IgE Reactivity of Potential Allergens from the Swimming Crab Portunus trituberculatus and the Research of ELISA Reagent for Detecting Crab Food Anaphylaxis
2022-02-24QIUXueniZHOUQiongyanZHUXiaoxiaLINWeiMUChangkaoLIRonghuaYEYangfangSONGWeiweiSHICeLIULeiWANGHuanWANGChunlinandXUSuling
QIU Xueni, ZHOU Qiongyan, ZHU Xiaoxia, LIN Wei, MU Changkao, *,LI Ronghua, YE Yangfang, SONG Weiwei, SHI Ce, LIU Lei,WANG Huan, WANG Chunlin, and XU Suling, *
IgE Reactivity of Potential Allergens from the Swimming Craband the Research of ELISA Reagent for Detecting Crab Food Anaphylaxis
QIU Xueni1), 2), ZHOU Qiongyan3), ZHU Xiaoxia3), LIN Wei3), MU Changkao1), 2), *,LI Ronghua1), 2), YE Yangfang1), 2), SONG Weiwei1), 2), SHI Ce1), 2), LIU Lei1), 2),WANG Huan1), 2), WANG Chunlin1), 2), and XU Suling3), *
1),,,315211,2),,315211,3),315020,
Crustacean is one of the major allergic foods. It is of great significance to identify more crab allergens and research the detection methods for crab food anaphylaxis. In this study, IgE reactivity to three recombinant proteins from, including tropomyosin (rPtTM), myosin light chain (rPtMLC), and pancreatic lipase (rPtPL), were detected by enzyme-linked im- munosorbent assay (ELISA). The expressions of tropomyosin (TM) in various tissues ofwere detected by Western blot (WB). Furthermore, microplates were coated with rPtTM and the ELISA conditions were optimized. The cut-off value was deter- mined by the receiver operating characteristic (ROC) curve. Among 51 crab-allergic sera, 21 (41.2%) showed positive IgE to rPtTM. Other 70 crab-allergic sera more frequently recognized rPtPL (9/70; 12.9%), followed by rPtMLC (1/70; 1.4%). WB results showed that TM was mainly expressed in the muscle, followed by the heart and a small amount in gills and the testis. The optimal results showed that the coating condition of rPtTM was 50ng per well with coating for 3h at 37℃. The optimal blocking condition was 1.2%BSA with blocking for 3h at 37℃, and the optimal dilution of the second antibody was 1:1500. The ROC curve showed that the ELISA reagent had high sensitivity (83.52%) and specificity (98.00%) when the cut-off value was 0.45. All results indicated that tropomyosin is the major allergen of, and myosin light chain and pancreatic lipase are the potential allergens. Addi- tionally, the ELISA reagent developed with the rPtTM was feasible for laboratory detection of crab anaphylaxis.
; potential allergens; food anaphylaxis; ELISA reagent
1 Introduction
Food allergy is an abnormal response of the mucosal im-mune system to food protein delivered through the oral route (Sampson, 2004). The main symptoms of food al-lergy are diarrhea, emesis, allergic rhinitis, eczema, asthma,., and the serious reactions are also accompanied bycollapse and shock and can even be life-threatening (Burks., 2012). Crustacean was designated as one of the eight major allergic foods by FAO/WHO (FAO/WHO, 2001). In recent years, the food allergy caused by crustaceans has increased. In the United States, Mexico, and Canada, food allergy from crustaceans affected 0.3%–4.2% of the ge- neral population (Moshe., 2010; Martín., 2015; Warren., 2019). In China, Vietnam, and Singapore, 3.0%–7.5% of people suffered with food allergies from crustaceans (Zeng., 2015; Goh., 2018; Thu., 2020).
Tropomyosin (TM) was reported as a major allergen in various crustacean species, such asBoone (Bai., 2016),(Liu., 2010)and(Nurul Izzah., 2015). Allergen of the second protein family, arginine kinase (AK), was de- scribed in(Sun., 2015)and(Yang., 2015). Sarcoplasmic cal- cium-binding proteins (SCPs) from(Suzanne., 2015) and(Mao., 2013) were described as novel shrimp allergens. Myosin light chain (MLC) was a new allergen that had been de- scribed in(Ayuso., 2008),(Zhang., 2015), and(Kerstin., 2011), but it was not described in crab. Other allergens, such as hemocyanin (HMC) (Piboonpocanun., 2011) and tro- ponin C (Tn C) (Wai., 2019) have also been charac- terized. Recently, it was found that pancreatic lipase (PL)from thecDNA library constructed by our laboratory had a lipase structure domain that was si- milar to phospholipase A1 (AAB48072.1), which was re- ported to be similar to bee venom allergen (King., 1996) (Fig.1). Therefore, PL was supposed to be a poten- tial crab allergen.
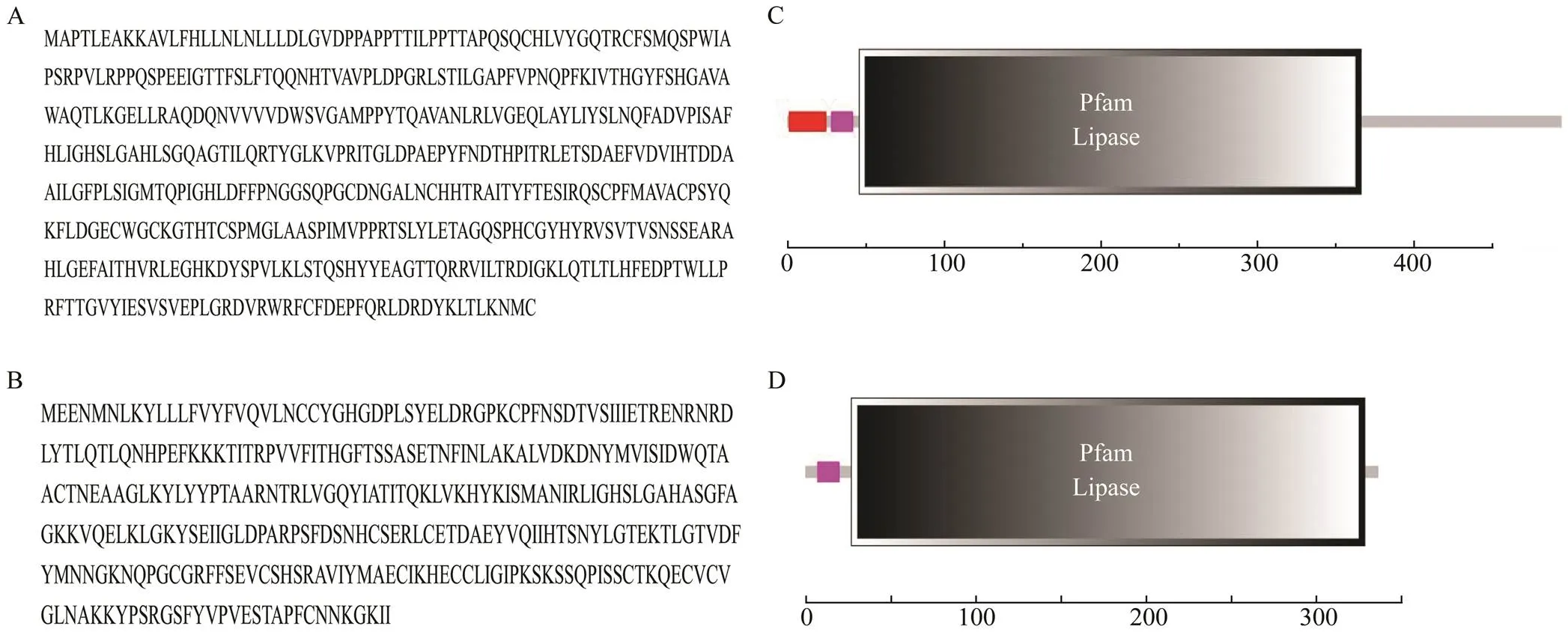
Fig.1 Product structural domain of pancreaticlipase from Portunus trituberculatus and phospholipase A1 from Vespula vulgaris. A, amino acids sequence of pancreaticlipase; B, amino acids sequence of phospholipaseA1; C, product structural domain of pancreaticlipase; D, product structural domain of phospholipaseA1.
As an important economic species in China’s marine aquaculture,is widely consumed as a delicious crustacean. However, it is recognized as one of the potential causes of food allergy. Knowing about the expression levels of allergen protein in different tissues ofcan provide an eating risk reference for people who are allergic to crabs.
Component-resolved diagnosis (CRD) provides a major step in improving the accuracy of diagnosing IgE-mediated food allergy, and it has allowed identifying single mole- cular allergen components responsible for sensitization (Guillermo., 2019). Instead of relying on the crude allergen extracts used in standard allergy diagnostics, the use of purified natural and recombinant allergens for CRDcan not only reveal co-sensitization and/or cross-sensitiza-tion, but also allow us to detect sensitization to allergensthat are not present in sufficient quantities in natural ex- tracts (Borres., 2016). It was reported that recombi- nant allergens may improve the diagnostic efficiency of spe-cific IgE (sIgE) in shrimp (Yang., 2010) and house dust mites (Pittner., 2004). Furthermore, recombinant allergens can be controlled in quality, purity, and yield ar- tificially compared to the purified natural allergens (Curin., 2017).
Recently, CRD was generally used in enzyme-linked im- munosorbent assay (ELISA) and Western blot (WB). Com- pared with WB, ELISA is a more sensitive, simple, and ra- pid test method that has considerable application in clini- cal diagnostics (Yeung, 2006). The critical diagnose value of positive or negative by ELISA is called the cut-off va- lue. To determine the cut-off value, the methods including standard deviation ratio, test to negative ratio, receiver op- erating characteristic (ROC), mean of negative control+2 or 3 SD,., were used, wherein the ROC curve is the bestmethod to set the cut-off value (Parkinson., 1988). The ROC curve shows the correlation between sensitivity and specificity of a certain method. The closer the curve to the upper left, the larger the area under the ROC curve (AUC), indicating that this method has higher sensitivity, specificity, and diagnostic value. Moreover, the cut-off va- lue is determined by the Youden index (Aycan., 2010)or the positive likelihood ratio(Woldetensay., 2018). At present, CRD had less research on the kit of crab food anaphylaxis, and the ROC curve analysis was always usedon the sIgE values to diagnose shrimp allergy (Tuano., 2017).
In this study, the sIgE reactivity of recombinant TM, MLC, and PL proteins fromwere detected by ELISA. The expressions of TM protein in the gonads, mu-scle, heart, gill, and hepatopancreas were analyzed by WB. Next, the ELISA reagent for detecting crab food anaphy- laxis was studied by using recombinant TM protein as an- tigen.
2 Materials and Methods
2.1 Human Sera
Sera were obtained from crab-allergic patients from the Affiliated Hospital of Medical School of Ningbo Univer- sity and Hangzhou Zheda Dixun Biological Gene Engi- neering Company. The experiments were carried out in ac- cordance with the ethical standards formulated in the Hel- sinki Declaration. The inclusion criteria of this study were as follows: 1) all patients had a history of crab allergy; 2) the allergic response was confirmed by the positive crab- specific IgE (sIgE>0.35IUmL−1). Sera from non-allergic healthy people with neither history of crab allergy nor sIgE- negative was used as a negative control. The study popu- lation comprised 121 crab-allergic individuals (68 male and 53 female patients; age: 6 months–66 years) and 50 non- allergic healthy individuals (10 male and 20 female peo-ple; age: 4 months–63 years; the other 20 cases had miss- ing information). All sera were stored at −20℃ until use.
2.2 Tissue Preparation and cDNA Synthesis
Adult pond-reared crabs and wild-caught crabswere pur- chased from an aquaculture company and market, respec- tively. The experiments were approved by the appropriate committee of the local animal institute. The individual live crab was dissected on ice. The abdominal muscle was im- mersed in liquid nitrogen immediately and then kept at −80℃ for RNA extraction, and six tissues (ovary, testis, heart, muscle, gill, and hepatopancreas) were kept at −80℃ for protein extraction.
Total RNA from the muscle was extracted by RNAiso Plus (Takara, Japan). The cDNA was synthesized from RNA with M-MLV reverse transcriptase (Promega, USA) and RNase Inhibitor (Thermo, USA) and stored at −80℃. The RNA loading amounts were adjusted according to the mea- sured concentration.
2.3 Expression and Purification of rPtTM, rPtMLC, and rPtPL
The expression and purification of the pET-21a(+)-PtX recombinant proteins (designated as rPtX, X indicates dif- ferent potential allergen including TM, MLC, and PL) were performed as described previously (Lu., 2017). Pri- mers used for recombinant proteins were shown in Table 1.

Table 1 Sequences and purposes of primers used in this study
2.4 ELISA for Serum IgE Reactivities to rPtTM, rPtMLC, rPtPL
Serum IgE reactivity of rPtX was detected by an aller- gen-specific IgE test kit (Zheda Dixun, Hangzhou) with mo- dification. The purified recombinant protein was used as the plate-coating antigen. The patient sera and standard so- lution (human IgE with the concentrations of 0.35, 0.70, 3.50, 17.50, 50.00, and 100.00IUmL−1) were added to the corresponding well and incubated at 37℃ for 45min. All plates were subsequently washed five times with wash buf- fer and horseradish peroxidase (HRP)-conjugated goat anti- human IgE was added and incubated at 37℃ for 45min.After washing five times, 3,3’,5,5’-tetramethylbenzidine (TMB) was used as a substrate and incubated for 15–20 min at 37℃. Then the reaction was immediately terminatedby the addition of stop solution (0.5molL−1HCl). The ab- sorbance was then measured at 450nm (A450) using a mi- croplate reader. For the calibration curve, the logarithm of the concentration of the IgE calibrator solution was taken as the abscissa, and the A450value was the ordinate.
2.5 The Expression of PtTM in Different Tissues
The anti-TM polyclonal antibody was provided by Hua- Bio. The purified rPtTM was injected into a New Zealand rabbit to generate a polyclonal antibody. The specificity of the polyclonal antibody was examined by WB. Six tissues were chosen in the WB assay: testis, ovary, hepatopancreas, heart, gill, and muscle. This procedure was performed as described previously (Xu., 2017). The protein signalswere detected by using Developer and Fixer Kit (Beyo- time) and ChemiScope 3300 (Shanghai, China). The sta- tistical gray level of each band was calculated using Im- age J software.
2.6 Optimization of the ELISA Reagent for Crab Food Anaphylaxis
To develop a qualitative detection reagent for food al- lergy ELISA, the reaction conditions, including the coat- ing concentration and the conditions of antigen and the blocking, and the dilution of the secondary antibody, were optimized. The highest A450for the positive serum or A450ratio for the positive and negative controls (P/N value) was regarded as the optimal pair.
2.6.1 Optimization of the rPtTM coating concentration
The rPtTM was diluted to a concentration of 16μgmL−1and used as the antigen. The first column of microtiter plates was coated with 50μL rPtTM and doubling diluted from the second column to the last one. Microplates were coated for 3h at 37℃ and overnight at 4℃ with different concen-trations of rPtTM. After blocked with 1% bovine serum albumin (BAS) for 3h at 37℃ and overnight at 4℃, the plates were washed three times by PBS (Double Helix Bio-technology, Shanghai, China), and the 1–11 columns of plates were incubated for 30min at 37℃ with an allergic serum which was collected from 113 crab-allergic people, while the same amount was taken from each patient and they were mixed thoroughly. The 12th column without se- rum was employed as the negative control. After washing, HRP-conjugated goat anti-human IgE (KPL, USA) (1:1500dilution) was added and incubated for 30min at 37℃. TMB (Solarbio, Beijing, China) was used as a substrate and in- cubated for 15min at 37℃, and the reaction was immediately terminated by the addition of HCl (1molL−1). Betweeneach incubation, the plates were washed with ELISA Wash Buffer (BBI, Canada). All experiments were conducted in duplicate. The plates were detected at 450nm using a mi- croplate detector.
2.6.2 Optimization of the rPtTM coating time and temperature
Based on the result of 2.6.1, the microplates were coatedwith rPtTM at the optimal concentration and incubated un- der three conditions. The first group was incubated for 3h at 37℃ and overnight at 4℃; the second group was incu- bated for 3h at 37℃; the third group was incubated over- night at 4℃. After blocking and washing, the microplates were incubated with allergic serum, or healthy people’s se- rum which was collected from 50 healthy people and mix-ed thoroughly, while the same amount was taken from each sample. All experiments were conducted in triplicate and were performed as described in 2.6.1.
2.6.3 Optimization of the blocking conditions
Based on the results of 2.6.1 and 2.6.2, the microplates were coated with rPtTM at the optimal conditions. Afterwashing, the microplates were blocked with 1%, 1.2%, 1.5% BSA (Solarbio) and 2%, 4%, 6% skimmed milk powder(Nestle, Switzerland). According to the blocking condi- tions, the experiments were divided into three groups asdescribed in 2.6.2. After blocking and washing, the micro-plates were incubated with allergic serum and healthy peo- ple’s serum. All experiments were conducted in triplicate and were performed as described in 2.6.1.
2.6.4 Optimization of the dilution of HRP-conjugated goat anti-human IgE
Based on the results of 2.6.1, 2.6.2, and 2.6.3, the micro- plates were coated, blocked, and incubated with optimal conditions. After washing, the microplates were incubated for 30min at 37℃ with allergic serum and healthy peo-ple’s serum. Then, the plates were washed five times andincubated for 30min at 37℃ with HRP-conjugated goatanti-human IgE (diluted at 1:1500, 0.67μgmL−1; 1:2000, 0.5μgmL−1; 1:2500, 0.4μgmL−1). All experiments were con- ducted in triplicate and were performed as described in 2.6.1.
2.6.5 Determination of cut-off value
Based on all the optimal conditions above, the sera of 113 patients with crab allergy and 50 healthy people were detected. The data were processed with SPASS 17.0 sta- tistical software. 1 or 0 were entered in the first column, which 1 represented the sera from patients with crab al- lergy and 0 represented the sera from healthy people. The detected A450values were entered in the second column. The ROC curve was made by using the first column as state variable and the second column as test variable. With dif- ferent thresholds in the ROC curve, the sensitivity, speci- ficity, positive likelihood ratio (sensitivity/1-specificity), and Jorden index (sensitivity+specificity-1) correspondingto each cut-off were calculated. The threshold with the high-est values of positive likelihood ratio and Jorden index was regarded as the optimal cut-off value.
3 Results
3.1 Recombinant Expression of PtTM, PtMLC,and PtPL
The recombinant plasmid pET-21a(+)-PtTM, pET-21a(+)- PtMLC and pET-21a(+)-PtPL were transformed intoOrigami (DE3) and expressed as described above. Follow- ing the IPTG induction, major bands of the expected pro- tein were observed (Fig.2). The molecular weights of pu- rified rPtTM, rPtMLC, and rPtPL were 28.27kDa, 17.61kDa, and 35.84kDa, respectively (Fig.2).
3.2 IgE Reactivity to rPtTM, rPtMLC, and rPtPL
The capability of recombinant protein to react with se- rum sIgE was determined by the relationship between sIgE concentration and grading standard (Table 2) and interna- tional grading standard curve (Fig.3). The international gra- ding standard showed that when the sIgE concentration was 0.35IUmL−1, A450attained 0.2; thus, 0.2 was regarded as the critical value to definite the ability of the recombi- nant protein to react with serum sIgE. The reactivity againstrPtTM, rPtMLC, and rPtPL by the serum IgE of crab-al- lergic patients was determined by ELISA. As shown in Fig.4, among the 51 crab-allergic sera, 21 (41.2%) had po- sitive IgE to rPtTM; among the other 70 crab-allergic sera, 1.4% (1/70) and 12.9% (9/70) were IgE-positive to rPtMLC and rPtPL, respectively.
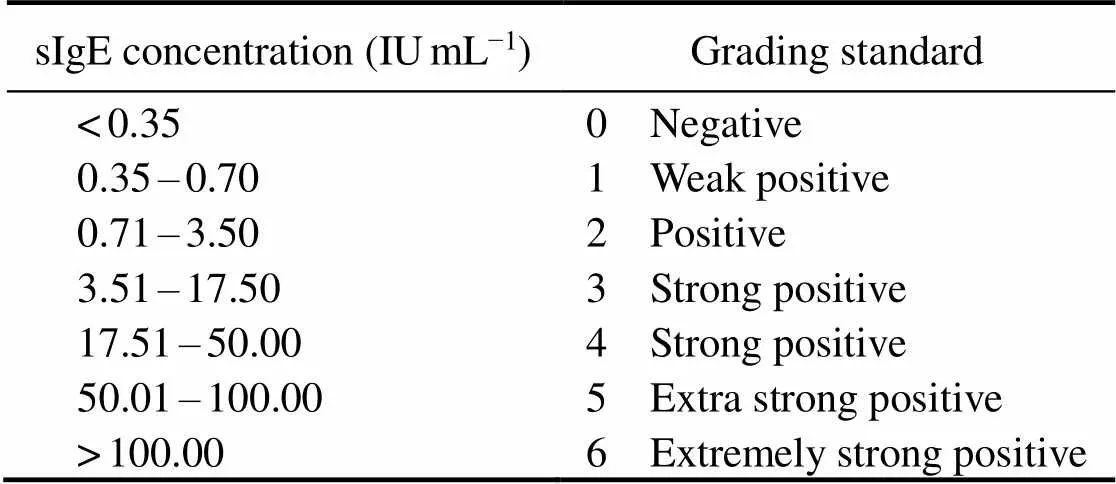
Table 2 Relationship between specific IgE concentration and international grading standard
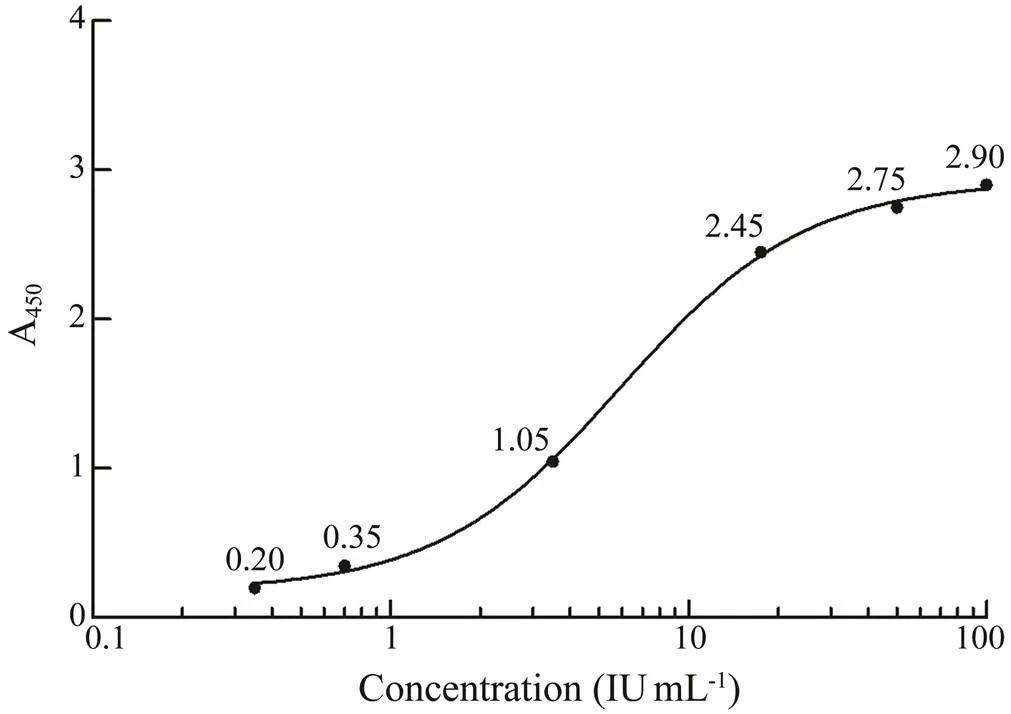
Fig.3 Standard curve of sIgE concentration.
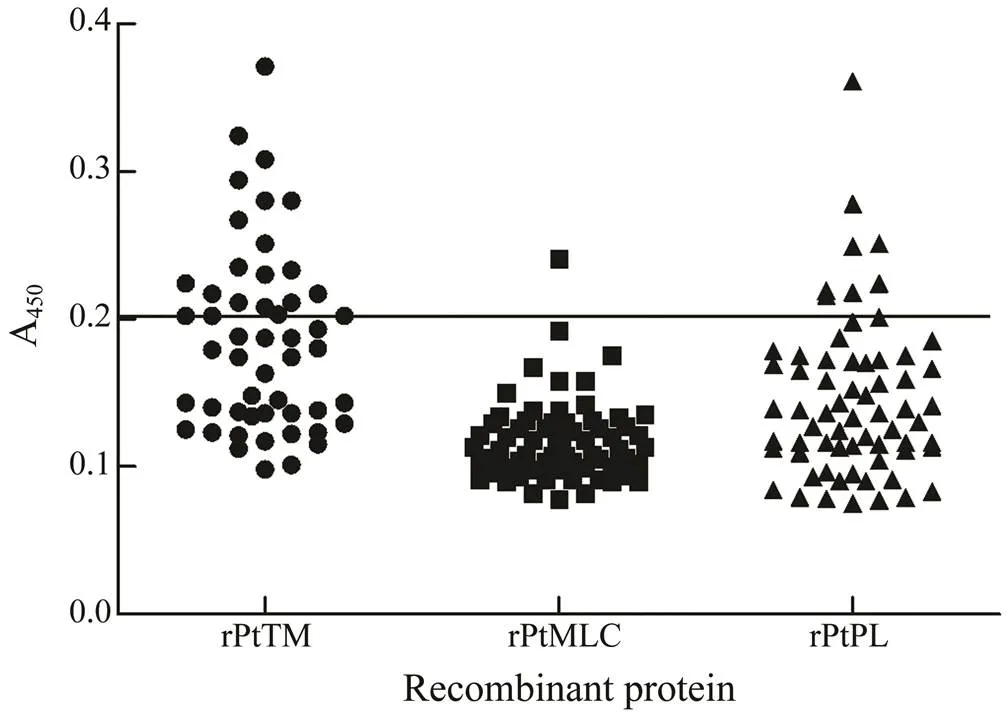
Fig.4 ELISA result of rPtTM, rPtMLC, rPtPL, and sera from patients with crab allergy.
3.3 Expression of PtTM in Different Tissues of Pond-Reared and Wild-Caught Crabs
WB results showed that the expression of TM was simi-lar in pond-reared crab and wild-caught crab, which was mainly expressed in the muscle, followed by the heart and a small amount in the gill (Fig.5A). The TM expression was detected in the testis of pond-reared crab, but was not detected in that of wild-caught crab. The β-actin and TMprotein were not detected in the hepatopancreas. More-over, the expression level of TM in the heart of pond-rear- ed crab was significantly higher than that of wild-caught crab (<0.05) (Fig.5B).
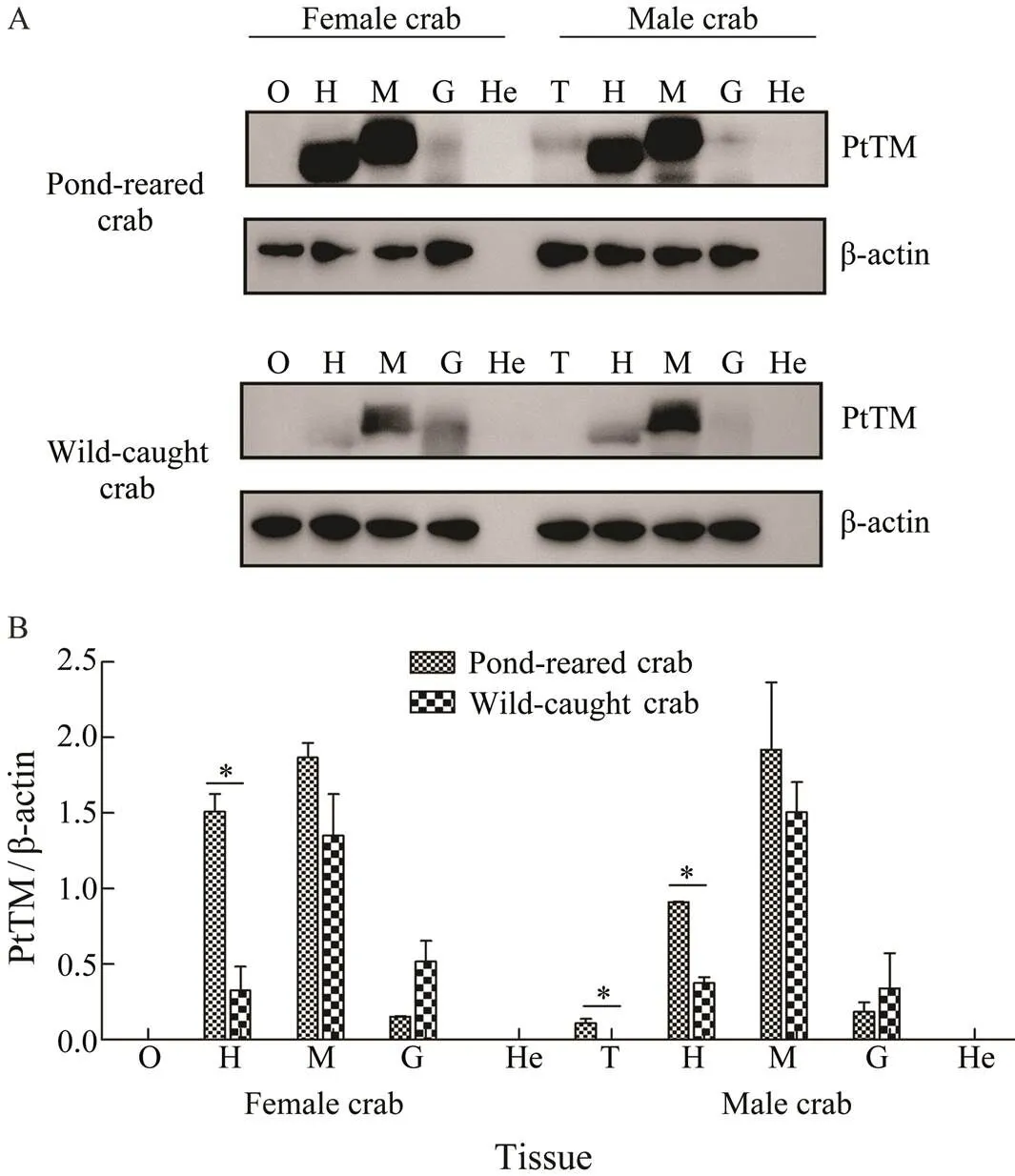
Fig.5 Expression of TM in different tissues of P. trituber- culatus. A, Western blot results of TM in different tissues; B, relative expression of TM in different tissues. O, ovary; T, testis; H, heart; M, muscle; G, gill; He, hepatopancreas; *P<0.05.
3.4 Optimization of the ELISA Reagent
The ELISA results of coating with different concentra- tions of rPtTM (Fig.6) showed that A450gradually reducedwhen the coating mass of rPtTM decreased, indicating that the specific bond between the antigen (TM) and the cor- responding antibody reduced. However, with the increas- ing of rPtTM coverage, the A450did not rise any more, whichwas because the binding of antigens to antibodies had reach- ed its limit. As the coating mass of rPtTM decreased from 50 to 25ng per well, the A450value significantly decreased. Therefore, 50ng per well was selected as the optimal coat- ing concentration for TM allergen. The P/N values of dif- ferent coating times and temperatures were shown in Fig.7, and the highest value was obtained. Therefore, 3h at 37℃ was selected as the optimal coating conditions for TM al- lergen.
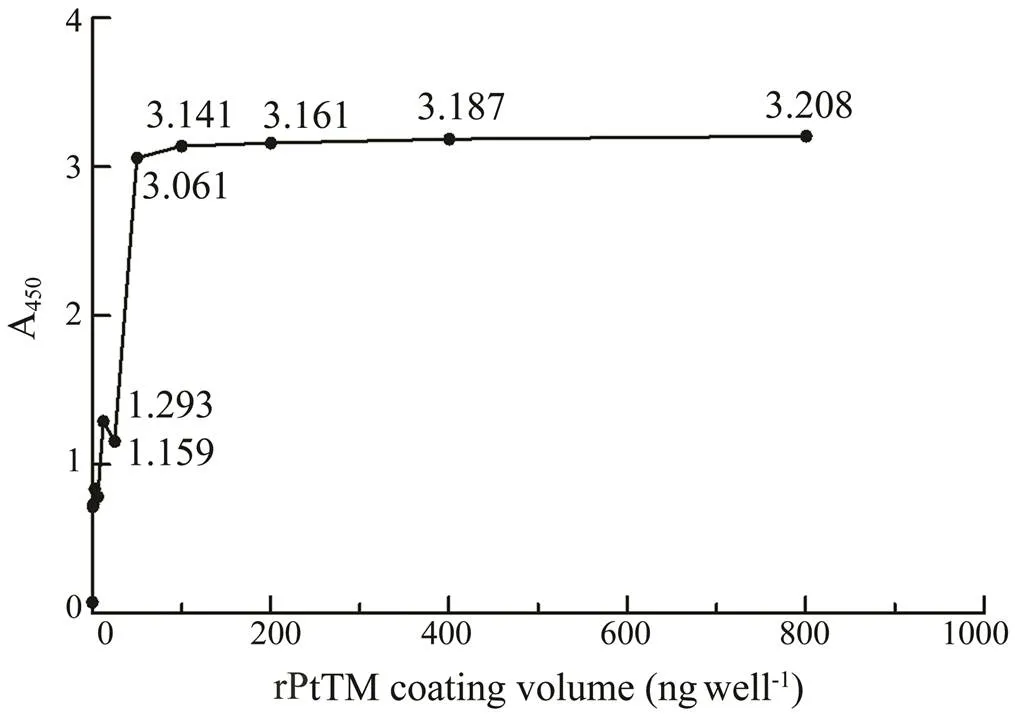
Fig.6 ELISA results (A450) of the rPtTM with different con- centrations.
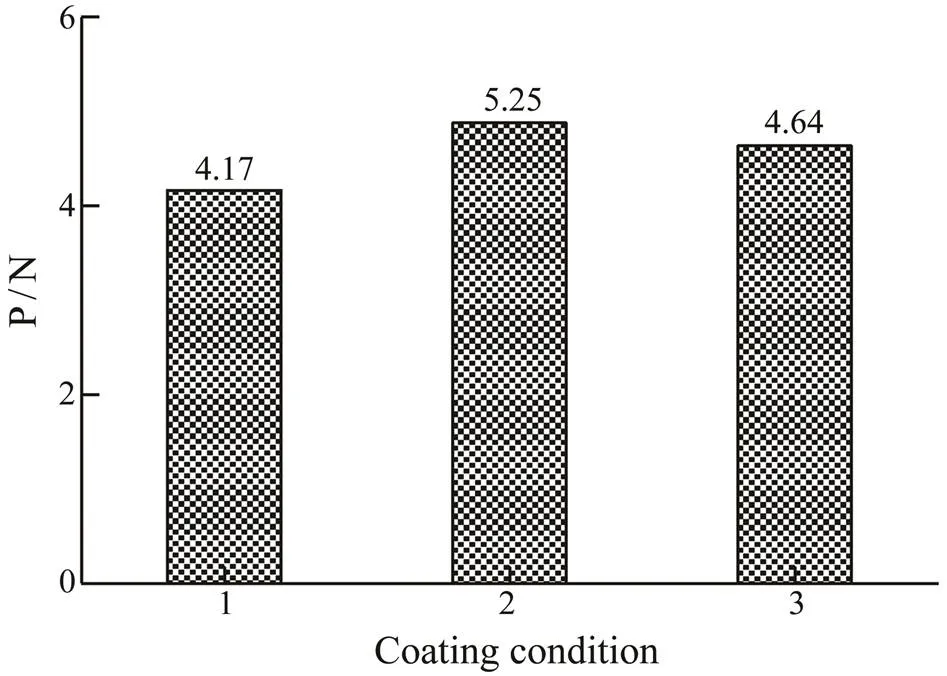
Fig.7 Ratio of ELISA results (A450) of pooled allergic sera to healthy sera reacted with the rPtTM in different coat- ing conditions. 1, coated for 3h at 37℃ then at 4℃ over-night; 2, coated for 3h at 37℃; 3, coated at 4℃ overnight.
The P/N values of different blocking conditions (Fig.8)showed that the blocking effect of BSA was better than thatof skimmed milk powder, and the optimal blocking con-ditions was incubating with 1.2% BSA for 37℃ at 3h. TheELISA results of HRP-conjugated goat anti-human IgE withdifferent dilutions (Table 3) showed that the A450values de- creased with the increase of the secondary antibody dilu- tion. Therefore, the 1:1500 dilution was selected as the op- timal concentration of secondary antibody.

Fig.8 Ratio of ELISA results (A450) of pooled allergic sera to healthy sera reacted with the rPtTM in different block- ing conditions. 1, coated for 3h at 37℃ then at 4℃ over- night; 2, coated for 3h at 37℃; 3, coated at 4℃ overnight.

Table 3 ELISA results of HRP labeled goat anti-human IgE antibody with different dilutions (A450, mean±SD)
The ROC curve was obtained (Fig.9), and the sensiti- vity and specificity were calculated for different thresh- olds (Table 4). Compared to other thresholds, the thresh- old of 0.45 yielded the highest positive likelihood ratio and Jordan index. Therefore, the cut-off value of ELISA was used to detect crab anaphylaxis. When the cut-off value was 0.45, the AUC was 0.979, which was the highest va- lue. The sensitivity, specificity, positive likelihood ratio, and Jordan index were 83.52%, 98.00%, 41.76, and 0.82, respectively. Moreover, the diagnostic efficiency of thismethod was 85.0% compared to the sensitization result (41.2%).
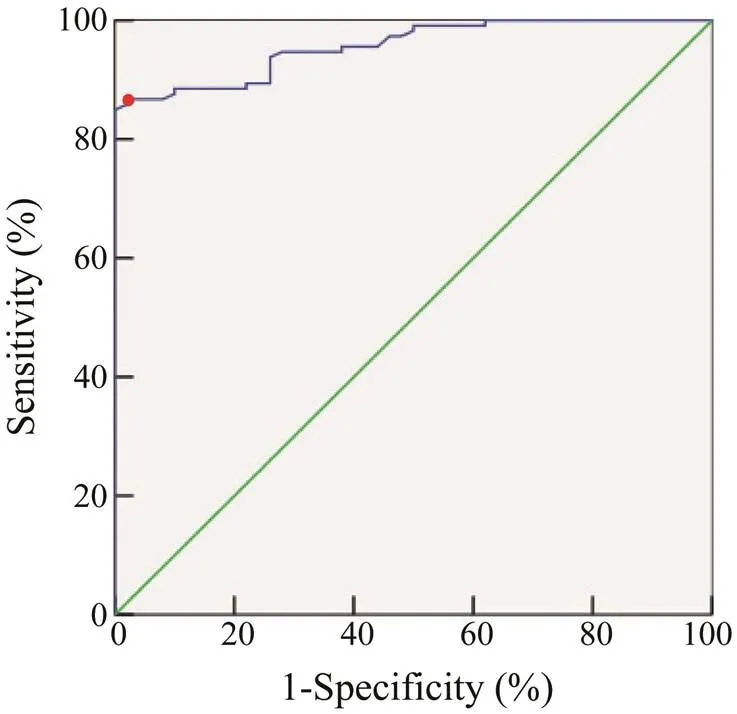
Fig.9 ROC curve of rPtTM in the detection of crab ana- phylaxis.
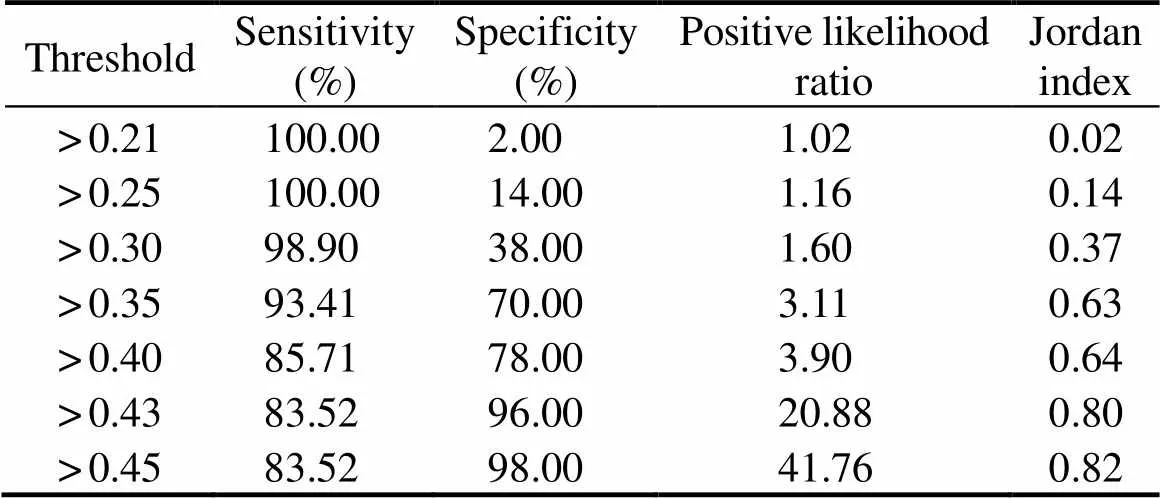
Table 4 Efficiency of diagnosing crab anaphylaxis at different thresholds
4 Discussion
In recent years, recombinant proteins have been widely used for the detection of sensitization of allergens. The sIgE reactivity of recombinant TM fromwas similar to that from(Yi., 2011),(Koeberl., 2014) and(Liang., 2009), indicating that TM is one of the ma- jor crab allergens, and it might be reliable to detect the sen-sitization by ELISA which used recombinant protein as coat- ing antigen. The weak sIgE reactivity of rPtMLC was si- milar to that of(Ayuso., 2008) and(Zhang., 2015), which indicated that MLC might also be a crab allergen. The immune response of rPtPL was obvious, but few reports could be referred to discuss the allergenic nature of PL. So, it was considered as a potential allergen temporarily.
TM is present in various muscle (skeletal, smooth, and cardiac) and non-muscle cells (Scellini., 2015). In the muscle, TM plays central role in the regulation of muscle contraction (Borovikov., 2011). In non-muscle cells, it plays vital role in maintaining the integrity of the cyto- skeleton (Khaitlina, 2015). In this study, the expression of TM protein in different tissues ofwas si- milar to that of(Miyazaki., 1992)and(Han., 2012). As an im-portant protein component, TM was mainly expressed in the muscle and heart, which had performed a long-time mu-scle contraction, and was also present in the gills. It was found that neither the reference protein nor target protein were detected in the hepatopancreas through repeated ex- periments. The antibody and experiment operations wouldnot be the main influencing factors according to the re- sults in other tissues, and the high fatty acids in the hepa- topancreas ofwould affect the quality of protein extraction. There were three layers, including up- per (fat), middle (protein), and lower (cell debris) layers, after lysis and centrifugation in the process of protein ex- traction. The fat can be removed by using a 0.22-μm or 0.45-μm centrifugal filter tube, or using filter paper or glass wool.
The muscle was the main edible part of the crab. That no significant difference in the expression of TM between the muscles of pond-reared crabs and wild-caught crabs indi- cated that the intake of wild-caught crabs did not increase the risk of allergy. The different expressions of TM in the hearts of pond-reared crabs and wild-caught crabs indicated that the hearts of pond-reared crabs had a higher demand for TM than wild-caught crabs. It was reported that themRNA level was different in the ovarian development of(Shui., 2016). In, the expression level ofgene was the highest in androgenic glands and higher in testes than in ovaries (Jin., 2014). It indicated that TM might be involved in thedevelopment of crustacean gonadal glands, though more investigations are needed to explore the role of TM in go- nad development of the pond-reared crabs and wild-caught crabs.
PtTM (ABS12234.1) shared high similarity with that from(A4URH3.1, 99.30% identity),(QHW05412.1, 98.94% identity),(BAF47268.1, 98.94% identity), and(QHW- 05411.1, 98.24% identity). Therefore, TM was used as an antigen of ELISA for analyzing crab food anaphylaxis. How- ever, there is strong homology of tropomyosin among the crustaceans, and it also shares sequence homology withhouse dust mites and cockroaches as they are all arthropods(Villalta., 2010; Abramovitch., 2013). Future in- vestigations are needed to consider cross-reactivity.
During the optimization of blocking conditions, the BSA was found to have a better blocking effect than skimmed milk powder, which was similar to the research of ELISA reagent foranaphylaxis (Chen., 2010). However, the reaction conditions in different reports were always different. For example, in a competitive ELISA,which was carried out to screen the hybridoma cell lines for the anti-glycinin activity of soybean, gelatin was used as a blocking reagent instead of BSA or skim milk pow- der (Xi., 2010). The incubating time and temperature of coating in the ELISA reagent foranaphyla- xis (Chen., 2012) was 37℃ for 3h and then 4℃ over- night instead of 37℃ for 3h. The incubating time of block-ing was also different between the ELISA in this study anda sandwich ELISA for the detection of shrimp tropomy- osin (Zeng., 2019). Therefore, it is still necessary to optimize the reaction conditions in order to obtain a better detection effect when developing an ELISA reagent for food allergy analysis.
The ROC curve was used to determine the optimal cut- off value of the ELISA reagent for analyzing crab food ana-phylaxis, and the cut-off value of 0.45 was inconsistent with the 0.159 ofanaphylaxis(Huang., 2010). It was reported that the cut-off values of hepatitis C antibody determined by two kinds of ELISA analysis systems were different (Tang., 2014), and the cut-off values were also different in two commercial ELISA kits which were used for serodiagnosis of pertussis (Waldemar., 2011). The reasons for this result may be as follows: firstly, the recombinant protein instead of crude protein ex-tract was used as the coating antigen in this study. Second- ly, the cut-off value was calculated by the ROC curve ins- tead of using the mean of negative control + 2 or 3 SD. Ad- ditionally, experimental reagents and conditions were dif- ferent. However, the limit of quantitation corresponding to the cut-off values could not be obtained due to the lack of human IgE standards substance (Werner., 2007).
5 Conclusions
In summary, the ELISA results revealed that MLC and PL are potential allergens of, but more studies are needed to verify the allergic characteristic of MLC and PL. The ELISA reagent developed with rPtTM had fine sensitivity and specificity, and may be applicable for laboratory detection of crab anaphylaxis. But the detec-tion limit and the cross-reactivity between crab, shrimp, and dust mite are worthy of further studies. It is the first time to reveal the expression of TM protein in different tissues ofin this study. The result of target pro- tein detection might be affected by the undetected internal reference protein in the hepatopancreas, which provides some clues for further investigations.
Acknowledgements
The authors would like to thank all the members of the Crustacean Study Laboratory for their technical advice and helpful discussions. This research was supported by Zhe- jiang Provincial Natural Science Foundation of China (No. LY18H110003), and the General Scientific Research Project of Zhejiang Education Department (No. Y201940887).
Abramovitch, J. B., Kamath, S., Varese, N., Zubrinich, C., Lopata, A. L., O’Hehir, R. E.,., 2013. IgE reactivity of blue swim- mer crab () tropomyosin, Por p 1, and otherallergens; cross-reactivity with black tiger prawn and effects of heating., 8 (6): e67487.
Aycan, K., Macmillan, N. A., and Rotello, C. M., 2010. Positive and negative remember judgments and ROCs in the plurals pa- radigm: Evidence for alternative decision strategies., 38 (5): 541-554.
Ayuso, R., Grishina, G., Bardina, L., Carrillo, T., Blanco, C., Ibá- ñez, M. D.,., 2008. Myosin light chain is a novel shrimp allergen, Lit v 3.,122 (4): 795-802.
Bai, G. L., Huang, L. Y., Du, B. J., Wang, Q. Q., and Ning, Y., 2016. Isolation and characterisation of tropomyosin from shrimp (Boone) and its association property at high ionic strength., 30 (1): 115-119.
Borovikov, Y. S., Avrova, S. V., Karpicheva, O. E., Robinson, P., and Redwood, C. S., 2011. The effect of the dilated cardiomyopa- thy-causing Glu40Lys TPM1 mutation on actin-myosin inter- actions during the ATPase cycle., 411 (3): 496-500.
Borres, M. P., Nobuyuki, M., Sakura, S., and Motohiro, E., 2016. Recent advances in component resolved diagnosis in food al- lergy., 65 (4): 378-387.
Burks, A. W., Tang, M., Sicherer, S., Muraro, A., Eigenmann, P. A., Ebisawa, M.,., 2012. ICON: Food allergy., 129 (4): 906-920.
Chen, W., Huang, H. B., Liu, Q. Y., Wu, M. C., and Wu, J., 2012. Research of ELISA reagent for crab food anaphylaxis., 31 (6): 42-44 (in Chinese with Eng- lish abstract).
Chen, W., Liu, P. J., Liu, Q. Y., You, C. X., Wu, M. C., Wu, J.,., 2010. Research of ELISA reagent of specific IgE foranaphylaxis., 29 (4): 22-27 (in Chinese with English abstract).
Curin, M., Garib, V., and Valenta, R., 2017. Single recombinant and purified major allergens and peptides., 119 (3): 201-209.
FAO/WHO, 2001. Evaluation of allergenicity of genetically mo- dified foods..New York, 22-25.
Goh, S. H., Soh, J. Y., Loh, W., Lee, K. P., Tan, S. C., Heng, W. J. K.,., 2018. Cause and clinical presentation of anaphylaxis in Singapore: From infancy to old age., 175 (1-2): 91-98.
Guillermo, T. P., Claudio, C., Pedro, L. S. E., Diego, A. T., and Ma-nuel, D. T. B., 2019. Sensitization profile in patients with re- spiratory allergic diseases: Differences between conventional and molecular diagnosis (a cross-sectional study)., 17 (1): 8, https://doi.org/10.1186/s12948-019-0112-4.
Han, F., Wang, Z. Y., and Wang, X. Q., 2012. Prokaryotic expres- sion, antibody preparation and tissue expression identification of tropomyosin in., 36 (6): 879-883 (in Chinese with English ab- stract).
Huang, H. B., Chen, W. W., Chen, W., Li, J., Li, X. D., and Liu, Q. Y., 2010. Detection of serum specific IgE to crab allergen in different group people with extractedaller- gens., 31 (2): 128-130 (in Chinese with English abstract).
Jin, S. B., Jiang, S. F., Xiong, Y. W., Qiao, H., Sun, S. M., Zhang, W. Y.,., 2014. Molecular cloning of two tropomyosin fa- mily genes and expression analysis during development in ori- ental river prawn,., 546 (2): 390-397.
Kerstin, B., Wangorsch, A., Garoffo, L. P., Reuter, A., Conti, A., Taylor, S. L.,., 2011. Generation of a comprehensive panelof crustacean allergens from the North Sea Shrimp., 48 (15-16): 1983-1992.
Khaitlina, S. Y., 2015. Tropomyosin as a regulator of actin dyna- mics., 31 (8): 255-291.
King, T. P., Lu, G., Gonzalez, M., Qian, N., and Soldatova, L., 1996. Yellow jacket venom allergens, hyaluronidase and phos- pholipase: Sequence similarity and antigenic cross-reactivity with their hornet and wasp homologs and possible implicationsfor clinical allergy., 98 (3): 588-600.
Koeberl, M., Kamath, S. D., Saptarshi, S. R., Smout, M. J., Rol- land, J. M., O’Hehir, R. E.,., 2014. Auto-induction for high yield expression of recombinant novel isoallergen tropomyosin from King prawn () for improved diag- nostics and immunotherapeutics., 415: 6-16.
Liang, Y. L., Cao, M. J., Guo, C., Su, W. J., Zhang, L. J., and Liu, G. M., 2009. Cloning and expression of tropomyosins from crabs., 33 (1): 24-29 (in Chinese with English abstract).
Liu, G. M., Cao, M. J., Huang, Y. Y., Cai, Q. F., Weng, W. Y., and Su, W. J., 2010. Comparative study ofdigestibility of major allergen tropomyosin and other food proteins of Chinese mitten crab ()., 90 (10): 1614-1620.
Lu, J. K., Yu, Z. B., Mu, C. K., Li, R. H., Song, W. W., and Wang, C. L., 2017. Characterization and functional analysis of a novel C-type lectin from the swimming crab., 64: 185-192.
Mao, H. Y., Cao, M. J., Maleki, S. J., Cai, Q. F., Su, W. J., Yang, Y.., 2013. Structural characterization and IgE epitope analysis of arginine kinase from., 56 (4): 463-470.
Martín, B. B., Tonatiuh Ramses, B. P., Nicole, M. R., Jaime, M. R.,and Martín, R. F., 2015. Prevalence of peanut, tree nut, sesame, and seafood allergy in Mexican adults., 67 (6): 379-386.
Miyazaki, J. I., Makioka, T., Fujiwara, Y., and Hirabayashi, T., 1992. Tissue specificity of crustacean tropomyosin., 263 (3): 235-244.
Moshe, B. S., Harrington, D. W., Soller, L., Fragapane, J., Joseph, L., St Pierre, Y.,., 2010. A population-based study on pea- nut, tree nut, fish, shellfish, and sesame allergy prevalence in Canada., 125 (6): 1327-1335.
Nurul Izzah, A. R., Rosmilah, M., Zailatul Hani, M. Y., Noorma- lin, A., Faizal, B., and Shangnaz, M., 2015. Identification of ma-jor and minor allergens of Mud crab ()., 10 (2): 90-97.
Parkinson, R. M., Conradie, J. D., Milner, L. V., and Marimuthu, T., 1988. The interpretation of ELISA results by means of the standard deviation ratio., 115 (1): 105-110.
Piboonpocanun, S., Jirapongsananuruk, O., Tipayanon, T., Boon- choo, S., and Goodman, R. E., 2011. Identification of hemocya- nin as a novel non-cross-reactive allergen from the giant fresh- water shrimp., 55 (10): 1492-1498.
Pittner, G., Vrtala, S., Thomas, W. R., Weghofer, M., and Valenta, R., 2004. Component-resolved diagnosis of house-dust mite allergy with purified natural and recombinant mite allergens., 34 (4): 597-603.
Sampson, H. A., 2004. Update on food allergy., 113 (5): 805-820.
Scellini, B., Piroddi, N., Flint, G. V., Regnier, M., Poggesi, C., and Tesi, C., 2015. Impact of tropomyosin isoform composition on fast skeletal muscle thin filament regulation and force develop- ment., 36 (1): 11-23.
Shui, Y., Xu, Z. H., and Zhou, X., 2016. Expression of the tro- pomyosin gene during the ovarian development of Red Swamp Crayfish ()., 43 (1): 42-46 (in Chinese with English abstract).
Sun, Y. F., Huang, J. F., Xiang, J. J., Wang, C. X., and Chen, C. F., 2015. Proteomics and immunological analysis of arginine ki- nase, an important shrimp allergen from., 36 (1): 170-173 (in Chinese with English abstract).
Suzanne, E. R., Wheatly, M. G., and Gillen, C., 2015. Calcium binding tosarcoplasmic calcium binding protein splice variants., 179: 57-63.
Tang, J., Bao, J. L., Meng, C. R., and Zhang, Z. X., 2014. Confir- mation and analysis of the cut-off value in hepatitis C antibody determined by ELISA with receiver operating characteristic curve., 29 (8): 826-830 (in Chinese with English abstract).
Thu, T. K. L., Thuy, T. B. T., Huong, T. M. H., An, T. L. V., Em- ma, M., and Lopata, A. L., 2020. The predominance of seafood allergy in Vietnamese adults: Results from the first population-based questionnaire survey., 13 (3): 100-102.
Tuano, K. T. S., Anvari, S., Hanson, I. C., Hajjar, J., Seeborg, F., Noroski, L. M.,., 2017. Utility of shrimp andspe-cific IgE for shrimp allergy diagnosis in non-house dust mite sensitized patients.: 199620, https://doi.org/10.1101/199620.
Villalta, D., Tonutti, E., Visentini, D., Bizzaro, N., and Mistrello, G., 2010. Detection of a novel 20kDa shrimp allergen showing cross-reactivity to house dust mites., 42 (1): 20-24.
Wai, C. Y., Leung, N. Y., Yin Leung, A. S., Lam, M. C., Xu, K., Shum, Y.,., 2019. Troponin C is the major shrimp allergen among Chinese patients with shellfish allergy., 143 (2): AB270.
Waldemar, R., Iwona, P. S., Paweł, S., and Aleksandra, A. Z., 2011. Reliability of the cut-off value in the routine serodiagnosis of pertussis performed by the commercial ELISA assays., 63 (1): 73-80.
Warren, C. M., Aktas, O. N., Gupta, R. S., and Davis, C. M., 2019. Prevalence and characteristics of adult shellfish allergy in the United States., 144 (5): 1435-1438.e5.
Werner, M. T., Fæste, C. K., and Egaas, E., 2007. Quantitative Sandwich ELISA for the determination of tropomyosin from crustaceans in foods., 55 (20): 8025-8032.
Woldetensay, Y. K., Belachew, T., Tesfaye, M., Spielman, K., and Scherbaum, V., 2018. Validation of the Patient Health Ques- tionnaire (PHQ-9) as a screening tool for depression in preg- nant women: Afaan Oromo version., 13 (2): e0191782.
Xi, M., Peng, S., He, P., Han, P., Wang, J., Qiao, S.,., 2010. Development of monoclonal antibodies and a competitive ELISAdetection method for glycinin, an allergen in soybean., 121 (2): 546-551.
Xu, Y. R., Fan, Y. S., and Yang, W. X., 2017. Mitochondrial pro- hibitin and its ubiquitination during spermatogenesis of the swimming crab., 627: 137-148.
Yang, A. C., Arruda, L. K., Santos, A. B. R., Barbosa, M. C. R., Chapman, M. D., Galvão, C. E. S.,., 2010. Measurement of IgE antibodies to shrimp tropomyosin is superior to skin prick testing with commercial extract and measurement of IgE to shrimp for predicting clinically relevant allergic reactions after shrimp ingestion., 125 (4): 872-878.
Yang, Y., Cao, M. J., Alcocer, M., Liu, Q. M., Fei, D. X., Mao, H. Y.,., 2015. Mapping and characterization of antigenic epi- topes of arginine kinase of., 65 (2): 310-320.
Yeung, J., 2006. Enzyme-linked immunosorbent assays (ELISAs) for detecting allergens in foods. In:.Koppelman, S. J., and Hefle, S. L., eds., Woodhead Publishing,Cambridge, 109-124.
Yi, H. T., Xia, L. X., Liu, F., Yan, H., Huang, Z., Tang, M. J.,.,2011. The cloning expression, purification and characterization of a fragment of red swamp crayfish major allergen tropomyo- sin., 35 (3): 280-285 (in Chinese with English abstract).
Zeng, G. Q., Luo, J. Y., Huang, H. M., Zheng, P. Y., Luo, W. T., Wei, N. L.,., 2015. Food allergy and related risk factors in 2540 preschool children: An epidemiological survey in Guang-dong Province, southern China., 11 (3): 219-225 (in Chinese with English abstract).
Zeng, L., Song, S., Zheng, Q., Luo, P., Wu, X., and Kuang, H., 2019. Development of a sandwich ELISA and immunochroma- tographic strip for the detection of shrimp tropomyosin., 30 (1): 606-619.
Zhang, Y. X., Chen, H. L., Maleki, S. J., Cao, M. J., and Liu, G. M., 2015. Purification, characterization, and analysis of the aller- genic properties of myosin light chain in., 63 (27): 6271-6282.
November 29, 2020;
March 1, 2021;
August 3, 2021
© Ocean University of China, Science Press and Springer-Verlag GmbH Germany 2022
E-mail: muchangkao@nbu.edu.cn
E-mail: xusuling@nbu.edu.cn
(Edited by Qiu Yantao)
杂志排行
Journal of Ocean University of China的其它文章
- Study of the Wind Conditions in the South China Sea and Its Adjacent Sea Area
- A Spatiotemporal Interactive Processing Bias Correction Method for Operational Ocean Wave Forecasts
- Characteristics Analysis and Risk Assessment of Extreme Water Levels Based on 60-Year Observation Data in Xiamen, China
- Underwater Target Detection Based on Reinforcement Learning and Ant Colony Optimization
- Polar Sea Ice Identification and Classification Based on HY-2A/SCAT Data
- Thermo-Rheological Structure and Passive Continental Margin Rifting in the Qiongdongnan Basin,South China Sea, China
