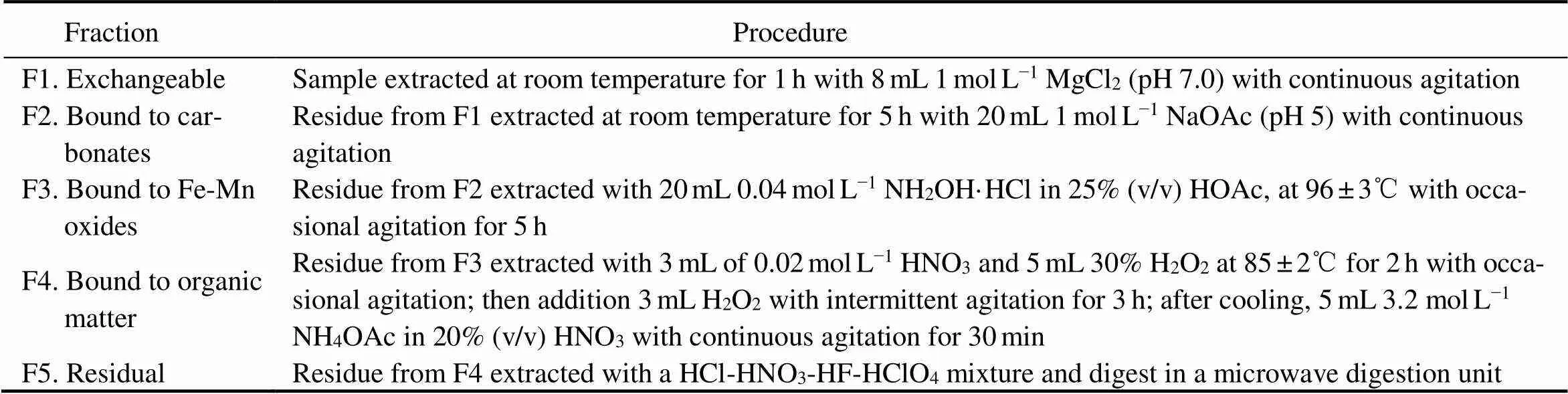
Table 2 The metal sequential extraction procedures for the sediment samples
The chemical partitioning of heavy metals in sediment samples were conducted following the method described by Tessier. (1979) and Jensen. (1992). The detailed results of the sediment geochemical fractionation procedure is presented in Supplementary materials (Table 2). The reference material (Offshore Marine Sediment, GBW07314, Chinese Academy of Sciences) was analyzed to determine the accuracy of the digestion of the F5 fraction, and the recovery was within 88%–118% for the heavy metals.
2.4 DGT Analysis
The DGT device (with an open window diameter of 20mm, Esaysensor, China) contained three layers membranes from bottom to top, which are respectively ZrO- Chelex binding gel layer, polyacrylamide diffusive gel layer and polyvinylidene fluoride filter membrane. The device was submerged in Milli-Q water and bubbled with nitrogen before use. The wet sediment sample was filled into the DGT-window, then transferred to semiclosed bag with a small amount of Milli-Q water for 24h at 20℃. After that, the DGT was rinsed with Milli-Q water and then disassembled. The ZrO-Chelex binding gel film was placed in tube with 1.8mL of 1molL−1nitric acid for 24 h to extract heavy metals. The extracting solution was analyzed by AAS. Detailed operational procedures were carried out according to the procedure described by Song. (2018).
The metal mass () accumulated by the gel layer during deployment can be calculated by Eq. (1) (Amato., 2016; Song., 2018):
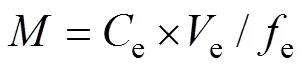
whereeis the concentration of metal studied in the extracts (µgL−1);eis the elution efficiency (0.8); andeis the volume of the diluted extracted nitric acid solution (mL).
According to, the metal concentration (DGT) measured by the DGT can be calculated by the following Eq. (2) (Costello., 2012; Amato., 2016):
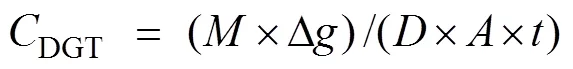
where Δis the aggregate thickness of three layer membranes (0.88cm) andis the diffusion coefficient of heavy metal in the diffusion layer (cm2s−1), which is influenced by temperature (Luo., 2010). At 20℃, the(×10−6cm2s−1) of Cu, Pb, Cd, Cr and Zn were 5.74, 7.32, 5.55, 6.92, and 6.00, respectively.is the deployment time (s) of the DGT, andis the DGT window area (cm2).
2.5 Analytical Methods for Chemical Parameters
The AVS contents of the sediment samples were determined according to the method described by Lin. (1997). Approximately 3g of wet sediment sample was subjected to a cold acid distillation with 6molL−1hydrochloric acid solution and the released hydrogen sulfide was trapped in 0.5molL−1sodium hydroxide with nitrogen gas as the carrier gas. Then, the AVS content was de- termined using UV/VIS spectrophotometer (UNICO, China). The organic matter (OM) in the sediment was mea- sured according to the method described by Jensen. (1992), which was the weight loss of burning at 550℃ for 4h. The pH and oxidation reduction potential (Eh) of sediments were measured by pH electrode and ORP electrode, respectively.
2.6 Statistical Analysis
The spatial differences were analyzed by one-way ANOVA, followed by pairwise comparisons using LSD’s post hoc tests using IBM-SPSS 24.0 software. The-tests (<0.05) was carried out to detect the differences between heavy metal concentrations in this study area and those in other areas. The correlations between the heavy metal contents of plants and those extracted by DGT technique were analyzed using Pearson’s rank correlation. If<0.05, a significant differences is considered. Origin 2018 software was used to plot the data.
2.7 Pollution Assessment
2.7.1 Geo-accumulation index (geo)
The geoaccumulation index (geo) was developed by Müller (1979) and has been widely applied to the assessment of metal contamination in sediments and soil (Bermejo., 2003). To quantify the degree of metal pollution in the sediment of tidal flats along the southern coast of Hangzhou Bay,geowas calculated according to Müller (1979) using Eq. (3).
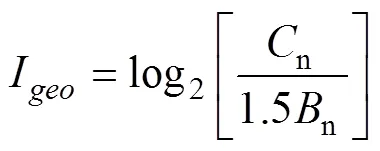
wherenis the concentration of the examined metal in the sediment,nis the geochemical background value of element n and 1.5 is a factor used to minimize the possible variations due to lithogenic variations. Since the sedi- ments formed by different sedimentary processes have different particle size and mineral compositions, the metal pollution information obtained by selecting ordinary shale as the background does not reflect the actual pollution status. Therefore, the metal background values in sediment from the Yellow Sea and East China Sea were selected to evaluate the metal contamination of sediments in this study.
geowas graded as follows: I (uncontaminated),geo<0; II (light), 0≤geo<1; III (moderately contaminated), 1≤geo<2; IV (moderately to strongly contaminated), 2≤geo<3; V (strongly contaminated), 3≤geo<4; VI (strongly to extremely strongly contaminated), 4≤geo<5; VII (extremely strongly contaminated), 5≤geo.
2.7.2 Ecological risk assessment
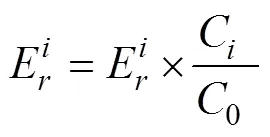
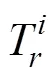
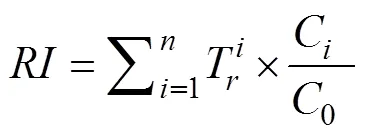
Eq. (5) was used to calculate the risk index () of sampling sites, which is the comprehensive potential ecological risk index of the metals.
3 Results and Discussion
3.1 Pollution Assessment Based on Total Metal Content in Sediment
The metal concentrations in sediments nearroots along the southern coast wetland of the Qiangtang Estuary are shown in Table 3. There is a significant difference (<0.05) among the sampling regions. The largest contents of Cu, Cd and Zn appeared in the B region, while the lowest values appeared in the D region (for Cu and Cd) and the A region (for Zn). In contrast, the Pb and Cr concentrations in the D region were significantly higher than those in the other regions. These sampling regions are located near some large industrial parks and are mainly influenced by coastal sewage discharge. For the B region, the higher Cu, Cd and Zn may be attributed to deep sewage treatment projects, fertilization and pesticides from nearby farmland. In addition, the D region may be mainly affected by wastewater containing Pb and Cr from Ningbo port, one of the major ports along China’s coast, which is used for the storage and transportation of iron ore, crude oil, liquid chemical, coal and so on.
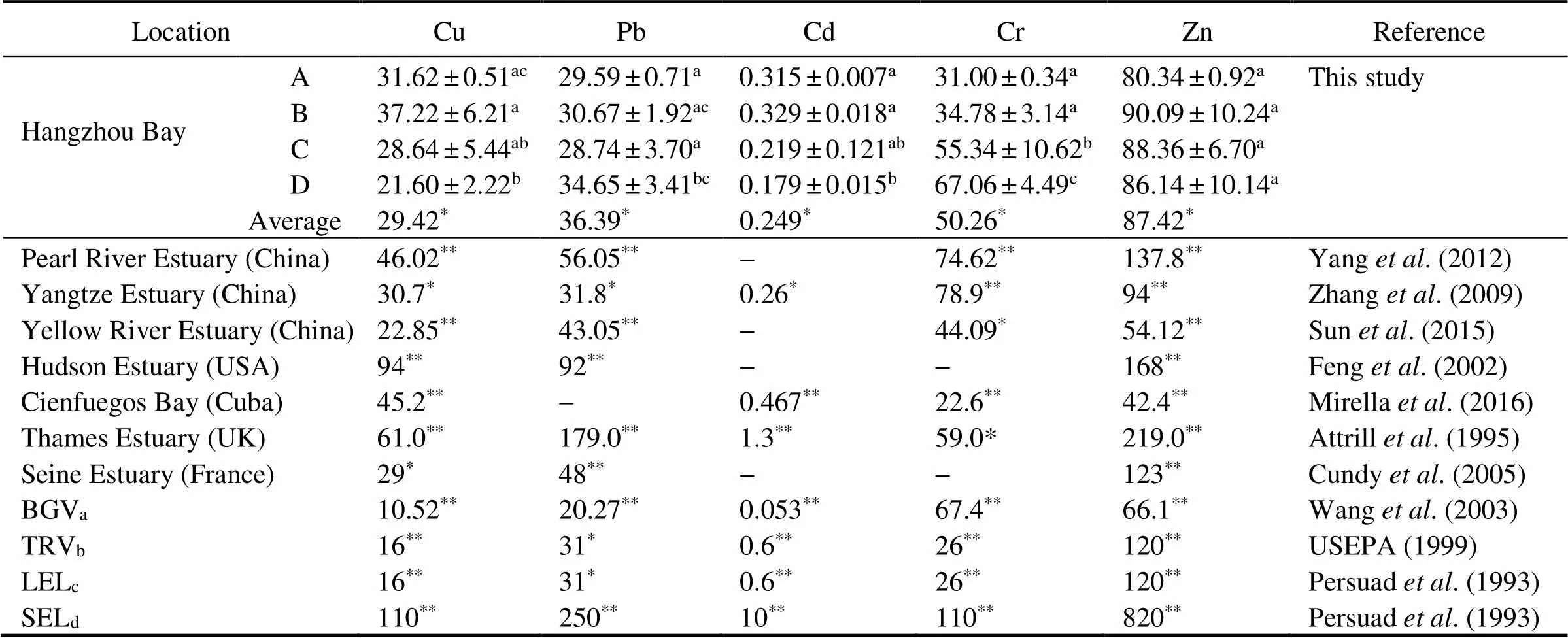
Table 3 Comparison of metal concentrations (µgg−1d.w.) in sediment with other areas in the world and different international guidelines
Notes: Values with the same superscript letter for each metal are not significantly different (>0.05).abackground values of metals in sediments of the Yellow Sea and East China Sea. TRV, toxicity reference value; LEL, lowest effect level; SEL, severe effect.*Superscript symbol for each metal are not significantly different (>0.05) between Hangzhou Bay and other areas in the world and different international guidelines, while**superscript symbol are significantly different (>0.05).
The metal contents in sediment of this study area were compared with those in other estuaries in the world (Table 3). The average content of Cu in this bay was higher in this bay than that in the Yellow River Estuary (<0.05) (Sun., 2015), but in agreement with that in the Seine Estuary (Cundy., 2005) and the Yangtze Estuary (Zhang., 2009) (>0.05), and significantly lower than that in other areas (<0.05). The mean concentrations of Pb and Cd were found to be lower than those in some previous studies. The mean concentrations of Cr and Zn in the Yellow River Estuary and Cienfuegos Bay were lower, but those in other areas had higher values than those in this study (<0.05). A comparison of the average values of metals with the background values of metals in the Yellow Sea and East China Sea (BGV) (Wang., 2003), indicated that the Cu, Pb, Cd and Zn values were significantly higher than BGV (<0.05). This results show that anthropogenic activities had some influence on the heavy metals in sediment (Chai., 2014). The contents of heavy metals in the sediment were compared with the toxicity reference value (TRV), lowest effect level (LEL) and severe effect (SEL) values (Table 3). The results indicated that Cu, Pb and Cr exceeded TRV and LEL, which suggested that Cu, Pb and Cr may occasionally have adverse effects on the ecosystem.
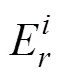
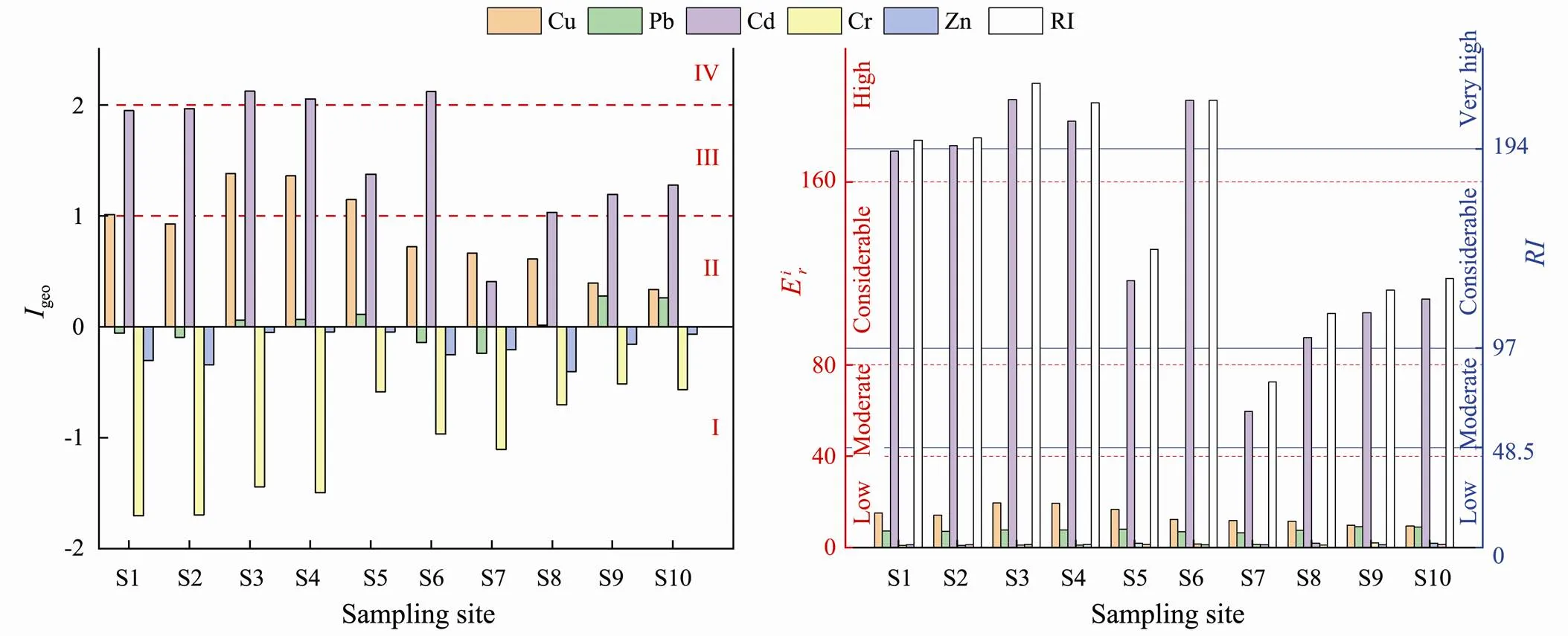
Fig.2 Geoaccumulation index (Igeo), ecological risk () and potential ecological risk index (RI) of metals in sediments from the southern coast wetland of the Qiantang Estuary.
3.2 Chemical Fractionation of Metals in Sediments
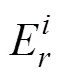
3.3 Metal Bioaccumulation in P. australis Roots
The distributions of Cu, Pb, Cd, Cr and Zn contents in the roots ofare shown in Fig.4. The average concentration followed the decreasing order of Zn>Pb> Cu>Cr>Cd. The contents of Zn and Pb were much higher than those of other metals; for example, Zn was on average 20 times higher than Cd. However, the P/S (content in plant roots/total content in sediments) values were in the decreasing order of Cd (1.06)>Pb (0.13)>Zn (0.08) >Cu (0.03)>Cr (0.01), which demonstrated that Cd was much more enriched than other metals, although its average concentration was the lowest. This result indicated ecological potential risk due to Cd enrichment (P/S>1). In terms of spatial distribution, the highest contents of Pb, Zn and Cd in the roots ofwere found in the B region, the highest contents of Cu and Cr were found in the C region. Obviously, this distribution was inconsistent with the distribution trend of total content or exchangeable fraction in the sediments, which meant that the uptake of metal bywas not only controlled by the content of metals in sediments but was also regulated by its own internal mechanism. Therefore, there is a certain deviation in the prediction of the metal bioaccumulation in plant by both total content and chemical fractionation in sediment.
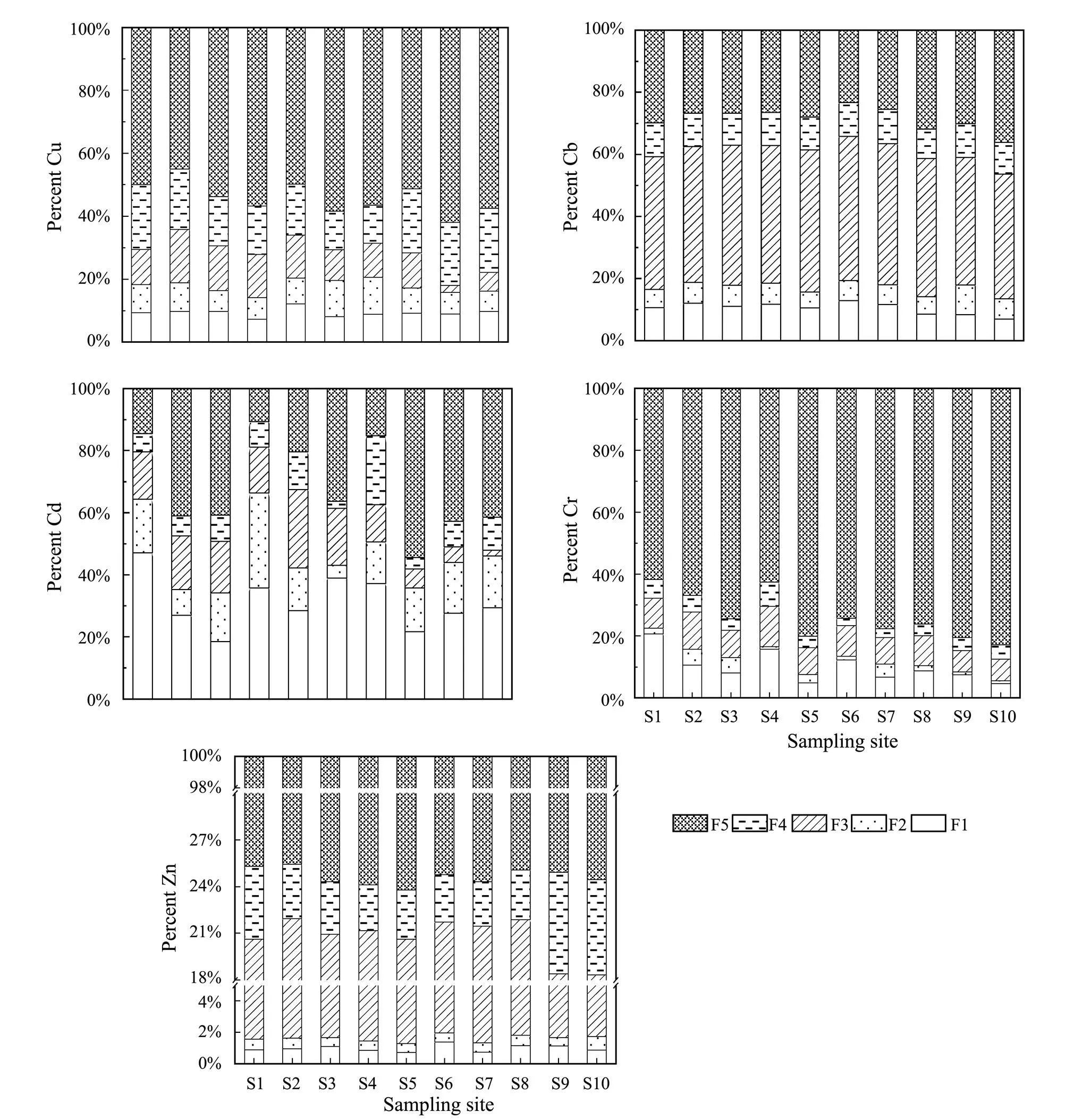
Fig.3 Relative distribution of metals in the sediments at different geochemical fractions from the southern coast wetland of the Qiantang Estuary. F1, exchangeable; F2, bound to carbonates; F3, bound to Fe-Mn oxides; F4, bound to organic matter; F5, residual.
3.4 Bioavailability Assessment Based on DGT Concentrations
The results of DGT metal concentrations in sediments nearroots along the southern coast wetland of the Qiangtang Estuary are displayed in Fig.4. The [M]- DGT concentrations (µgL−1) for Cu, Pb, Cd, Cr and Zn were 3.44–7.34, 3.02–1.40, 0.78–3.47, 8.50–15.62 and 15.94–30.04, respectively. The values in this study area were significantly higher than those of the western region of western Xiamen Bay (Yao., 2010), and the opposite result was found in the Shaocun River, China (Song., 2018). The largest values occurred in the B region for Pb, Zn and Cd; and in the C region for Cu and Cr. This outcome was consistent with the distribution trends of the same metals inroots.
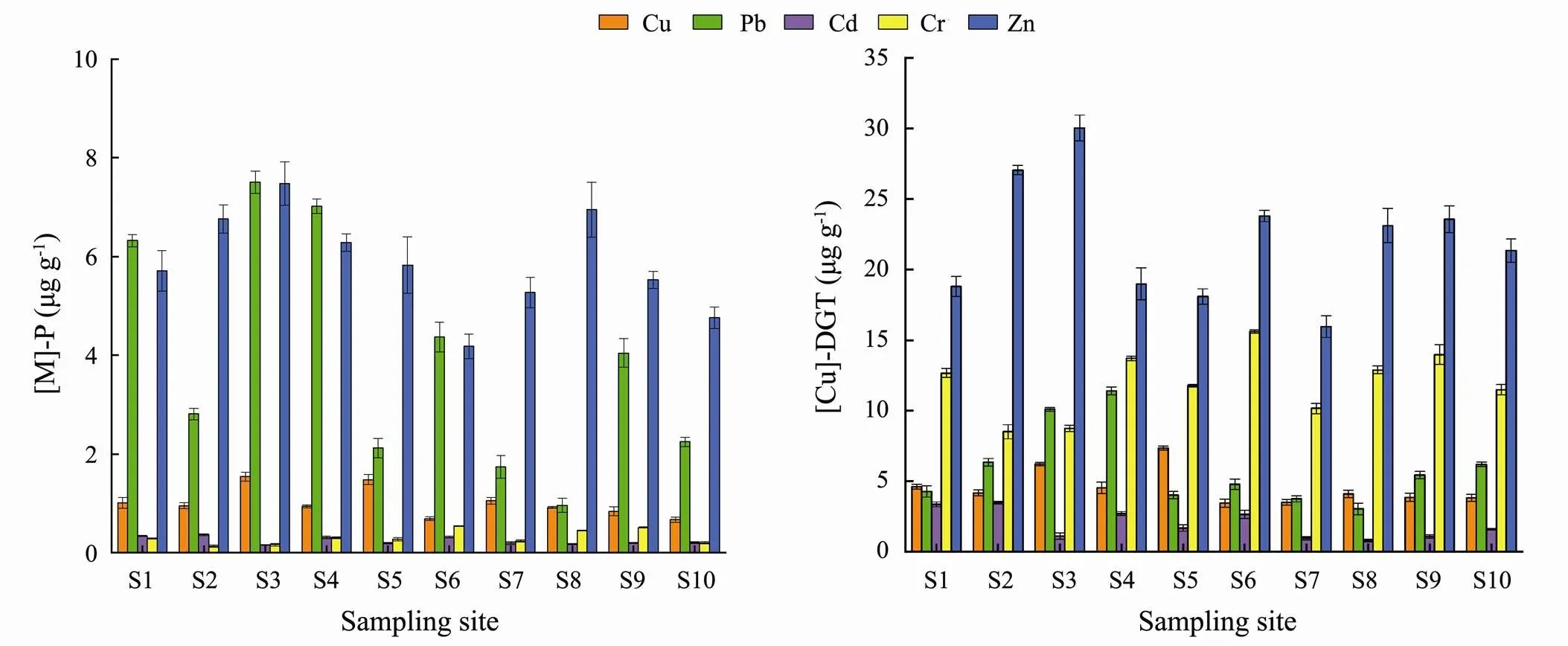
Fig.4 Distribution characteristics of the metal contents in P. australis root ([M]-P) and diffusive gradients in thin-films extracted metal concentrations ([M]-DGT).
The correlations between the metal contents in the plant roots ([M]-P) and [M]-DGT were analyzed (Fig.5). There were significant correlations between [Cu]-P and [Cu]-DGT (=0.847,=0.002), [Pb]-P and [Pb]-DGT (=0.592,=0.072), [Cd]-P and [Cd]-DGT (=0.789,=0.007), [Cr]-P and [Cr]-DGT (=0.754,=0.011). This result was similar to previous studies (Song., 2018; Zhang., 2019). However, there was no significant relationship between [Zn]-P and [Zn]-DGT (=0.181,=0.52), which was contrary to other studies (Song., 2018; Zhang., 2019), suggesting that different species of plants may have different metal accumulation capacities. This result indicated that it was difficult to predict the Zn content inwith DGT, while it is a simple and reliable technique for evaluating the bioavailability of metal (Cu, Pb, Cd and Cr) pollutants in contaminated sediments. Therefore, the use of DGT to predict bioaccumulation is not applicable to all metals, which may also be affected by plant species, chemical and physical properties of sediment.
The toxicity of pollutants in sediments is closely related to their bioavailability, because the bioabsorbable fraction of pollutants may be potentially toxic to organisms. Previous study results have shown that metal fractionation was important to its bioavailability and the degree of potential harm to the environment (Luoma, 1983; Islam., 2015). Chen and Huang (1998) found that there was a strong affinity between organisms and the water-soluble, exchangeable and carbonate-bound fractions of metals which had a great influence on bioaccumulation. In addition, Fe-Mn oxide bound forms of metals can moderately influence bioaccumulation, while little effect is observed for the carbonate and residual bound forms.
This study found that there were positive correlations between the exchangeable fraction of metals in the sediments and their [M]-DGT or [M]-P values, except [Zn]-P. The total contents of Cu and Cd were positively correlated with [M]-DGT and [M]-P, while Pb, Cr and Zn were not significantly correlated (Fig.6). This result indicates that it is not reliable to use the total contents of metals to assess their potential biological hazards. The absorption of metals by plant tissues largely depends on the metal bioavailability in sediments, which is influenced by pH, redox potential, organic matter, ion exchange capacity, plant species and other factors (Olaniran., 2013). To evaluate the effect of these environmental factors on the metal DGT concentrations and metal contents in plant tissues, we analyzed the correlations between the factors using thecorrelation coefficient (Fig.6). For Cu, Pb, Cd and Zn, [M]-DGT and [M]-P were positively correlated with organic matter (OM) to some extent, while for Cr, there was no significant correlation. Low-molecu- lar-weight OM can form soluble complexes with metals, while high-molecular-weight OM can reduce the biological efficiency of heavy metals (Du., 2009). However, humic acid, produced by the degradation of OM, can lead to a decrease in pH, which will increase the release of metals. In addition, CO2will be produced by organic matter during anaerobic decomposition, which can also cause metals to be rereleased into pore water through decalcification, thus increasing the possibility of metal absorption by plants (Du., 2009; Nobi., 2010). Sulfur ions can combine with metal cations and then form metal sulfides that are poorly soluble in water. Under anaerobic conditions, sulfide can hold more metal cations and reduce toxicity to organisms due to the high concentration of AVS, but under aerobic conditions, AVS loses its ability to hold onto metal (Nizoli and Luiz-Silva, 2012; Hernandez-Crespo and Martin, 2013; Zhuang and Gao, 2014). In this study, there were negative relationships between the AVS concentrations in sedi- ments and the [M]-DGT of Cu, Pb and Cd, or their [M]-P values, which was consistent with the results of Zhang. (2019). Acidity can strongly influence the solubility of metals in sediment. At an alkaline pH, adsorption and pre- cipitation of metals can be promoted, while at an acidic pH, the binding forces between groups can be weakened leading to metal release (Belzile., 2004; Guven and Akinci, 2013). According to Martínez and Motto (2000), under alkaline pH, Pb, Cu and Zn are metallic carbonate and phosphate, insoluble in sediments. Under acidic pH, the solubility and bioavailability of these metals increase. In this study, there were negative correlations between the contents of Cu, Zn and Pb in the plant tissues and pH in the sediment, while there was no significant correlation between [M]-DGT and pH value. The redox potential (Eh) can affect the migration and transformation ability of metals. When the Eh value increases, the oxidation rate of sulfide increases and the uptake by organisms decreases, thus accelerating the release of metal (Peng., 2009; De Jonge., 2012). For example, an increased Eh value in sediments can reduce the stable cadmium sulfide content from 65% to 30%, transforming it into a more easily migratory form of cadmium ions (Kelderman and Osman, 2007). Additionally, iron and manganese oxides can absorb or co-precipitate metals under oxidation conditions (Dong, 2000; Munger., 2016), whereas iron and manganese oxides release metals by reducing dissolution under anoxic conditions (Du Laing., 2009; Munger., 2016). Under anoxic conditions, the release of metals also leads to the formation of stable metal sulfide precipitation with AVS (De Jonge., 2010; De Jonge., 2012). This process leads to the decrease of metal concentration in the sediment pore water and the decrease of metal bioavailability (De Jonge., 2012). In addition, Pb was strongly absorbed by DOM in sediments, which significantly increased the mobilization of Pb in sediments (Hashimoto., 2009a, 2009b). However, in the present study, there was significantly positive correlation between [Cu]-DGT concentration, [Cu]-P tissue content and redox potential in sediments, but for other metals, there were no significant or negative relationships.
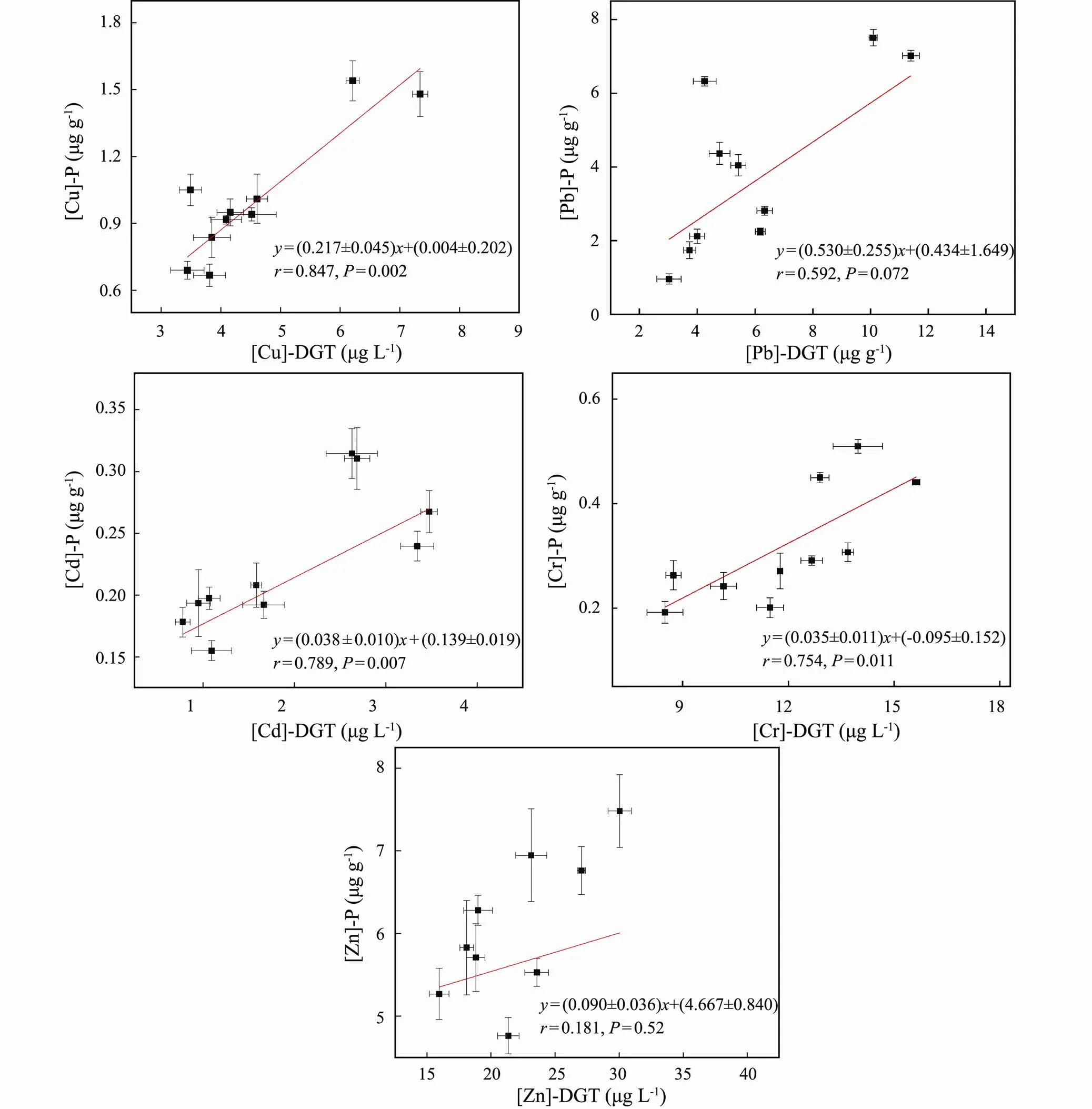
Fig.5 Correlation of metal contents in P. australis roots with diffusive gradients in thin-films extracted metal concentrations.
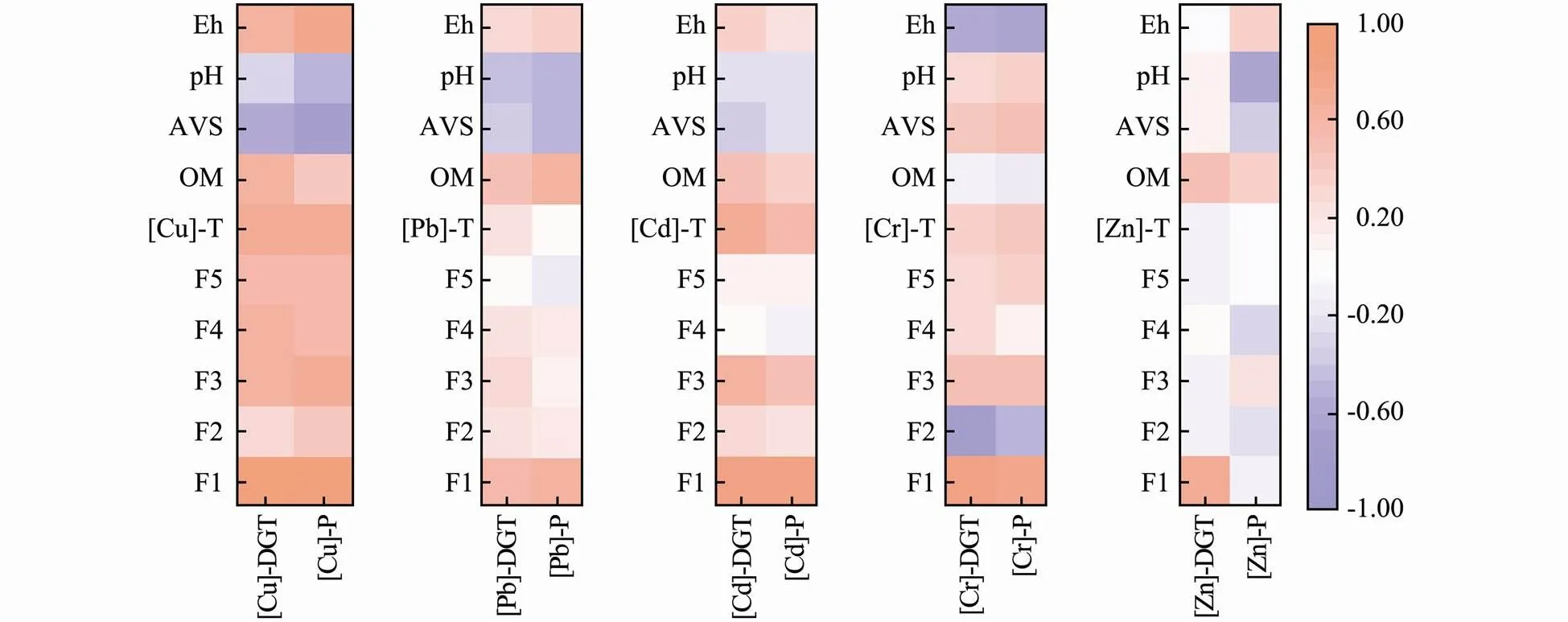
Fig.6 Pearson’s correlation between [M]-DGT, [M]-P and metal fractionation, sediment characteristic parameters.
3.5 Uncertainty
Our study is partially limited by having a little previous scientific database about assessing the bioavailability of metal in estuaries wetlands using DGT technology, with only the studies made by Song (2018) and Amato (2016) relating to its use in rivers. It made few comparisons in order to make our results more convincing. On the one hand, this study has limitations in terms of plant species, metals and sampling sites, since we currently only have information on five metal elements infrom ten sampling sites.
4 Conclusions
This is the first study that evaluate the bioaccumulation of metals in sediment from the southern coastal wetland of the Qiantang Estuary by DGT technique. We found some differences in the evaluation results between using DGT concentrations and total contents.
By using DGT technique to determine the bioavailable share of metal in sediment, we found positive correlations between [M]-DGT and the exchangeable fraction or [M]- P for Cu, Pb, Cd, and Cr, while a weak relationship for Zn. It is concluded that the DGT technique is capable of predicting some metals (Cu, Pb, Cd and Cr) bioaccumulation inroot. However, there is uncertainty about the types of metals and plants suitable for DGT technology.
This study highlights the severity of Cd pollution along the southern coast wetland of the Qiantang Estuary regardless of the assessment method. Furthermore, the combined assessment evidenced the wetlands towards the upstream region are potentially more vulnerable to the anthropogenic pollution. This may be harmful to wetland plants, birds and benthonic animal.
Acknowledgements
The authors are grateful to the Natural Science Foundation of China (No. 41776119) and Zhejiang Provincial Natural Science Foundation of China (No. LY15D060004) for the financial support.
Alves, J. P. H., Passos, E. A., and Garcia, C. A. B., 2007. Metals and acid volatile sulfide in sediment cores from the Sergipe River Estuary, Northeast, Brazil.,18: 748-758.
Amato, E. D., Simpson, S. L., Remaili, T. M., Spadaro, D. A., Jarolimek, C. V., and Jolley, D. F., 2016. Assessing the effects of bioturbation on metal bioavailability in contaminated sediments by diffusive gradients in thin films (DGT)., 50: 3055-3064.
Attrill, M. J., and Myles, T. R., 1995. Heavy metal concentrations in sediment from the Thames Estuary, UK.,30(11): 742-744.
Baptista, M. S., Vasconcelos, V. M., and Vasconcelos, M. T. S. D., 2014. Heavy metal concentration in a temperate freshwater reservoir seasonally subjected to blooms of toxin-producing cyanobacteria.,68: 671-678.
Belzile, N., Chen, Y. W., Gunn, J. M., and Dixit, S. S., 2004. Sediment heavy metal profiles in lakes of Killarney Park, Canada: From regional to continental influence., 130: 239-248.
Bermejo, J., Beltrán, R., and Gomez-Ariza, J. L., 2003. Spatial variations of heavy metals contamination in sediments from Odiel River (Southwest Spain).,29 (1): 69-77.
Bi, C., Chen, Z. L., and Xia, S. Y., 2003. Chemical associations of heavy metals in the sediments near Bailonggang sewage discharge outlet of Shanghai.,5: 52- 58.
Chai, M., Shi, F., Li, R., and Shen, X., 2014. Heavy metal contamination and ecological risk inmarsh in intertidal sediments of Bohai Bay, China.,84: 115-124.
Chen, M., Ding, S., Lin, J., Fu, Z., Tang, W., Fan, X.,., 2019. Seasonal changes of lead mobility in sediments in algae- and macrophyte-dominated zones of the lake.,660: 484-492.
Chen, R. S., and Huang, Y. K., 1998.. China Environmental Science Press, Beijing, 1-44.
Costello, D. M., Burton, G. A., Hammerschmidt, C. R., and Taulbee, W. K., 2012. Evaluating the performance of diffusive gradients in thin films for predicting Ni sediment toxicity.,46: 10239-10246.
Cundy, A. B., Hopkinson, L., Lafite, R., Spencer, K., Taylor, J. A., Ouddane, B.,., 2005. Heavy metal distribution and accumulation in twosp.-dominated macrotidal salt marshes from the Seine Estuary (France) and the Medway Estuary (UK).,20: 1195-1208.
De Jonge, M., Blust, R., and Bervoets, L., 2010. The relation between Acid Volatile Sulfides (AVS) and metal accumulation in aquatic invertebrates: Implications of feeding behavior and ecology.,158: 1381-1391.
De Jonge, M., Teuchies, J., Meire, P., Blust, R., and Bervoets, L., 2012. The impact of increased oxygen conditions on metal- contaminated sediments part II: Effects on metal accumulation and toxicity in aquatic invertebrates., 46: 3387-3397.
Dong, D., 2000. Adsorption of Pb and Cd onto metal oxides and organic material in natural surface coatings as determined by selective extractions: New evidence for the importance of Mn and Fe oxides.,34: 427-436.
Du Laing, G., Rinklebe, J., Vandecasteele, B., Meers, E., and Tack, F. M., 2009. Heavy metal behaviour in estuarine and riverine floodplain soils and sediments: A review.,407: 3972-3985.
Ernstberger, H., Zhang, H., Tye, A., Young, S., and Davison, W., 2005. Desorption kinetics of Cd, Zn, and Ni measured in soils by DGT., 39 (6): 1591- 1597.
Feng, H., Cochran, J. K., and Hirschberg, D. J., 2002. Transport and sources of metal contaminants over the course of tidal cycle in the turbidity maximum zone of the Hudson.,36: 733-743.
Feng, H., Kirk Cochran, J., Lwiza, H., Brownawell, B. J., and Hirschberg, D. J., 1998. Distribution of heavy metal and PCB contaminants in the sediments of an urban estuary: The Hudson River.,45: 69-88.
Galán, E., Gómez-Ariza, J. L., González, I., Fernández-Caliani, J. C., Morales, E., and Giráldez, I., 2003. Heavy metal partitioning in river sediments severely polluted by acid mine drainage in the Iberian Pyrite Belt.,18: 409-421.
Gambrell, R. P., Wiesepape, J. B., Patrick, W. H., and Duff, M. C., 1991. The effects of pH, redox, and salinity on metal release from a contaminated sediment.,57-58: 359-367.
García-Hernández, J., García-Rico, L., Jara-Marini, M. E., Barraza-Guardado, R., and Hudson Weaver, A., 2005. Concentrations of heavy metals in sediment and organisms during a harmful algal bloom (HAB) at Kun Kaak Bay, Sonora, Mexico.,50: 733-739.
Gaw, S. K., Kim, N. D., Northcott, G. L., Wilkins, A. L., and Robinson, G., 2008. Uptake of ΣDDT, arsenic, cadmium, cop- per, and lead by lettuce and radish grown in contaminated horticultural soils., 56 (15): 6584-6593.
Guven, D. E., and Akinci, G., 2013. Effect of sediment size on bioleaching of heavy metals from contaminated sediments of Izmir Inner Bay.,25: 1784-1794.
Hakanson, L., 1980. An ecological risk index for aquatic pollution control: A sedimentological approach.,14(8): 975-1001.
Hamilton, R. S., Revitt, D. M., and Warren, R. S., 1984. Levels and physico-chemical associations of Cd, Cu, Pb and Zn in road sediments.,33: 59-74.
Harper, M. P., 2000. A modelling and simulation tool for DGT induced trace metal remobilisation in sediments and soils., 35 (12): 2602-2607.
Hashimoto, Y., Takaoka, M., Oshita, K., and Tanida, H., 2009a. Incomplete transformations of Pb to pyromorphite by phosphate-induced immobilization investigated by X-ray absorption fine structure (XAFS) spectroscopy.,76: 616-622.
Hashimoto, Y., Taki, T., and Sato, T., 2009b. Extractability and leachability of Pb in a shooting range soil amended with poultry litter ash: Investigations for immobilization potentials., 44: 583-590.
He, J. Y., Ren, Y. F., Wang, F. J., Pan, X. B., Zhu, C., and Jiang, D. A., 2009. Characterization of cadmium uptake and trans- location in a cadmium-sensitive mutant of rice (L. ssp.)., 57: 299-306.
Hernandez-Crespo, C., and Martin, M., 2013. Mid-term variation of vertical distribution of acid volatile sulphide and simultaneously extracted metals in sediment cores from Lake Albufera (Valencia, Spain).,65: 654-664.
Islam, M. S., Ahmed, M. K., Raknuzzaman, M., Habibullah-Al- Mamun, M., and Islam, M. K., 2015. Heavy metal pollution in surface water and sediment: A preliminary assessment of an urban river in a developing country., 48: 282-291.
Jensen, H. S., Kristensen, P., Jeppesen, E., and Skytthe, A., 1992. Iron: Phosphorus ratio in surface sediment as an indicator of phosphate release from aerobic sediments in shallow lakes., 235: 731-743.
Kelderman, P., and Osman, A. A., 2007. Effect of redox potential on heavy metal binding forms in polluted canal sediments in Delft (the Netherlands).,41: 4251-4261.
Lin, Y., Guo, M., and Zhuang, Y., 1997. Determination of acid volatile sulfide and simultaneously extracted metals in sediment.,17: 353-358.
Liu, B., Hu, K., Jiang, Z., Yang, J., Luo, X., and Liu, A., 2010. Distribution and enrichment of heavy metals in a sediment core from the Pearl River Estuary.,62: 265-275.
Luo, J., Zhang, H., Santner, J., and Davison, W., 2010. Perfor- mance characteristics of diffusive gradients in thin films equipped with a binding gel layer containing precipitated ferrihydrite for measuring arsenic(V), selenium(VI), vanadium (V), and antimony(V).,82: 8903-8909.
Luoma, S. N., 1983. Bioavailability of heavy metals to aquatic organisms–A review.,28: 1-22.
Maiz, I., Arambarri, I., Garcia, R., and Millán, E., 2000. Evaluation of heavy metal availability in polluted soils by two sequential extraction procedures using factor analysis.,110: 3-9.
Martı́nez, C. E., and Motto, H. L., 2000. Solubility of lead, zinc and copper added to mineral soils., 107: 153-158.
Müller, G., 1979. Schwermetalle in den sediments des Rheins- Veranderungenseitt 1971., 79: 778-783
Munger, Z. W., Carey, C. C., Gerling, A. B., Hamre, K. D., Doubek, J. P., Klepatzki, S. D.,., 2016. Effectiveness of hypolimnetic oxygenation for preventing accumulation of Fe and Mn in a drinking water reservoir.,106: 1- 14.
Nedell, D. B., and Taffaelli, D. G., 1999.. Academic Press, London, 22-42.
Nizoli, E. C., and Luiz-Silva, W., 2012. Seasonal AVS-SEM relationship in sediments and potential bioavailability of metals in industrialized estuary, southeastern Brazil.,34: 263-272.
Nobi, E. P., Dilipan, E., Thangaradjou, T., Sivakumar, K., and Kannan, L., 2010. Geochemical and geo-statistical assessment of heavy metal concentration in the sediments of different coastal ecosystems of Andaman Islands, India.,87: 253-264.
Olaniran, A. O., Balgobind, A., and Pillay, B., 2013. Bioavailability of heavy metals in soil: Impact on microbial biodegradation of organic compounds and possible improvement stra- tegies.,14: 10197- 10228.
Pardo, R., Barrado, E., Lourdes, P., and Vega, M., 1990. Determination and speciation of heavy metals in sediments of the Pisuerga River.,24: 373-379.
Peña-Icart, M., Pereira-Filho, E. R., Fialho, L. L., Joaquim A. Nóbrega, and Pomares-Alfonso M. S., 2016. Combining contamination indexes, sediment quality guidelines and multivariate data analysis for metal pollution assessment in marine sediments of Cienfuegos Bay, Cuba.,168: 1267-1276.
Peng, J. F., Song, Y. H., Yuan, P., Cui, X. Y., and Qiu, G. L., 2009. The remediation of heavy metals contaminated sediment.,161: 633-640.
Persuad, D., Jaagumagi, R., and Hayton, A., 1993.. Ontario Ministry of the Environment and Energy, Canada.
Rees, J. G., Ridgway, J., Knox, R. W. O. B., Wiggans, G., and Breward, N., 1999. Sediment-borne contaminants in rivers discharging into the Humber Estuary, UK.,37: 316-329.
Ren, L., Li, X., Yang, S., Yan, Z., and Huang, X., 2014. The impact of salt marsh change on sediment accumulation and wave attenuation at the East Chongming Island.,84: 3350-3358 (in Chinese with English abstract).
Ren L. Y., Wu, C. F., Yue, W. Z., Liu, Y., and Lu, Z. W., 2008. Impact of urban planning and industrial development on wetlands in Hangzhou Bay.,63: 1055- 1063.
Richau, K. H., Kozhevnikova, A., Seregin, I. V., and Vooigs, R., 2009. Chelation by histidine inhibits the vacuolar sequestration of nickel in roots of the hyperaccumulator., 183 (1): 106-116.
Riedel, G. F., Sanders, J. G., and Osman, R. W., 1999. Biogeochemical control on the flux of heavy elements from estuarine sediments: Effects of seasonal and short-term hypoxia.,47: 349-372.
Shaifullah, K., Mezbahuddin, M., Sujauddin, M., and Haque, S., 2008. Effects of coastal afforestation on some soil properties in Lakshmipur coast of Bangladesh.,19: 32-36.
SOA, 2007. The specification for marine monitoring (GB 17378-2007). Standardization Administration of the People’s Republic of China (SAC), Beijing.
Song, Z., Shan, B., and Tang, W., 2018. Evaluating the diffusive gradients in thin films technique for the prediction of metal bioaccumulation in plants grown in river sediments., 344: 360-368.
Sun, Z., Mou, X., Tong, C., Wang, C., Xie, Z., Song, H.,., 2015. Spatial variations and bioaccumulation of heavy metals in intertidal zone of the Yellow River Estuary, China.,126: 43-52.
Suresh, G., Ramasamy, V., Meenakshisundaram, V., Venkatacha- lapathy, R., and Ponnusamy, V., 2011. Influence of mineralogical and heavy metal composition on natural radionuclide concentrations in the river sediments.,69 (10): 1466-1474.
Tessier, A., Campbell, P. G. C., and Bisson, M., 1979. Sequential extraction procedure for the speciation of particulate heavy metals.,51: 844-851.
USEPA, 1999. Screening level ecological risk assessment protocol for hazardous waste combustion facilities. Appendix E: Toxicity Reference Values. EPA 530-D99-001C, Vol. 3.
Wang, F. L., Song, N. N., Zhao, Y. J., Zhang, C. B., Shen, Y., and Liu, Z. Q., 2012. Predicting the cadmium bioavailability in the soil of sugarcane field based on the diffusive gradients in thin films with binding phase of sodium polyacrylate., 33 (10): 3562-3568 (in Chinese with English abstract).
Wang, J. Y., Ma, D. Y., Bao, Y. E., Liu, G. Y., and Liu, J., 2003. Evaluation on sediment quality in Yellow Sea and East China Sea.,22: 21-24 (in Chinese with English abstract).
Warnken, K. W., Davison, W., and Zhang, H., 2008. Interpretation ofspeciation measurements of inorganic and organically complexed trace metals in freshwater by DGT., 42: 6903-6909.
Williams, P. N., Islam, S., Islam, R., Jahiruddin, M., Adomako, E., Soliaman, A. R. M.,., 2009. Arsenic limits trace mineral nutrition (selenium, zinc, and nickel) in Bangladesh Rice Grain., 43: 8430- 8436.
Xu, D., Chen, Y., Ding, S., Sun, Q., Wang, Y., and Zhang, C., 2013. Diffusive gradients in thin films technique equipped with a mixed binding gel for simultaneous measurements of dissolved reactive phosphorus and dissolved iron., 47 (18): 10477-10484.
Yang, Y., Chen, F., Zhang, L., Liu, J., Wu, S., and Kang, M., 2012. Comprehensive assessment of heavy metal contamination in sediment of the Pearl River Estuary and adjacent shelf.,64 (9): 1947-1955.
Yao, F., Zhang, Y., Hu, X., and Liu, X., 2010. Occurrence states and potential mobility of heavy metals in sediments of Western Xiamen Bay., 29: 532-538 (in Chinese with English abstract).
Yin, H., Cai, Y., Duan, H., Gao, J., and Fan, C., 2014. Use of DGT and conventional methods to predict sediment metal bioavailability to a field inhabitant freshwater snail () from Chinese eutrophic lakes.,264: 184-194.
Zhang, H., and Davison, W., 1998.measurements of solution concentrations and fluxes of trace metals in soils using DGT., 32 (5): 704- 710.
Zhang, H., Zhao, F. J., Sun, B., Davison, W., and Mcgrath, S. P., 2001. A new method to measure effective soil solution concentration predicts copper availability to plants., 35 (12): 2602-2607.
Zhang, T., Liu, S., Song, Y. M., Pan, J. C., and Guo, P. R., 2019. Bioavailability and ecological risk assessment of heavy metals in sediments of marine aquaculture in Zhelin Bay.,39: 706-715 (in Chinese with English abstract).
Zhang, W., Feng, H., Chang, J., Qu, J., Xie, H., and Yu, L., 2009. Heavy metal contamination in surface sediments of Yangtze River intertidal zone: An assessment from different indexes., 157: 1533-1543.
Zhuang, W., and Gao, X., 2014. Assessment of heavy metal im- pact on sediment quality of the Xiaoqinghe Estuary in the coastal Laizhou Bay, Bohai Sea: Inconsistency between two commonly used criteria.,83: 352- 357.
February 10, 2021;
November 20, 2021;
December 11, 2021
© Ocean University of China, Science Press and Springer-Verlag GmbH Germany 2022
. Tel: 0086-571-81963293
E-mail:xuht@sio.org.cn
(Edited by Ji Dechun)
杂志排行
Journal of Ocean University of China的其它文章
- Probabilistic Methods for Reliability Assessment of Floating Structures Based on Extreme Wave Simulation
- Structure of the Potential Vorticity of an Explosive Cyclone over the Eastern Asian Region in Late November 2013
- Enzyme-Induced Carbonate Precipitation for the Protection of Earthen Dikes and Embankments Under Surface Runoff: Laboratory Investigations
- Isolation, Identification, and Quantitative Determination of Saponin in Apostichopus japonicus by HPLC-DAD
- Transcriptome Analysis of Pacific White Shrimp (Litopenaeus vannamei) Under Prolonged High-Salinity Stress
- The Combined Effect of Plastic Particles Size and Concentration on Rotifers’ (Brachionus plicatilis) Performance
